Abstract
Standard cancer therapies for solid malignancies, such as chemotherapy and radiotherapy, are not target specific against cancer cells and are often not fully efficacious. Chemotherapy and radiotherapy may cause side effects, and the need to develop additional strategies for cancer treatment is urgent. MicroRNAs (miRNAs) are small non-coding RNAs with heterogeneous functions and have been described in almost every known cancer model. Besides their basic tumor-suppressive and oncogenic functions, they also have the potential to modulate chemotherapy and radiotherapy and to be manipulated with chemical compounds to make them chemically suitable for efficient delivery to cancer cells. It has been suggested that the level of expression of specific miRNAs could increase treatment efficacy by determining the stage of chemotherapy/radiotherapy sensitivity. Application of miRNAs alone or in combination with standard therapeutic strategies may significantly improve the success of cancer treatments in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
One microRNA (miRNA) can be used as both a target for cancer therapy or be therapeutic itself. |
miRNAs as therapeutics or drug targets may significantly increase therapy success and modulate the side effects of the therapy. |
Combinations of miRNAs and standard therapeutic strategies may be an important tool for cancer treatment. |
1 Introduction
Malignant transformation results from the combined effect of genetic and epigenetic changes that lead to cancer formation of various types of cells. Along with these genetic and epigenetic changes, such as promoter hypermethylation, cancer pathogenesis is frequently associated with small non-protein-coding genome elements, known as microRNA (miRNA) molecules. miRNAs are single-stranded molecules, approximately 19–24 nucleotides long, that tune gene expression at the posttranscriptional level by binding to the 3′ untranslated region (UTR) of their partially complementary messenger RNA (mRNA) targets. The effect of their activity is translational repression and reduced protein synthesis [1]. mRNA levels and the levels of translation of mRNA into the protein depend on miRNA levels, and the recognition of 3’UTR by the ‘seed’ sequence on miRNA molecule [2, 3]. Since miRNAs repress translation of tumor suppressor and oncogenes, they can be both oncogenic and tumor-suppressive [4], and this can result in the formation of different cancer phenotypes and disease progression.
Diverse miRNA signatures (changes in combinations of various miRNA expression levels) have been linked with both hematological and solid malignancies [5]. According to Li et al. [6], miRNA expression level profiling, rather than mRNA expression profiling, may also soon be used as an additional factor and criterion in the classification of human cancers; this suggests they may be potential therapeutic targets in cancer research.
Besides the miRNA features used in tumor classification and prediction of disease progression, miRNA molecules have been discussed, examined, and proposed as a powerful tool for the development of additional drug and therapeutic options in cancer treatment. The heterogeneity of their function and behavior in different cancer types and subtypes make them suitable for various therapeutic approaches and strategies. miRNA molecules can be modified chemically and used in replacement therapy as miRNA mimics or can be inhibited by different chemical compounds and genetic systems.
2 Limitations of Current Cancer Therapies
Classical chemotherapy disrupts the activity of vital cellular elements, such as the cytoskeleton and ribosomes; blocks key enzyme action to inhibit replication, transcription, or translation; or simply harms DNA to arrest cancer cell proliferation and induce production of free radical cells. Yet, classical cancer therapy is not target specific against cancer cells. It also causes toxicity in rapidly dividing normal tissue cells in the gastrointestinal tract and in bone marrow, causing side effects.
Next, target-specific approaches, such as tyrosine kinase inhibitors and growth factor receptor inhibitors, were introduced to inhibit molecular targets, managing cancer formation and progression [7]. Unlike chemotherapy, which cannot discriminate between tumor cells and rapidly proliferating non-tumorous cells, targeted therapy has greater specificity to kill tumor cells than non-transformed cells because their targets have intracellular localization. They can also be easily combined with conventional chemotherapy because their cytotoxicity affects different mechanisms and can work synergistically. The inhibitors bind to a wide range of receptors and downstream elements, decreasing specificity and increasing toxicity [7]. However, targeted therapy also has limitations, because it does not have sufficient specificity. In fact, targeted therapy frequently cannot target and identify early events in malignant transformation, development of drug resistance via the formation of tumor cell sub clones, and the selection of appropriate patients according to the required target, so these therapies serve as second- and third-line tumor defenses [7].
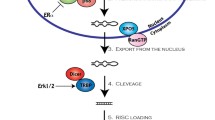
More than 50% of patients with solid malignancies undergo radiotherapy [8]. Radiotherapy prevents the highly proliferative cancer cells from dividing. Irradiation also induces cell and DNA damage and activates repair mechanisms. If the activation of cell repair systems and the microenvironment is too strong, the tumor may become resistant to radiation treatment. Resistance to radiation leads to earlier tumor recurrence and poor prognosis [8]. The introduction of therapeutics such as miRNA mimics or anti-miR molecules could soon overcome problems such as radioresistance and radiotoxicity, or could be used to modulate radiotherapy in various cancers, such as prostate cancer, glioblastoma (GB), breast cancer (BC), etc. [9]. Given the heterogeneity of miRNAs involved in the biology of malignant tumors, as described in our previous article [10], it is also important not to neglect their heterogeneity when discussing drug resistance. The heterogeneity of miRNAs means they may be used (1) either as drugs or as drug targets in cancer treatment, (2) as the major therapeutic, and as adjuvant therapy to sensitize tumors to treatment, (3) to prevent or reduce resistance to therapeutics/therapy, and (4) to reduce the toxic effects of treatment. The long-term goal of improving therapeutic efficacy involves identifying agents or molecules to enhance cancer cell sensitivity to therapy [11], as summarized in Fig. 1.
3 Advantages of MicroRNA (miRNA)-Based Cancer Therapies
miRNAs can simultaneously control the levels of a wide range of genes used in cancer therapy. It has been suggested that targeting a group of associated oncogenic pathways or genes concurrently was associated with good outcomes in patients with cancer [12]. Furthermore, miRNAs, which are natural antisense nucleotides, displayed lower toxicity and a more decreased immune response than protein-based drug complexes and plasmid DNA-based gene therapy. Therefore, miRNAs may have a remarkable function in cancer treatment [13].
