Abstract
Human endogenous retroviruses (HERV) represent about 8 % of the human genome. Some of these genetic elements are expressed in pathological circumstances. A HERV protein, the multiple sclerosis–associated retrovirus (MSRV) envelope protein (MSRV-Env), is expressed in the blood and active brain lesions of multiple sclerosis (MS) patients. It possesses pro-inflammatory and myelinotoxic properties. The patterns of expression and pathogenic properties of MSRV-Env make it a relevant drug target for MS therapeutics—in particular for preventing neurodegeneration, a key component of progressive forms of MS. An immunoglobulin G4 monoclonal antibody (mAb), called GNbAC1, has been developed to neutralize this pathogenic target. After showing neutralizing effects in vitro and in mouse models of MS, GNbAC1 is now in phase II clinical development. MSRV-related biomarkers such as MSRV-Env and MSRV polymerase (MSRV-Pol) gene transcripts are overexpressed in the blood and cerebrospinal fluid of patients with MS. These biomarkers may have prognostic value for long-term MS evolution, and their transcription levels in blood decline during treatments with GNbAC1, which has also been reported in patients administered reference MS drugs such as natalizumab or interferon-β. GNbAC1 as a new MSRV-Env-antagonist mAb could be a specific and causal treatment for MS, with a particular application for progressive forms of the disease. For possible use in companion diagnostic tests, MSRV-associated biomarkers could open the door to a personalized therapeutic approach for MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
GNbAC is an immunoglobulin G4 monoclonal antibody targeting a protein of endogenous retroviral origin. |
The target protein appears to play a critical role in the pathophysiology of multiple sclerosis. |
Biomarkers related to the target could play a role in companion diagnostic tests. |
1 Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating, and neurodegenerative disorder of the central nervous system (CNS), of unknown etiology. An autoimmune process underlies the pathophysiology of the disease; however; inflammation and neurodegeneration are closely associated during the disease: autoimmunity seems to predominate at the beginning of the disease evolution, while neurodegeneration prevails at the late stages and is associated with the progression of disability. The interrelations between both pathophysiological processes are complex and far from being fully understood [1].
Clinically, MS can manifest with different symptoms, but the most frequent ones include acute and subacute motor dysfunction of the limbs, sensory disturbance, gait disorders, partial or complete visual loss, autonomic dysfunction, fatigue, cognitive symptoms, and psychiatric abnormalities [2].
There are different clinical phenotypes of MS, three of which are the most prevalent. The prevalence figures are variable according to different studies [3, 4]. The most frequent form, with a prevalence of about 60 %, is defined as “relapsing–remitting MS” (RRMS), in which patients have recurrent attacks of neurological symptoms followed by symptomatic improvement ranging from complete remission to significant remaining neurological dysfunction. After 10–20 years of evolution, about 75–85 % of these patients convert to “secondary progressive MS” (SPMS), in which the neurological dysfunction progresses slowly and the cycle of exacerbations/remissions progressively wanes. The actual prevalence of SPMS is around 20 %. The third group, representing about 10 % of MS patients, have the “primary progressive MS” (PPMS) form, in which progressive neurological disability tends to accumulate from the time of clinical onset. Other less prevalent phenotypes of MS have also been described.
Pathologically, MS is characterized by inflammatory infiltrates associated with focal demyelination lesions (plaques) in the brain and spinal cord white matter, as well as in the gray matter. Inflammation is dominated by T-lymphocyte cells and activated microglia. In active lesions, this process is accompanied by disruption of the blood–brain barrier and local expression of pro-inflammatory cytokines and their receptors. Complete demyelination is accompanied by a variable degree of axonal injury and loss. These acute inflammatory plaques dominate the pathology in RRMS [5]. In the chronic progressive forms of MS (SPMS and PPMS), the pathology differs somewhat. Focal demyelinated white matter lesions are present, but active demyelinating plaques are rare. Pre-existing plaques show evidence of slow and gradual expansion of the lesions at their margins, characterized by moderate inflammatory infiltrates, mainly composed of perivascular macrophages and profound microglia activation. The demyelination process is slow: the normal-appearing white matter is affected by a diffuse inflammatory process and generalized activation of microglia, associated with diffuse axonal injury and destruction, also followed by secondary demyelination. Extensive cortical demyelination associated with inflammatory infiltrates in the leptomeninges is seen in the forebrain and the cerebellum [5].