Several strategies have been developed to target oncogenic miRNAs in cancer therapy, such as miRNA inhibitors, which bind to mature overexpressed miRNA, disabling their processing with either RNA-induced silencing complex (RISC), or their maturation, or the process of translational repression [14, 15].
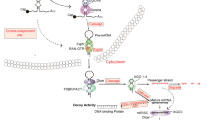
The basic strategy lies in the oncogenic feature of miRNAs overexpressed in several cancers. Their oncogenic ability can be repressed with various molecules, named miRNA inhibitors. These inhibitors represent synthetic single-stranded oligonucleotides, mainly represented as anti-sense oligonucleotides (ASOs) with full or partial complementary binding to the endogenous miRNA. Several synthetic anti-miRNA strategies with either immature or mature miRNA molecules as targets have been described: locked nucleic acid (LNA) anti-miRNAs, anti-miR oligonucleotides (AMOs), small-molecule chemical compounds (small molecule inhibitors of miRNAs [SMIRs]), antagomiRs, miRNA-zippers [16], and miRNA sponges [15, 17], as shown in Fig. 2.
Modulation and targeting of specific miRNAs in cancer using a range of anti-miRNA techniques can block miRNAs with altered and undesirable activities and free up previously silenced gene transcripts to translate into proteins. These are usually tumor suppressors, and they activate their downstream regulatory networks, including pro-apoptotic or DNA-repairing signaling pathways, or can increase drug/therapy sensitivity, thus improving treatment outcome and survival.
4 miRNAs in Drug, Chemotherapy, and Radiotherapy Resistance and Sensitivity
Because cancer is a very heterogeneous disease, and malignant tumors frequently comprise genetically heterogeneous cells, the tumor might develop resistance to chemotherapy [18]. Recent reports indicate that miRNAs have an important role in inducing resistance to anti-cancer drugs. Specific miRNA alterations occur selectively in cancer cells, rendering these cells resistant to various chemotherapeutic agents. For example, resistance to 5-fluorouracil is mediated by alterations in miR-21, miR-27a/b, and miR-155, and sensitivity to docetaxel is influenced by miR-98, miR-192, miR-194, miR-200b, miR-212, miR-424, and miR-214 [18].
miR-451 was overexpressed in tissue from non-small-cell lung carcinoma (NSCLC) when compared with normal lung tissue, and overexpression of miR-451 increased cisplatin (DDP)-based chemosensitivity in A549 cells by suppressing cell development and triggering apoptosis formation. Bian et al. [19] showed that miR-451 upregulation increased caspase-3-dependent apoptosis via Akt signaling cascade inactivation, which in turn reduced B-cell lymphoma 2 (Bcl-2), while elevating Bcl-2-associated X (Bax) protein levels. Moreover, miR-31 is overexpressed in NSCLC cell lines and has been shown to trigger resistance to DDP. To prove this, Glavinas et al. [20] transfected DDP-sensitive human lung cancer SPC-A-1 cells with miR-31 mimics, which caused a remarkable elevation in the resistance of the SPC-A-1 cell line, whereas transfection with miR-31 inhibitors rendered the initially resistant NSCLC line (NCI-H1299) sensitive to DDP therapy.
Adjuvant treatment is used after or alongside initial treatment to boost efficiency and enhance disease management. It would be advantageous if adjuvant therapy could prevent general resistance mechanisms from appearing, generally via genetic and epigenetic alterations in the cancer cell or in their microenvironment, by making the resistant cancer cells sensitive to a related agent. Numerous preclinical studies have demonstrated miRNA-based therapy agents as hopeful targets for this type of adjuvant treatment. For instance, colorectal carcinoma (CRC) cells were rendered sensitive to methotrexate by miR-192 [21] and to 5-fluorouracil by miR-143 [22], whereas miR-222 has been thought to take a role in drug resistance via controlling a disintegrin and metalloprotease 17 (ADAM17) [23]. In lung cancer, miR-379 increases chemosensitivity to cisplatin via eukaryotic translation initiation factor 4 gamma 2 (EIF4G2) [24].
FOLFOX (folinic acid, fluorouracil, and oxaliplatin) is the most common primary chemotherapy used in advanced CRC; however, only half of the patients benefit from this therapy, and there is no perfect way to anticipate the development of resistance [25]. Chen et al. [25] proved the upregulation of serum miR-19a in FOLFOX chemotherapy-resistant patients, proposing that the serum miR-19a level may be a molecular biomarker for anticipating and screening resistance to primary FOLFOX chemotherapy in advanced CRC [25].
miRNA may be targeted in radiotherapy to reduce radioresistance, induce radiosensitivity, or predict or reduce acute or late radiotoxicity [9]. miR-21 is an important regulator of radioresistance in a plethora of solid tumors [26]. The same authors showed the importance of miR-21 avoiding radiation-induced cell death in leading to radioresistance in a malignant glioma model. Conversely, using anti-miR-21 to inhibit miR-21 activity sensitized glioma cells to radiation, describing a novel therapeutic clue for the future treatment of glioma malignancies [26]. In an NSCLC model, miR-7 and miR-885 were described as potential prognostic biomarkers of better overall survival after chemotherapy and radiotherapy [27]. Another study in NSCLC showed that radiotherapy-resistant cases had significantly lower levels of miR-126 and let-7a than radiotherapy-sensitive cases. Lower levels of miR-126 and let-7a were also associated with poor overall survival. miR-126 overexpression in a lung cancer cell line model induced sensitivity of cells to radiation by inducing apoptosis [28]. miRNAs may be used as indicators of radiotoxicity in surrounding non-transformed tissue and in personalized approaches to radiotherapy application (dosage) and prediction of response to therapy.