Because of the immunoinflammatory characteristics of MS, which are particularly prevalent in the RRMS form, treatments for MS that act by inhibiting lymphocyte activation have played a critical role for a long time. Corticosteroids were first introduced as a treatment of choice for MS; however, they appeared to have no real impact on the long-term course of the disease. Major progress in MS treatment was made with the introduction of the first disease-modifying therapies (DMTs), i.e., drugs that are able to alter the course of MS [6]. The first of these drugs were the β-interferons (β-IFNs), introduced in the 1990s. Since then, several different DMTs have been registered for MS as injectable drugs and more recently as oral formulations: all of them possess an immunomodulatory or immunosuppressive mode of action (for a review, see Curtin and Hartung [7]), and they are efficacious in treating RRMS, at least by decreasing the frequency of relapses. However, none of them has shown real efficacy in the treatment of either PPMS or SPMS. Currently, most MS drugs in clinical development are based on immunomodulation or immunosuppression. However, a couple of new molecules aimed at promoting remyelination are now at the early clinical development stage [8].
We present here a new therapeutic approach for the treatment of MS, which does not affect the immune system and is intended to neutralize a protein of human endogenous retroviral (HERV) origin, called the multiple sclerosis–associated retrovirus (MSRV) envelope protein (MSRV-Env), which is likely to play a key role in the pathophysiology of MS. We describe the development of a specific treatment against this target, based on an immunoglobulin (Ig) G4 monoclonal antibody (mAb), called GNbAC1, co-developed with a panel of MSRV-related biomarkers, which may have potential for use in companion diagnostic tests.
2 Human Endogenous Retrovirus and Molecular Pharmacology
2.1 MSRV-Env as a TLR4 Activator
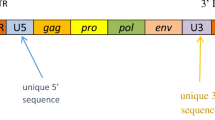
The gene encoding the MSRV-Env protein belongs to the HERV-W family of retroviral elements, which are widely and heterogeneously dispersed in the human genome, following the integration events of a corresponding exogenous retrovirus, which first infected the germ line of primates (Catharrhines) about 25 million years ago [9, 10]. The HERV elements represent approximately 8 % of the human genomic sequences. From a phylogenic perspective, HERV-W elements, to which MSRV belongs, share homologies with other endogenous or exogenous retroviruses, as shown in Fig. 1 (for a review, see Weiss [11]). The role of these elements in the causation of human disorders and, in particular, in autoimmune diseases is beginning to be better understood and is now a subject of active research [12, 13].
Phylogeny of endogenous and exogenous retroviruses, according to Weiss [11]. ALV avian leukosis virus, BaEV baboon endogenous virus, BFV Barmah Forest virus, BLV bovine leukemia virus, EIAV equine infectious anemia virus, FeLV feline leukemia virus, FFV feline foamy virus, FIV feline immunodeficiency virus, GALV gibbon ape leukemia virus, HERV human endogenous retrovirus, HIV human immunodeficiency virus, HTLV human T-cell lymphotropic virus, JRSV Jaagsiekte sheep retrovirus, MLV murine leukemia virus, MMTV mouse mammary tumor virus, MVV Maedi-visna virus, PERV porcine endogenous retrovirus, RSV respiratory syncytial virus, SIVmac macaque simian immunodeficiency virus, SnRV snakehead retrovirus, SRV simian retrovirus, SFVagm simian foamy virus type 3 (strain LK3), SFVcpz chimpanzee foamy virus, WDSV walleye dermal sarcoma virus
The MSRV-Env protein comprises three domains: the signal peptide, the surface (SU) domain or ectodomain, and the transmembrane (TM) domain. The SU domain is an agonist of Toll-like receptor (TLR)-4. Following the first observation that the in vitro abnormal immune response characterized by T-lymphocyte polyclonal activation was associated with MSRV-Env [14], the cytokines induced by the SU domain of MSRV-Env protein were evaluated in peripheral blood mononuclear cells (PBMCs). This revealed an early increase in interleukin (IL)-1β, IL-6, and IL-12p40, reflecting innate immune activation, followed by IFN-γ production reflecting delayed but potent T cell immune reaction, as expected from previous studies. This also unraveled the key role of TLR4 as an upstream receptor in the immunoinflammatory cascade when it is exposed to MSRV-Env. MSRV-Env itself was revealed to be a potent agonist of TLR4-mediated immune activation pathways, leading to both acute and chronic inflammation, as well as to autoimmunity [15, 16]. MSRV-Env appears pathogenic to all human PBMCs tested in vitro, and an interesting observation is that the magnitude of the IL-6 and IL-12p40 release appears to correlate positively, and in a statistically significant way, with MS disease severity as measured by the Expanded Disability Status Scale (EDSS) score [17]. It was shown that the mechanism of release of pro-inflammatory cytokines, such as IL-1β, IL-6, or tumor necrosis factor (TNF)-α, from PBMCs and microglial cells from the CNS was mediated through engagement of CD14 and TLR4, which are pattern recognition receptors of primary importance to innate immunity. MSRV-Env appears to also trigger a maturation process in human dendritic cells and to endow these cells with the capacity to support a T-helper-1 (Th1)-like type of T-helper cell differentiation [15, 17]. It has been further shown that MSRV-Env induces a concentration-dependent release of IL-6 and TNF-α by human PBMCs in culture, with affinities in the picomolar or low nanomolar range (internal report).
In a study comparing the cytokine profile of PBMCs stimulated by MSRV-Env in samples from 30 patients with RRMS (of whom 13 were presenting with a clinical relapse and 17 others were in a stable clinical condition at the time of blood collection), inflammatory cytokines, such as TNF-α and IFN-γ, were produced by cells from patients in a relapse state, whereas the anti-inflammatory cytokine IL-10 was produced by the T lymphocytes and CD14+ cells of patients who were in a stable condition. These data suggest a clinically correlated balance between patients’ immune systems and the peripheral reactivity status to this endogenous retroviral protein [18].
Beyond its potent agonist effects on native human TLR4, MSRV-Env appears to exert superantigen-like effects downstream from the innate immune activation [14, 19]. The ability of superantigens to cause antigen-independent polyclonal activation of T lymphocytes can result in abnormal autoreactive T-cell activation [20] and oversecretion of pro-inflammatory cytokines [21]. Superantigen properties of bacterial or viral antigens have been suggested as a causative factor in autoimmune diseases [22–24]. Nonetheless, the MSRV-Env superantigen-like effect appears to be dependent upon upstream TLR4 activation, as it was no longer observed after blockade of TLR4 receptors with an appropriate antibody, which resulted in inhibition of both TNF-α [17] and IFN-γ secretion (H. Perron, personal communication).
Addressing TLR4 expression in myelin-producing cells, immunostaining and gene expression analyses evidenced transient expression of TLR4 on oligodendroglial precursor cells (OPCs) in human brain tissue and in culture. Cultured primary OPCs were stimulated with MSRV-Env protein to determine the effects of the ligand-receptor interaction in such cells. It was shown that human and rat OPCs expressed TLR4 in a window period preceding their differentiation. It was then shown that MSRV-Env-mediated activation of TLR4 led to release of pro-inflammatory cytokines and, via inducible nitric oxide synthase, to release of nitric oxide radicals. This caused formation of nitrotyrosine groups and paralleled the subsequent blockade of myelin protein expression. These observations showed that induction of nitrosative stress by MSRV-Env through TLR4 signaling inhibits oligodendroglial differentiation capacity, which results in failure of remyelination by migrated OPCs—a critical pathophysiological process in MS [25]. This remyelination failure is a pathophysiological mechanism of particular importance for progressive forms of MS [26–31]. The findings regarding the OPC toxicity of MSRV-Env were confirmed independently (internal report).
Consequently, both the pro-inflammatory effect with cytokine release and the oligodendrocyte toxicity induced by MSRV-Env indicate that the MSRV-Env protein is a key element in the pathophysiology of MS.
3 Pathophysiology
3.1 Expression in MS Lesions
Several studies have shown that the MSRV-Env protein is systematically expressed in active MS brain lesions, where the expression takes place in macrophages and microglial cells [32–34], while there is no or only faint expression in normal-appearing white matter from patients or control subjects without MS. The level of immunostaining also appears to correlate with the acute characteristics of the brain lesions [32, 34], notably at the start of a lesion, as shown in Fig. 2 [35].
A new perivascular multiple sclerosis lesion is associated with envelope protein (Env)-positive infiltrating perivascular macrophages, colored in brown by immunostaining [35]
In a study by Perron and Van Horssen [35], brain samples from 20 patients with clinically diagnosed and neuropathologically confirmed MS, and from ten control subjects without MS, were analyzed. Immunodetection of specific proteins was done with specific antibodies to MSRV-Env or GAG proteins, to proteolipid protein of myelin, to human leukocyte antigen (HLA) class II antigens, and to microglia, macrophage, and lymphocyte-specific markers. Both MSRV proteins were detected in the same cells in lesions from all MS brains (20/20), but only rare and faint detection of MSRV-Env was seen in a few control subjects or in normal-appearing white matter from MS brains. MSRV-Env was strongly and essentially expressed in perivascular macrophages and in brain microglia, and this was particularly seen in actively demyelinating areas and at all stages of MS plaques. It was occasionally detected in astrocytes within lesions and was only faintly detected in inactive (burnt-out) plaques with diffuse staining retained on an extracellular matrix of fibrotic areas. Globally, MSRV-Env detection appears to be associated with an active demyelination process, whatever the stage of the MS disease is.