5 Selected Examples of miRNAs as Drug/Therapeutic Targets of miRNA Inhibitors
The characterization of specific miRNAs involved in oncogenesis has enabled the formulation of new miR-based anticancer therapies finalized to rehabilitate normal physiological functions of deregulated miRNAs. These therapeutic approaches focus on inhibiting oncogenic miRNA activity (miRNA inhibitors) or restoring the function of tumor-suppressor miRs (miRNA mimics). Selected examples of miRNAs as targets of drugs and therapeutics of miRNA inhibitors are summarized in Table 1.
5.1 Anti-miR oligonucleotides (AMOs), AntagomiRs, Locked Nucleic Acid Anti-miR Molecules, and Chemically Modified Anti-Sense Oligonucleotides
AMOs are a type of anti-sense miRNA-inhibiting oligonucleotide, chemically modified and fully complementary to the desired miRNA molecules. AntagomiRs are conjugated with cholesterol [15], completely complementary to the mature miRNA sequence of interest, and can prevent miRNAs from binding to mRNAs and introduce them into the RISC complex. They are chemically modified to increase the stability of mRNA to prevent degradation. AMOs and antagomiRs have different lengths and chemical modifications, and to date, both are undergoing preclinical studies in animal models [15]. LNA anti-miRNAs represent a type of AMO molecule containing an additional methylene bridge that locks the ribose into the more thermodynamically stable conformation.
For example, Song and Rosi [29] found antagomiR-21 targeting miR-21 on two levels: transcriptional and post-transcriptional in a human colon carcinoma HCT-116 cell line model. AntagomiR-21 also significantly increased rates of mature intracellular miR-21 degradation by competing for and compensating RISC components, necessary for complete miRNA maturation. Interestingly, antagomiR-21 also decreased rates of pro-angiogenic miR-30, ‘hitting’ two oncomiRs with one type of molecule. These results indicate the benefits of and potential uses for miR-21 as a drug target in colon cancer and the multifunctional roles of antagomiR-21 [29].
Dereani et al. [30] described miR-17/92, members of the oncogenic miR-17–92 cluster, as potentially good targets for anti-miR therapy. The authors introduced antagomiR-17 as having the ability to bind to miR-17. The introduction of antagomiR-17 molecules significantly lowered miR-17 levels and the proliferation rates of chronic lymphocytic leukemia (CLL)-like MEC-1 cells. Additionally, in an in-vivo experiment, the induction of tumor formation by injecting MEC-1 cells into severe combined immunodeficient (SCID) mice showed that the AMO significantly reduced disease progression and significantly improved mouse survival [31].
miR-21 was proposed as a potentially good anti-miR therapeutic target in BC models, especially in combination with 4-hydroxytamoxifen (4-OHT), delivered by poly(d,l-lactide-co-glycolide)-block-poly(ethylene glycol) PLGA-b-PEG polymer-coated nanoparticles [32]. Devulapally et al. [32] combined ASO anti-miR-21-PS with 4-OHT and administered it in estrogen receptor-positive (ER+) MCF7, BT-474, and ZR-75-1 human BC cell line models and 4T1 mouse BC cells in vitro. Anti-miR-21 administered by PLGA-b-PEG polymer nanoparticles did not show significant antiproliferative features but significantly influenced proliferation rates when delivered with 4-OHT. Furthermore, they observed significant reductions in proliferative rates when 4-OHT was combined with anti-miR-21 in nanoparticles, suggesting PLGA-b-PEG polymer nanoparticles may be a good choice for co-delivery of these two molecules and that the combination of these two drugs may be effective for the treatment of ER+ BC.
The application of antisense-miR-21 in BC MCF7 cells has been shown to suppress tumor cell growth in a cell culture model and to suppress tumor growth in a xenograft mouse tumor model [33]. The authors also showed that inhibition of cell proliferation depends on the dose of anti-miR agent and that reduced miR-21 levels rendered tumor cells more sensitive to anticancer factors, and increased apoptosis rates by neutralizing translational silencing of Bcl-2 [33]. This example is more evidence for the potential significance of miR-21 targeting in cancer therapy. In another experimental confirmation of how miR-21 might be targeted by antagomiR molecules in cancer, Griveau et al. [34] conducted an in vitro experiment, silencing miR-21 with LNA-modified ASO complexed with lipid nanocapsule (LNC). The authors observed significantly lower levels of miR-21 molecules and higher levels of caspases, which reduced U87MG GB cell viability by sensitizing GB cells to radiation, resulting in radiated cell deaths.
Brognara et al. [35] targeted miR-221 in an MDA-MB-231 BC cell line with a peptide nucleic acid (PNA), which resulted in a significant reduction of oncomiR-221 binding to its target mRNA. In this case mRNA translates into cyclin-dependent kinase inhibitor 1B (p27Kip1) protein, which allows the control of cell cycle progression. Targeting miR-221/222 with ASOs AS-miR-221 and AS-miR-222 can restore sensitivity to tamoxifen treatment [36] and regenerate the tumor-suppressing activity of miR-221/222 targets such as TIMP3 tumor suppressor, which is associated with the progression of ER+ invasive BC [37, 38].
Zhang et al. [39] used a negatively charged liposomal delivery system as a target for antisense therapy to apply antisense miR-221 oligonucleotide (anti-miR-221) in hepatocellular carcinoma (HCC)-derived HepG2 cells overexpressing the transferrin receptor. In an in vivo experimental model, chemically modified antisense miR-221 molecules coated with transferrin-targeted negatively charged liposome were injected into a xenograft mouse tumor model. This resulted in increased expression levels of phosphatase and tensin homolog (PTEN), cyclin-dependent kinase inhibitor 1B (CDKN1B), and metalloproteinase inhibitor 3 (TIMP3), suggesting the feasibility of the delivery system for targeted anti-miR therapy for HCC [39].
miR-10b is overexpressed in numerous tumor types. Ma et al. [40] showed that miR-10b suppression, with the activity of a particular antagomiR, did not decrease primary tumor development but blocked the formation of lung metastases and elevated the expression of the target Hoxd10 in 4T1 mammary tumor cells transplanted in a Balb/c mouse model. The molecule was extremely specific and well tolerated by healthy animals that received the antimiR-10b therapy under similar conditions. Plummer et al. [13] showed that miR-10b and miR-196b targeting of miR-10b and miR-196b by LNA-modified ASO negatively influenced angiogenesis and tumor formation and progression in mouse models suggesting that their targeting may be used in future novel therapeutic approaches to inhibit angiogenesis in BC [13].