These different neuropathological studies show the systematic presence of MSRV-Env protein in MS brain lesions, from the earliest perivascular stage to the latest active rim of microglia in progressive plaques—in particular linked to the demyelination process. This systematic expression of such a protein with inflammatory and myelinotoxic properties in lesions suggests that MSRV-Env plays a key pathogenic role in the MS lesional process, and makes it a highly relevant drug target. As it is present in lesions of all types of MS—RRMS as well as progressive forms—its potential role in the neurodegeneration process suggests that the neutralization of this target could be particularly valuable for treatment of progressive forms of MS.
4 Therapeutics
4.1 Development of GNbAC1, a Monoclonal Antibody
The above-described pathophysiological findings led to a search for a specific MSRV-Env-antagonist drug, which could specifically bind to this endogenous retroviral protein and neutralize its pathogenic activity. A mAb was isolated, which selectively binds to a linear epitope of the SU domain of the MSRV-Env protein and neutralizes its TLR4 binding potential without interacting with this receptor. Following murine and chimeric antibody versions, a humanized recombinant antibody of the IgG4/κ subclass, called GNbAC1, was produced. Site-directed mutagenesis within the corresponding encoding nucleotide sequence was performed to stabilize the interchain disulfide bridges of the core region of IgG4. GNbAC1 has a molecular weight of approximately 147 kDa. Details of the discovery and preclinical development of GNbAC1 have been described by Curtin et al. [36].
4.2 In Vitro Evidence
GNbAC1 was tested in vitro for its ability to block the release of pro-inflammatory cytokines induced by MSRV-Env. It was shown that release of TNF-α by PBMCs stimulated by MSRV-Env was inhibited by GNbAC1, but that GNbAC1 did not suppress PBMC activation by lipopolysaccharide (LPS), a TLR4 agonist: this showed that the mode of action of GNbAC1 is specific to the target and does not involve any TLR4 inhibition [17]. The neutralizing activity of GNbAC1 does not interfere with the receptor, neither by itself nor when it is bound to the target epitope on its anti-ligand.
Another study used HEK-Blue™ human TLR4 cells designed for studying the stimulation of recombinant human TLR4 by monitoring the activation of nuclear factor (NF)-κB and activator protein (AP)-1. In this model, it was confirmed that MSRV-Env induces strong and highly potent TLR4 activation (with a half-maximal effective concentration [EC50] in the picomolar range). It was further shown that the MSRV-Env effect is significantly and concentration-dependently inhibited by GNbAC1 but not by corresponding GNbAC1 vehicle or another control mAb (internal report).
In a recent study [37], the in vitro effect of GNbAC1 on OPCs was tested. It was shown that GNbAC1 significantly diminished the induction of nitrosative stress due to MSRV-Env in OPCs and allowed rescue of the expression of myelin proteins by differentiated OPCs, which are reduced by MSRV-Env. This additional effect on glial cell pathology therefore indicates that GNbAC1 can provide a protective effect on OPCs, and this suggests the potential to prevent the defect in remyelination associated with MS lesions [38]. In this experiment, GNbAC1 also decreased pro-inflammatory cytokines—notably TNFα, which is known to induce myelin and oligodendroglial damage [39]. These findings indicate that GNbAC1 can display a double therapeutic effect, protecting OPC differentiation capacity and inhibiting the pro-inflammatory signaling cascades induced by MSRV-Env in the CNS.
4.3 In Vivo Evidence
Assessment of the therapeutic efficacy of the different forms of GNbAC1 was assessed in an MSRV-Env experimental allergic encephalitis (EAE) mouse model [16]. The results of the inhibition of MSRV-Env effects in the EAE model were described by Curtin et al. [36]. It was shown that a reversal of clinical score kinetics toward healing was observed in all animal groups treated with GNbAC1 or other chimeric human isotypes binding to the same epitope. In those experiments, some untreated animals died or had to be euthanized because of complete paralysis after 4 weeks, while all of the GNbAC1-treated mice survived. In another experiment in an EAE model in severe combined immunodeficiency (SCID) mice, after several weeks post-treatment with a chimeric human antibody similar to GNbAC1, it was possible to observe remyelination tracts in CNS lesions, such as those shown in Fig. 3 [40].
Signs of remyelination in experimental allergic encephalitis mouse brain lesions after treatment with chimeric human antibodies similar to GNbAC1, observed with 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) labeling with a specific monoclonal antibody [40]
These results support the concept that GNbAC1 could be an efficacious treatment for MS. In addition, the observation of remyelination in mice confirms the data obtained in vitro with OPCs. These results suggest that GNbAC1 could be of particular interest for treatment and prevention of demyelination and axonal death, as are particularly observed in progressive forms of MS.