Given that GB-initiating stem-like cells (GSCs) are highly resistant to standard chemotherapy/radiotherapy, Teplyuk et al. [41] investigated the properties of miR-10b GB in GB-GSCs as future therapy targets and demonstrated that downregulation of miR-10b by ASO lowered the levels of several mRNA molecules [41] in human xenograft and mouse allograft models.
5.2 miRNA-Zippers, miRNA Sponges, and SMIRs
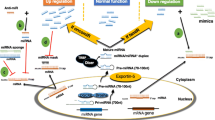
Recently, miRNA-zippers were tested as another approach to targeting miRNAs. miRNA-zippers are miRNA inhibitors that result in an miRNA loss-of-function phenotype. An miRNA-zipper contains a nucleotide gap between two miRNAs generating the space to ensure the formation of a stable form and assure specificity in binding for miRNA [16] (Fig. 3). Meng et al. [16] tested miR-17 and miR-221 in human BC cell lines and found miRNA levels to be reduced by almost 90%. Moreover, the authors concluded that, with this strategy, the miR-221 zipper reversed doxorubicin resistance with greater efficiency than anti-miR-221 in BC cell lines.
Small RNA zipper molecule: collecting, and connecting (a) microRNA molecules by binding to the 3′ end of one microRNA (b) and to the 5′ end of another microRNA molecule (c). Small miRNA zipper molecules contain nucleotide gaps (d) to stabilize and improve the specificity of binding to microRNA molecules. miRNA microRNA
miRNAs can also be the targets of miRNA sponges in cancer. Long non-coding RNAs represent a class of epigenetic endogenous elements that saturate miRNA molecules like “sponges,” and—by binding to them—prevent miRNAs from binding to their target genes, preventing translational repression or transcript degradation [42]. LncRNAs usually contain several binding sites on its sequence for miRNA molecules. Liu et al. [43] showed that (LncRNA) LncSPRY4-IT1 positively regulated the expression of histone-lysine N-methyltransferase-enhancer of zeste homolog 2 (EZH2) via sponging of miR-101-3p, an oncogenic molecule in bladder cancer, and suggested this lncRNA as a therapeutic option that effects miR-101-3p activity on its downstream targets. The second example of miRNA targeted by sponge-acting molecules was described by Li et al. [44] in BGC823 and SGC7901 gastric cancer (GC) cell lines. Li et al. [44] proposed long non-coding RNA PVT1 as a sponge molecule for suppressor miRNA miR-152. miR-152 overexpression suppresses translation of cluster of differentiation 151 (CD151) and fibroblast growth factor 2 gene (FGF2). In this case, miR-152 is not the desired target; this would be the long non-coding RNA PVT1, which could be targeted by a drug to prevent sponging and miR-152 activity [44].
SMIRs are presented as potential drugs targeting oncomiRNA molecules. First, Melo et al. [45] described them as small compounds targeting specific miRNAs, with the role of inhibiting predominantly oncomiRNA activities. Small molecules can inhibit miRNA oncogenic activities on three levels—pre-transcriptional, transcriptional, and post-transcriptional—by preventing their maturation. These small molecules would target either primary (pri-miRNA), precursor (pre-miRNA), or mature miRNA sequences, on one hand and—on the other—molecules and factors involved in miRNA processing and biogenesis. In addition, these small molecules may also target the promoter region of miRNA genes, thus changing/decreasing the transcription rates of a specific miRNA [46].
Gumireddy et al. [47] described inhibitors of components of the miRNA biogenesis pathway and described the action of small-molecule inhibitors of miRNA function. For example, small molecules regulate transcriptional regulation of miR-21 rather than inhibition of target recognition by miR-21. Young et al. [48] reduced the viral replication of hepatitis C virus (HCV), known to increase the chance of HCC developing in liver cells, by introducing small inhibitory molecule of miR-122, which is shown to be highly overexpressed in HCC cells. Furthermore, small-molecule inhibitors reduced levels of pri-miR-122 in liver cancer cells and induced apoptosis, which might emphasize its potential role in potential combined treatment with chemotherapeutics [48].
Monroig et al. [46] described an additional feature of small molecules: the ability to increase the levels of tumor-suppressive miRNAs. Small molecules can restore levels of tumor-suppressive miRNAs, which may lock down translation of oncogenes. For example, Shan et al. [49] showed the effects of the small molecule enoxacin, which can enhance the maturation of specific pri-miRNAs. They detected twofold increased levels of examined miRNAs and suggested that the quantity of restored expression probably depends on the initial levels of precursor miRNAs.
Watashi et al. [50] investigated two non-toxic small molecules, trypaflavine (TPF) and polylysine (PLL), with the ability to suppress the activity of miRNA-RISC complex or maturation of miRNA molecules. In fact, PLL has the potential to silence processing of pre-miRNA to mature miRNA by Dicer and Drosha, whereas the TPF molecule reduces the formation of miRNA AGO2 complex. As a result, cells treated with PLL contained fewer mature miRNAs and more pri-miRNA, whereas TPF-treated cells contained more miRNA levels that could not have been properly associated with their target genes. Their results suggested that TPF and PLL cells significantly decreased tumorigenic features of examined cells [50] by decreasing the levels of two oncomiRs, miR-93 and miR-130b. Those compounds block miRNA activity and RISC-miRNA-mRNA formation and release the translation of miRNA target genes.
Small molecules that modulate miRNA activity may be used to restore the activity and amounts of deregulated miRNAs in cancer pathology, both decreasing and increasing levels of specific miRNA.
6 Systemic Delivery Options of miRNAs as Therapeutic Molecules
miRNAs encoded by expression vectors or miRNA mimics can be utilized to restore the physiological function of miRNAs [51]. Delivery mechanisms, including viral or non-viral (polymers, liposomes) approaches to targeting cells in vivo, are now being investigated to increase the efficiency of the molecules suggested in Sect. 5.1. Nanotechnology-based strategies have been improved and analyzed for their probable clinical usage in solid tumors. Transportation by nanoparticles presents numerous benefits in vitro and, particularly, in vivo, because of their immunogenicity, low toxicity, target specificity, and uniform size. Much research on pre-clinical in vivo models has defined the possible efficiency of miR-based treatment choices in various tumor types [52].
Virus-based transporters, such as adenoviruses, adeno-associated viruses, or lentiviruses, can more effectively carry miRNAs to the cells of interest and save them from the activity of nucleases, extending the half-life of miRNAs in the blood. Unfortunately, viral transporters have drawbacks, such as limited DNA packaging capacity, restricted vector generation, and life-threatening consequences such as immunogenicity reactions or systemic toxicity [53].
Nanoparticle-based non-viral transport mechanisms were designed to optimize transport with fewer problems. Nanoparticles protect miRNAs from lysosomal and/or endosomal degradation and can transport miRNAs to the nucleus or the cytoplasm of the target cell without producing high levels of toxicity or powerful immune responses. Cationic polymers are the most widely preferred nanoparticles; these are positively charged molecules that can readily be combined with nucleic acids and display low immunogenic reaction and toxicity [54]. The artificially derived polymer polyethylenimine (PEI) and its conjugates have commonly been used for gene transport objectives because of their low molecular weight, which supports their efficiency and rapid uptake and release of the nucleic acid inside the cell [55]. However, the main restriction to the use of PEI is its weak biodegradability inside the cell, which causes aggregation and cytotoxicity.
7 Selected Examples of miRNA Molecules as Treatment Options
miRNA mimics or miRNAs coded by expression vectors can be the best choices to restore the physiological function of miRNAs. Their biological features and functions mean that miRNAs can be used as suitable agents for therapeutic purposes and as biomarkers of chemotherapy, radiotherapy, and targeted therapy success. Suppression or restoration of miRNA action has great potential for the control of cancer. A great deal of research on pre-clinical patterns has shown the efficiency and applicability of miRNA-based treatments. Nevertheless, despite the exciting potential, a number of difficulties must be overcome to achieve passage to clinical administration, including issues of bio-distribution or probable hazardous effects [56]. Selected examples of miR mimics combined with various delivery systems are summarized in Table 2.
Lung cancer is one of the deadliest cancer types, globally. Therefore, the need to uncover the pathological molecular mechanisms and create innovative treatment choices is urgent. For example, let-7 can directly suppress the progression of lung carcinoma both in vitro and in vivo in xenograft immunodeficient mice [57]. Moreover, intranasal application of an adenovirus expressing let-7 was found to inhibit growth and lung carcinoma production in LSL-K-ras G12D mice with G12D K-Ras mutations. Furthermore, Trang et al. [58] verified these outcomes, highlighting the therapeutic potency of let-7 in NSCLC. Systemic transport of let-7 and miR-34a mimics to lung carcinomas in mice was realized via intravenous administration of miR mimics coated with neutral lipid emulsion (NLE), a neutral lipid-based carrier. These complexes do not include cationic lipids and can make it easier to transport miRNAs within the tumor mass. Mice exposed to miR-34a and let-7 presented with substantially decreased tumor size, supporting the efficiency of the therapy [58].
miR-124 was defined as a downstream stimulator of the hepatocyte nuclear factor 4α (HNF-4α). miR-124 is an important molecule that activates hepatocytes and liver growth. It is a central component of an inflammatory feedback mechanism consisting of miR-629, miR-24, signal transducer and activator of transcription 3 (STAT3), and interleukin 6 receptor (IL6R), responsible for initiating tumorigenesis by repressing HNF-4α when activated [59]. Administration of miR-124 with liposome complexes restored its activity in an HCC mouse model and blocked tumor growth via tumor-specific apoptosis and tumor progression [59]. In addition, miR-122 is a liver-specific tumor suppressor generally downregulated in HCC [60]. Cationic lipid LNP-DP1 nanoparticles enclosing miR-122 were used in an in vivo miR-122-knockout mouse model exposed to the carcinogenic DEN and a Sk-Hep-1 xenograft nude mice model. miR-122 mimics coated with LNP-DP1 were convenient, lacked toxicity, and, when administered intra-tumor, were associated with a 50% decrease in xenograft tumor development, indicating a possible use for this nano-complex in HCC therapy [40].
Furthermore, miR-145 has been found to be downregulated in BC and to control the regulation of insulin-like growth factor 1 receptor (IGF-1R), fascin-1, SMAD2/3, and myelocytomatosis oncogene (c-myc). Ad-miR-145 adenoviral assembly was applied to MDA-MB-231 BC mice. Upregulation of miR-145 substantially reduced the development of BC. Therapy with combined 5-fluorouracil and Ad-miR-145 strengthened the curative efficiency [61]. To evaluate the curative potency of miR-34a, Li et al. [62] constructed a T-VISA-miR-34a plasmid that was triggered in BC. With the help of a liposomal transport mechanism, T-VISAmiR-34a was intravenously administered to an MDA-MB-231 human BC mouse model. T-VISA-miR-34a administration provided miR-34a activity within cancer cells and reduced tumor mass without noteworthy side effects [62].