4.4 Early Clinical Testing
A phase I, first-in-man clinical study, performed in a double-blind, placebo-controlled, dose-escalating titration design, enrolled 33 healthy subjects who did not express the MSRV-Env target. In dose cohort 0 at a dose of 0.0025 mg/kg, three subjects received an intravenous GNbAC1 infusion and one subject received a placebo. In dose cohort 1 at a dose of 0.025 mg/kg, four subjects received an intravenous GNbAC1 infusion and one subject received a placebo. For the following four dose cohorts (receiving respective doses of 0.15, 0.6, 2, or 6 mg/kg), four subjects received GNbAC1 intravenously and two subjects received a placebo in a sequential manner. All 33 healthy subjects received the scheduled injections. In that study, the safety profile of GNbAC1 appeared favorable without infusion reactions or serious adverse events. There was no evidence of induction of anti-drug antibodies. The single-administration pharmacokinetics were in line with those expected for normal IgG, with apparent dose-linear pharmacokinetics and an elimination half-life of 19–26 days [41].
A phase IIa study was performed as a single-blind, placebo-controlled, dose-escalating randomized study in ten MS patients: one had RRMS, three had PPMS, and six had SPMS. In each of two dose cohorts (2 and 6 mg/kg), four patients received GNbAC1 and one patient received a placebo (randomization ratio 4:1) in a sequential manner. Then, the patients entered a repeated-dose extension study, where all ten patients received GNbAC1 in an open-label setting for 11 additional intravenous infusions on a 4-week administration schedule. Eight of them remained in the study until the study end. In that study, the safety profile of GNbAC1 was also favorable, and no particular trends toward specific types of adverse events were observed; in particular, no hypersensitivity or infusion reactions were observed. The two dropouts were not related to safety events [42, 43]. A pharmacodynamic response with a statistically significant decline in MSRV-Env biomarkers was observed (see below) [42]. Repeated-dosing pharmacokinetics confirmed the initial observation of the phase I study, with dose-linear pharmacokinetics and an accumulation factor of about 3 [43]. No anti-drug antibodies were detected during the whole duration of the study. An experiment involving immunomonitoring of monocytes and T cells in the ten enrolled patients and matched healthy control subjects showed that administration of GNbAC1 did not lead to a decrease in the T-cell response to viral antigens and did not impact TLR4 activation by LPS, supporting the expected mechanism of action of GNbAC1 [44]. Blood levels of IL-6, TNF-α, and IFN-γ were monitored during the treatment, and no consistent changes were observed during the treatment.
Interestingly, from a clinical and brain-imaging perspective, the MS patients remained stable over 1 year of treatment [42, 43]. The open-label setting and the small sample size somewhat limited the conclusions that could be drawn; however, these results suggested that there was a pharmacodynamic response to GNbAC1 in line with the expected mechanism of action of the mAb.
5 Biomarkers
5.1 MSRV Biomarker Prevalence
Different studies in MS patients and control populations have shown that MSRV expression, assessed by RNA polymerase chain reaction (PCR) in blood as well as in cerebrospinal fluid (CSF), is more prevalent in MS patients than in control populations.
In a preliminary study [45] analyzing sera from MS patients and control subjects, MSRV RNA was detected in nine of 17 patients with MS; a negative association between MS therapy and RNA expression was observed. A positive RNA detection was noted in three of 36 apparently healthy control subjects and in none of eight patients with non-neurological disorders.
In a European multicenter study reported by Arru et al. [46], the presence of MSRV, identified by the pol gene, using RNA PCR quantification, was more frequent and the viral load was greater, with a prevalence of 71 % in plasma from 147 MS patients, compared with 17 % in plasma from 98 healthy control subjects (17 %) and 40 % in plasma from 57 patients with other inflammatory and non-inflammatory neurological diseases, some of whom suffered from chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) which also appears to be associated with expression of MSRV [34]. In another study [47], blood and CSF from 113 Sardinian patients with MS and neurological control subjects were analyzed by PCR. MSRV positivity was detected in the CSF of 50 % of the MS patients at clinical onset—increasing to about 90 % in those with RRMS and 100 % in those with SPMS with temporal disease progression—and in 40 % of patients with other neurological diseases (MSRV was positive in 50 % of patients with CNS inflammatory disease and in 33.3 % of patients with non-CNS inflammatory disease). In blood, MSRV was detected in 100 % of MS patients, 63 % of patients with inflammatory neurological diseases (four of five patients with inflammatory CNS diseases, three of four patients with inflammatory peripheral neuropathy, and neither of two patients with myasthenia gravis), and 13 % of healthy blood donors. In a study by Perron et al. [34], 199 healthy control subjects and control subjects with other neurological diseases (n = 28), chronic infections (n = 30), or autoimmunity (n = 30) were analyzed with an immunoassay detecting MSRV-Env protein in serum. MSRV-Env RNA or DNA copy numbers in PBMCs were also determined by quantitative PCR. RNA expression in PBMCs and DNA copy numbers were significantly elevated in patients with MS versus healthy control subjects (p < 0.001). In patients with MS, DNA copy numbers were greater in those with chronic progressive MS than in RRMS patients. This observation was reproduced independently by two other studies [48, 49].