CRC is the third most frequent cancer type worldwide [63]. It has been determined that p53 activation triggers the cyclin-dependent kinase inhibitor p21, which can further move to block p53-mediated apoptosis [64]. A recombinant adenoviral vector, which facilitated co-cistronic expression of p53 and synthetic miRNAs targeting p21 (Ad-p53/miR-p21), was constructed to assess its therapeutic efficiency in an in vivo model of nude mice administered with DLD1 or SW480 CRC cells. When the tumor reached a stable size, adenoviral constructs were applied directly inside the tumor mass. A remarkable increase in apoptosis, chemosensitivity, and tumor downsizing were seen in animals receiving Ad-p53/miR-p21 compared with those exposed to Ad-p53 alone [65]. Moreover, PEI (polyethylenimine)-complexed miR-145, an miR downregulated in colon cancer that targets c-myc and extracellular receptor kinase (ERK5) was administered intraperitoneally in an HCT116 or LS1741T colon carcinoma cell xenograft nude mouse model, resulting in a 50% decrease in tumor progression. The same animal model was further exposed to PEI-miR33a, leading to Pim-1 oncogenic kinase suppression and blocking of tumor development [66]. Dai et al. [67] engineered a vector-based plasmid to evaluate the anti-cancer efficiency of tumor suppressor miR-15a/16-1, whose activity was inversely related to cyclin B1 (CCNB1) in CRC [67]. Systemic transport of structures enclosed in cationic liposomes led to remarkable suppression of angiogenesis and tumor development. Zhai et al. [68] analyzed the function of miR-502, an miRNA downregulated in CRC, which inhibits autophagy via targeting Rab1B, on CRC tumor development in an HCT116 xenograft mouse model. They showed that miR-502 intra-tumor administration decreased tumor development [68].
8 Application of miRNAs in Therapy Success Prediction
In a study investigating the possible usage of circulating miR-451 in serum to predict neoadjuvant chemotherapy (NACT) resistance in BC, the relative expression of miR-451 was remarkably reduced in both the NACT-sensitive group and the NACT-resistant group compared with controls [66]. The authors also observed that relative miR-451 expression was decreased in the NACT-resistant group compared with the NACT-sensitive group. Therefore, these findings suggest that the circulating miR-451 level might be of functional importance in predicting NACT resistance in patients with BC [69]. Anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (anti-EGFR mAb) are used in the treatment of metastatic CRC (mCRC), but patients with a Kirsten rat sarcoma viral oncogene homolog/v-raf murine sarcoma viral oncogene homolog B1 (KRAS/BRAF) mutation, and nearly one-half of those without the mutation do not benefit from the treatment [70]. Mosakhani et al. [70] used miRNA profiling to predict the therapeutic efficiency of (anti-EGFR) mAB in patients with and without the KRAS/BRAF mutation and found significant miR-592 underexpression and miR-31 overexpression in progressive disease compared with the control group and upregulation of let-7 miRNA family members in patients with poor overall survival. Moreover, miR-1224-5p underexpression and miR-140-5p overexpression were associated with poor overall survival. In patients with mCRC with wild-type KRAS/BRAF, miRNA profile may effectively predict the success of anti-EGFR mAb therapy [70].
The treatment success ratio of preoperative chemoradiotherapy (CRT) ranges from complete regression to resistance in locally advanced rectal carcinoma. Local resection (LR) applications were recently investigated to lessen surgical morbidity and to enhance functional consequences for patients responding well to CRT. Suitable grading processes are necessary to maintain oncologic efficacy, but the latest clinical evaluation and imaging methods require further enhancement. Five miRNAs related to rectal carcinoma (miR-31, miR-18b, miR-17, miR-193-3p, and miR-20a) were investigated in the plasma of patients with rectal cancer. Expression levels were evaluated before, during, and after CRT and were tested with respect to lymph node status. Four miRNAs had trustworthy outcomes in plasma. Levels of miR-17, miR-18b, miR-20a, and miR-193-3p changed at previously described time points. The expression of miR-20a and miR-18b during CRT was associated with negative lymph node status. The coincidence of decreased miR-20a and miR-18b expressions with lymph node negativity after preoperative CRT may assist in stratification of surgical procedures in terms of complete meso-rectal excision [71].
9 Conclusions
The worth of miRNAs as potential therapeutic agents is widely recognized among researchers worldwide. Recently, the precise molecular specification of cancers has made it feasible to anticipate response to treatments and generate targeted drugs and personalized schemes to manage cancer. The expression status of miRNAs is associated with tumor growth and aggressiveness, and the estimation of chemotherapy/radiotherapy/targeted treatment success. In vivo and in vitro experimental studies have displayed the applicability of rehabilitating the normal or, conversely, repressing the abnormal activity of deregulated miRNAs in cancer: miRNA formation presents low antigenicity, and mimics have been readily transported via effective and well-tolerated vectors, such as nanoparticles. miRNA activity has been repressed independently by short synthetic structures particularly designed to enhance specificity, stability, and binding efficiency to target. According to the literature listed in this review, targeting of miRNAs might be much more effective in cancer treatment when combined with standard therapy approaches, suggesting that miRNA targeting may also improve existing therapeutic models in the future. Some miRNA molecules can be excellent targets for various types of drugs that silence their oncogene activity and revert resistance to other drugs and radiotherapy, or both, adding a new level to cancer treatment strategies treatment.
Using a range of approaches to silence miR-21, either LNA anti-miRs or SMIRs, might be a promising model for future combined therapeutic approaches, with CRT for GB, BC, and lung cancer; combined with radiotherapy in patients with prostate cancer; and combined with chemotherapy in patients with colon cancer. Silencing of miR-122 by SMIRs or antagomiRs may prevent the development of HCC in patients with HCV infection and improve therapy success rates in patients with HCC, as well as ASO-based miR-221/222 silencing combined with tamoxifen in the breast carcinoma treatment.
miRNAs may be very valuable targets for various therapeutic mechanisms, increasing the success of cancer treatment, but the miRNA therapeutic research niche needs further characterization and improvement, including the specificity and selectivity of silencing, choice of delivery system, or potential side effects (because miRNAs can silence multiple targets), before use in clinical practice.
References
Kim DH, Sætrom P, Snøve O, Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci. 2008;105:16230–5.
Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–14.
Bartel DP. MicroRNA target recognition and regulatory functions. Cell. 2009;136:215–33.
Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8.
Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971.
Moertl S, Mutschelknaus L, Heider T, Atkinson MJ. MicroRNAs as novel elements in personalized radiotherapy. Transl Cancer Res. 2016;5:S1262–9.