All of these results show that MSRV-related biomarkers—in particular those related to MSRV-Env (RNA, DNA, or proteins)—are more prevalent in patients with MS than in healthy control subjects. The sensitivity and specificity of these biomarkers are high; however, as the measurement techniques used in the different studies were not standardized (i.e., as the analytical validity has not been established), no attempts were made to establish the definitive clinical validity (i.e., establishing a receiver operating characteristic [ROC] curve based on the sensitivity and specificity of the test as a function of defined cut-off points).
5.2 MSRV Elements as a Prognostic Factor
Interestingly, expression of MSRV biomarkers could have prognostic value for the evolution of MS. The original findings come from a longitudinal cohort study performed in Sardinia, Italy. Following a preliminary study, which showed that the presence of MSRV-Pol RNA in the CSF of early MS patients was indicative of a poor 3-year follow-up prognosis [50], a long-term longitudinal observational study was launched: at study entry, 18 of 23 patients could be categorized according to MSRV-Pol status in their CSF, assessed with nested PCR: ten were MSRV positive and eight were MSRV negative; both groups were of a similar mean age and had similar EDSS scores (1.9–2.1) at study entry. A 6-year follow-up study showed that the mean EDSS scores differed between the MSRV-positive and MSRV-negative cohorts (4.3 versus 2.2; p = 0.004) in a statistically significant way, and that the annual relapse rate was higher in MSRV-Pol-positive patients (0.5 versus 0.3 in MSRV-Pol-negative patients; p = 0.01) [51]. The same cohort was followed up for 10 years [52], and the results showed that MSRV-Pol-positive patients had a statistically significantly higher average EDSS score (6.2) than MSRV-Pol negative patients (3.3). When the patients were dichotomized on the basis of an EDSS score higher than 5, 88 % of MSRV-Pol-positive patients had an EDSS score higher than 5, while only 12 % of MSRV-Pol-negative patients did (Fisher’s exact test p < 0.0004). In addition, six of 14 MSRV-Pol-positive patients (42 %) had converted to SPMS, whereas no MSRV-Pol negative patients had converted (Fisher exact test p < 0.01). These data suggest that the MSRV-Pol biomarker in the CSF may indicate a more severe course of MS disease and a higher risk of conversion to SPMS. This was a small study, which certainly deserves confirmation with larger cohorts, with adjustment for concomitant MS treatments—in particular to confirm the positive predictive value (PPV) and negative predictive value (NPV) of the biomarker detection for conversion to SPMS and especially within which timeframe. If clinical validity for prognosis were to be confirmed, this biomarker could be of real importance for the therapeutic approach used for patients at the onset of the disease.
Interestingly, in the patient cohort of the phase IIa study of GNbAC1 [42, 43], the level of MSRV-Env RNA presented a trend toward an inverse correlation (Pearson’s r value −0.55, p = 0.12) with the duration of the MS disease before the start of the trial (unpublished GeNeuro data on file) in nine patients. This observation also requires confirmation but can be put in parallel with findings from a large transverse study in Spain, including 178 MS patients and 124 control subjects, where it was observed that the MSRV-Env DNA load was significantly associated with a higher EDSS score and a higher Multiple Sclerosis Severity Score (MSSS) in women [49]—the latter score also taking into consideration the speed of the evolution [53].
5.3 MSRV as a Predictor of Response
In the MS study in 10 patients, MSRV-Env and MSRV-Pol transcripts obtained from PBMCs were analyzed at baseline and at regular intervals: the distributions of MSRV transcript levels at inclusion were homogeneous in both cohorts. Figure 4, adapted from Derfuss et al. [42], presents measurements of MSRV-Env and MSRV-Pol messenger RNA (mRNA) transcripts relative to GUS-B gene expression before the first, third, and sixth GNbAC1 administrations. Decreases in MSRV-Env and MSRV-Pol transcript levels at 3 months and at 6 months of treatment were observed. These findings were not expected, as GNbAC1 neutralizes the MSRV-Env protein only when binding to its target peptidic epitope and does not target RNA, nor is it known to interfere with a molecule activating proviruses from the related HERV-W family. At 6 months, the differences were statistically significant (repeated-measures analysis of variance [ANOVA] on MSRV-Env: p = 0.029; on MSRV-Pol: p = 0.044) [42].