Korpela E, Vesprini D, Liu SK. MicroRNA in radiotherapy: miRage or miRador? Br J Cancer. 2015;112:777–82.
Petrovic N, Ergun S, Isenovic ER. Levels of microRNA heterogeneity in cancer biology. Mol Diagn Ther. 2017;21:511–23.
Wu W. MicroRNA: potential targets for the development of novel drugs? Drugs RD. 2010;10:1–8.
Chen Y, Gao D-Y, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–41.
Plummer PN, Freeman R, Taft RJ, Vider J, Sax M, Umer BA, Gao D, Johns C, Mattick JS, Wilton SD, Ferro V, McMillan NA, Swarbrick A, Mittal V, Mellick AS. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013;73:341–52.
Schmidt MF. Drug target miRNAs: chances and challenges. Trends Biotechnol. 2014;3:578–85.
Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer—an emerging concept. EBioMedicine. 2016;12:34–42.
Meng L, Liu C, Lü J, Zhao Q, Deng S, Wang G, et al. Small RNA zippers lock miRNA molecules and block miRNA function in mammalian cells. Nat Commun. 2017;8:13964.
Rothschild SI. microRNA therapies in cancer. Mol Cell Ther. 2014;2:7.
Geretto M, Pulliero A, Rosano C, Zhabayeva D, Bersimbaev R, Izzotti A. Resistance to cancer chemotherapeutic drugs is determined by pivotal microRNA regulators. Am J Cancer Res. 2017;7:1350–71.
Bian H-B, Pan X, Yang J-S, Wang Z-X, De W. Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549). J Exp Clin Cancer Res. 2011;30:20.
Glavinas Hristos, Krajcsi Peter, Cserepes Judit, Sarkadi Balazs. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv. 2004;1:27–42.
Song B, Wang Y, Kudo K, Gavin EJ, Xi Y, Ju J. miR-192 regulates dihydrofolate reductase and cellular proliferation through the p53-miRNA circuit. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:8080–6.
Borralho PM, Kren BT, Castro RE, Moreira da Silva IB, Steer CJ, Rodrigues CMP. MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS J. 2009;6:6689–700.
Xu K, Liang X, Shen K, Sun L, Cui D, Zhao Y, et al. MiR-222 modulates multidrug resistance in human colorectal carcinoma by down-regulating ADAM-17. Exp Cell Res. 2012;318:2168–77.
Hao G, Hao H, Ding Y, Wen H, Li X, Wang Q, et al. Suppression of EIF4G2 by miR-379 potentiates the cisplatin chemosensitivity in nonsmall cell lung cancer cells. FEBS Lett. 2017;591:636–45.
Chen QXH, Zhang YC, Tang QL, Bi F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pac J Cancer Prev. 2013;14(12):7421–6.
Gwak H-S, Kim TH, Jo GH, Kim Y-J, Kwak H-J, Kim JH, et al. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One. 2012;7:e47449.
Bi N, Schipper MJ, Stanton P, Wang W, Kong F-M. Serum miRNA signature to identify a patient’s resistance to high-dose radiation therapy for unresectable non-small cell lung cancer. J Clin Oncol. 2013;31:7580.
Wang X-C, Du L-Q, Tian L-L, Wu H-L, Jiang X-Y, Zhang H, et al. Expression and function of miRNA in postoperative radiotherapy sensitive and resistant patients of non-small cell lung cancer. Lung Cancer. 2011;72:92–9.
Song M-S, Rossi JJ. The anti-miR21 antagomir, a therapeutic tool for colorectal cancer, has a potential synergistic effect by perturbing an angiogenesis-associated miR30. Front Genet. 2013;4:301.
Dereani S, Macor P, D’Agaro T, Mezzaroba N, Dal-Bo M, Capolla S, et al. Potential therapeutic role of antagomiR17 for the treatment of chronic lymphocytic leukemia. J Hematol Oncol J Hematol Oncol. 2014;7:79.
Dal Bo M, Bomben R, Hernandez L, Gattei V. The MYC/miR-17-92 axis in lymphoproliferative disorders: a common pathway with therapeutic potential. Oncotarget. 2015;6:19381–92.
Devulapally R, Sekar TV, Paulmurugan R. Formulation of anti-miR-21 and 4-hydroxytamoxifen co-loaded biodegradable polymer nanoparticles and their antiproliferative effect on breast cancer cells. Mol Pharm. 2015;12:2080–92.
Si M-L, Zhu S, Wu H, Lu Z, Wu F, Mo Y-Y. miR-21-mediated tumor growth. Oncogene. 2006;26:2799–803.
Griveau A, Bejaud J, Anthiya S, Avril S, Autret D, Garcion E. Silencing of miR-21 by locked nucleic acid-lipid nanocapsule complexes sensitize human glioblastoma cells to radiation-induced cell death. Int J Pharm. 2013;454:765–74.
Brognara E, Fabbri E, Aimi F, Manicardi A, Bianchi N, Finotti A, et al. Peptide nucleic acids targeting miR-221 modulate p27Kip1 expression in breast cancer MDA-MB-231 cells. Int J Oncol. 2012;41:2119–27.
Gan R, Yang Y, Yang X, Zhao L, Lu J, Meng QH. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther. 2014;21:290–6.
Petrovic N, Sami A, Martinovic J, Zaric M, Nakashidze I, Lukic S, Jovanovic-Cupic S. TIMP-3 mRNA expression levels positively correlates with levels of miR-21 in in situ BC and negatively in PR positive invasive BC. Pathol Res Pract. 2017. https://doi.org/10.1016/j.prp.2017.08.012.
Petrovic N, Davidovic R, Jovanovic-Cupic S, et al. Changes in miR-221/222 levels in invasive and in situ carcinomas of the breast: differences in association with estrogen receptor and TIMP3 expression levels. Mol Diagn Ther. 2016;20:603–15.
Zhang W, Peng F, Zhou T, Huang Y, Zhang L, Ye P, et al. Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes. Int J Nanomed. 2015;10:4825–36.
Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson E, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–7.
Teplyuk NM, Uhlmann EJ, Gabriely G, et al. Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: first steps toward the clinic. EMBO Mol Med. 2016;8(3):268–87.
Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–89.
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X, et al. LncRNA SPRY4-IT1 sponges miR-101-3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett. 2017;388:281–91.
Li T, Meng X-L, Yang W-Q. Long noncoding RNA PVT1 acts as a “sponge” to inhibit microRNA-152 in gastric cancer cells. Dig Dis Sci. 2017. https://doi.org/10.1007/s10620-017-4508-z.
Melo S, Villanueva A, Moutinho C, Davalos V, Spizzo R, Ivan C, et al. Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc Natl Acad Sci USA. 2011;108:4394–9.
Monroig PDC, Chen L, Zhang S, Calin GA. Small molecule compounds targeting miRNAs for cancer therapy. Adv Drug Deliv Rev. 2015;81:104–16.
Gumireddy K, Young DD, Xiong X, Hogenesch JB, Huang Q, Deiters A. Small molecule inhibitors of microRNA miR-21 function. Angew Chem Int Ed Engl. 2008;47:7482–4.
Young DD, Connelly CM, Grohmann C, Deiters A. Small molecule modifiers of microRNA miR-122 function for the treatment of hepatitis C virus infection and hepatocellular carcinoma. J Am Chem Soc. 2010;132:7976–81.
Shan G, Li Y, Zhang J, Li W, Szulwach KE, Duan R, Faghihi MA, Khalil AM, Lu L, Paroo Z, Chan AW, Shi Z, Liu Q, Wahlestedt C, He C, Jin P. A small molecule enhances RNA interference and promotes microRNA processing. Nat Biotechnol. 2008;26(8):933–40.
Watashi K, Yeung ML, Starost MF, Hosmane RS, Jeang K-T. Identification of small molecules that suppress microRNA function and reverse tumorigenesis. J Biol Chem. 2010;285:24707–16.
Saumet A, Lecellier C-H. microRNAs and personalized medicine: evaluating their potential as cancer biomarkers. In: Santulli G, editor. microRNA: medical evidence: from molecular biology to clinical practice. Cham: Springer International Publishing; 2015. p. 5–15.
Shu Y, Pi F, Sharma A, Rajabi M, Haque F, Shu D, et al. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv Drug Deliv Rev. 2014;66:74–89.
Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58.
Zhu L, Mahato RI. Lipid and polymeric carrier-mediated nucleic acid delivery. Expert Opin Drug Deliv. 2010;7(10):1209–26. https://doi.org/10.1517/17425247.2010.513969.
Catela Ivkovic T, Voss G, Cornella H, Ceder Y. microRNAs as cancer therapeutics: a step closer to clinical application. Cancer Lett. 2017;407:113–22.
Tessitore Alessandra, Cicciarelli Germana, Mastroiaco Valentina, Del Vecchio Filippo, Capece Daria, Verzella Daniela, et al. Therapeutic use of microRNAs in cancer. Former Curr Med Chem Anti-Cancer Agents. 2016;16:7–19.
Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M, et al. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2010;29:1580–7.
Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–22.
Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, et al. An HNF4α-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147:1233–47.
Hayes CN, Chayama K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma. Santanam N, Cho WC, eds. Int J Mol Sci. 2016;17:280.
Kim S-J, Oh J-S, Shin J-Y, Lee K-D, Sung KW, Nam SJ, et al. Development of microRNA-145 for therapeutic application in breast cancer. J Control Release. 2011;155:427–34.
Li L, Xie X, Luo J, Liu M, Xi S, Guo J, et al. Targeted expression of miR-34a using the T-VISA system suppresses breast cancer cell growth and invasion. Mol Ther. 2012;20:2326–34.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91.
Chattopadhyay D, Ghosh MK, Mal A, Harter ML. Inactivation of p21 by E1A leads to the induction of apoptosis in DNA-damaged cells. J Virol. 2001;75:9844–56.
Idogawa M, Sasaki Y, Suzuki H, Mita H, Imai K, Shinomura Y, et al. A single recombinant adenovirus expressing p53 and p21-targeting artificial microRNAs efficiently induces apoptosis in human cancer cells. Clin Cancer Res. 2009;15:3725.
Ibrahim AF, Weirauch U, Thomas M, Grünweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71:5214.
Dai F, Zhang Y, Zhu X, Shan N, Chen Y. Anticancer role of MUC1 aptamer–miR-29b chimera in epithelial ovarian carcinoma cells through regulation of PTEN methylation. Target Oncol. 2012;7:217–25.
Zhai H, Song B, Xu X, Zhu W, Ju J. Inhibition of autophagy and tumor growth in colon cancer by miR-502. Oncogene. 2013;32:1570–9.
Gu X, Xue J-Q, Han S-J, Qian S-Y, Zhang W-H. Circulating microRNA-451 as a predictor of resistance to neoadjuvant chemotherapy in breast cancer. Cancer Biomark Sect Dis Markers. 2016;16:395–403.
Mosakhani N, Lahti L, Borze I, Karjalainen-Lindsberg M-L, Sundström J, Ristamäki R, et al. MicroRNA profiling predicts survival in anti-EGFR treated chemorefractory metastatic colorectal cancer patients with wild-type KRAS and BRAF. Cancer Genet. 2012;205:545–51.
Azizian A, Kramer F, Jo P, Wolff HA, Beißbarth T, Skarupke R, et al. Preoperative prediction of lymph node status by circulating Mir-18b and Mir-20a during chemoradiotherapy in patients with rectal cancer. World J Surg. 2015;39:2329–35.
Acknowledgements
This work was supported by the Ministry of Education and Science, Republic of Serbia, Grants OI173049, OI175011, and Ordu University, Scientific Research Projects Coordination Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
NP and SE have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Petrovic, N., Ergun, S. miRNAs as Potential Treatment Targets and Treatment Options in Cancer. Mol Diagn Ther 22, 157–168 (2018). https://doi.org/10.1007/s40291-017-0314-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-017-0314-8