Multiple sclerosis–associated retrovirus (MSRV) envelope protein (Env) and polymerase (Pol) transcript expression proportional to a reference glucuronidase, beta (GUS B) gene transcript, before the first, third, and sixth administrations of GNbAC1. Adapted from Derfuss et al. [42]
This pharmacodynamic response shows an effect not only on the target itself but also on the endogenous retroviral transcriptional activity associated with MSRV-Env protein expression. This suggests that neutralization of the MSRV-Env protein would also downregulate expression of the corresponding endogenous retroviral genome(s). This observation is of particular interest, as two reference MS treatments induce similar responses at the level of MSRV-Env transcripts. In an Italian longitudinal study, which included 11 RRMS patients, IFN-β induced a decrease in MSRV-Env RNA measured by reverse transcriptase (RT)-PCR, which was observed at 3 months and persisted at follow-up at 12 months. Incidentally, one patient, who was non-responder to IFN-β and had progression of his disease, presented with an increase in MSRV-Env levels after an initial decrease [54]. In another study in 22 RRMS patients, it was shown that natalizumab induced a decrease in MSRV-Env mRNA, measured by RT-PCR, observed later after 6 months of treatment. This effect was maintained at 12 months of treatment in all patients who were all clinically stable over this period [55]. The pharmacodynamic response observed with GNbAC1, paralleling the response observed with the MS treatments natalizumab and IFN-β, was suggestive of the potential therapeutic effect of GNbAC1. In addition, it suggested that MSRV-Env RNA measurement is an interesting tool for monitoring the response to treatment, which could be used for adjustment of MS treatment in terms of the dose, schedule or duration—typical specificities of a companion diagnostic [56, 57].
Apart from one patient in a study performed by Mameli et al. [54], where an increase in the MSRV biomarker was associated with treatment failure, it is unclear whether the level of the biomarker response correlates with endpoints of efficacy, as the above-mentioned studies were not powered or designed to allow a comparison between the magnitude of the biomarker response and the clinical response. Exploration of these markers in a future phase II efficacy trial of GNbAC1 should allow assessment of whether these MSRV markers could be predictors of therapeutic responses to treatment and whether they would correlate with clinical endpoints.
5.4 Further Clinical Development
Following the results of the phase I and phase IIa studies showing good safety profiles and initial pharmacodynamic responses, there is a need to further assess GNbAC1 efficacy in a phase IIb study. There are current plans to launch a double-blind, placebo-controlled study in patients with RRMS and to base the efficacy evaluation of the drug on magnetic resonance imaging (MRI) of the brain, as this is currently considered an acceptable endpoint for exploratory studies in regulatory guidelines on MS drug development [58]. The study should be launched soon. The assessment of MSRV-related biomarkers in a parallel development with GNbAC1 is envisaged from a personalized medicine perspective. According to the results already cited [42, 54, 55], MSRV biomarkers could allow monitoring of a pharmacodynamic response to MS treatments, although the clinical correlation of this observation remains unknown. It is unclear whether the status of MSRV biomarkers at baseline will be predictive of a given response to GNbAC1 and, in particular, its magnitude. As described in the US Food and Drug Administration (FDA) guideline [57], one of the purposes of a companion diagnostic is to identify patients who are most likely to have the best benefit-to-risk profile with a therapeutic product. This is one of the questions that the future clinical development of GNbAC1 will address.
6 Discussion
The mAb GNbAC1 is the first therapeutic product developed as an antagonist of a protein of endogenous retroviral origin from the HERV-W family, called MSRV-Env. In the field of MS, this is one of the rare therapeutic developments whose aim is to neutralize a target considered as being directly involved in the pathophysiological cascade of MS. This differs from the vast majority of registered drugs or those in development, which are trying to control the disease by a modulatory or suppressing action on the immune system [7, 59]. The MSRV-Env target is particularly interesting, as it appears to be closely associated with the evolution of the active pathological process and has been found so far in all active regions of MS brain plaques from different forms of MS. The target—expressed on monocytes, B lymphocytes, and microglial cells—has a pathophysiological effect associated with inflammation, as well as neurodegeneration. The latter point is particularly relevant for the chronic progressive forms of MS, where neurodegeneration predominates, and where drugs promoting or “unblocking” remyelination are particularly needed [8], yet only a few are in early clinical development. The results collected in various in vitro and in vivo relevant models have shown that GNbAC1, by acting in the periphery as well as within the CNS, may promote remyelination in MS lesions while also acting on inflammation—the two pathogenic pillars of MS. The high target specificity expected with a mAb is also valuable in terms of safety, as the MSRV-Env target is not known to play any role in normal human physiology [60]. Initial clinical studies have shown a favorable safety profile for GNbAC1, which has no negative impact on immunological function. This is a clear advantage over current immunomodulators used in MS, as long-term immunosuppression is a preoccupying safety issue with these treatments [61]. Of course, the safety profile of GNbAC1 has to be reassessed and confirmed during further clinical development.
GNbAC1 is currently in early clinical development and will soon enter a phase IIb study, in which the therapeutic approach will be accompanied by a panel of RNA and/or protein biomarkers directly related to the therapeutic target protein and to the disease. The importance of biomarkers to aid in the therapeutic choice and in optimal monitoring of MS treatments has been highlighted recently in this journal [62]—in particular because of heterogeneity in the clinical and pathological phenotypes of the disease, highlighting the need for panels of various biomarkers. MSRV-related biomarkers appear to have some prognostic properties, at least when they are measured in CSF; they are also markers of the pharmacodynamic response for the ones measured in PBMCs; however, these preliminary findings need to be confirmed in larger studies. Now, it should be investigated whether these biomarkers could play a predictive role in the therapeutic response to GNbAC1, and their potential role in treatment monitoring should be evidenced to support a role of companion diagnostics. If this role were to be confirmed, a personalized medicine approach could be envisaged for GNbAC1 treatment. This would be all the more interesting for MS as a chronic disorder, where patients must be treated over the long term with minimal safety risks and optimal long-term treatment compliance (see Gotovac et al. [63]).
There is accumulating evidence showing that endogenous retroviruses of different families have a close association with the etiopathogeny of several disorders: beyond MS, HERV-Env appears to also be associated with other disorders, as data have now been obtained on an association of HERV-W with schizophrenia [64], as well as with CIDP [65], autoimmune disorders such as systemic lupus erythematosus (associated with HTLV-1 related endogenous sequence [HRES-1]) [66], rheumatoid arthritis (associated with HERV-K) [67, 68], amyotrophic lateral sclerosis (associated with HERV-K [69]), and Sjögren syndrome [70]. The GNbAC1 therapeutic development program is the first therapeutic development program—and, so far, the only therapeutic development program—specifically targeting a HERV-related target. If the assumptions presented above are confirmed, this therapeutic approach could be a complete change of paradigm not only for MS but for several fields of medicine.
References
McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–9.
Sadiq SA. Multiple sclerosis. In: Rowland LP, editor. Merrit’s neurology. Philadelphia: Lippincott, William & Wilkins; 2005. p. 941–59.
Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126:770–82.
Pugliatti M, Rosati G, Carton H, Riise T, Drulovic J, Vécsei L, Milanov I. The epidemiology of multiple sclerosis in Europe. Eur J Neurol. 2006;13:700–22.
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–8.
Spain RI, Cameron MH, Bourdette D. Recent developments in multiple sclerosis therapeutics. BMC Med. 2009;7:74.
Curtin F, Hartung HP. Novel therapeutic options for multiple sclerosis. Expert Rev Clin Pharmacol. 2014;7:91–104.
Kremer D, Küry P, Dutta R. Promoting remyelination in multiple sclerosis: current drugs and future prospects. Mult Scler. 2015;21:541–9.
Voisset C, Blancher A, Perron H, Mandrand B, Mallet F, Paranhos-Baccala G. Phylogeny of a novel family of human endogenous retrovirus sequences, HERV-W, in humans and other primates. AIDS Res Hum Retroviruses. 1999;15:1529–33.
Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A, Tristem M. Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci USA. 2004;101:4894–9.
Weiss RA. The discovery of endogenous retroviruses. Retrovirology. 2006;3:67.
Volkman HE, Stetson DB. The enemy within: endogenous retroelements and autoimmune disease. Nat Immunol. 2014;15:415–22.
Feschotte C, Gilbert C. Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet. 2012;13:283–96.
Perron H, Jouvin-Marche E, Michel M, Ounanian-Paraz A, Camelo S, Dumon A, Jolivet-Reynaud C, Marcel F, Souillet Y, Borel E, Gebuhrer L, Santoro L, Marcel S, Seigneurin JM, Marche PN, Lafon M. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology. 2001;287:321–32.
Rolland A, Jouvin-Marche E, Saresella M, Ferrante P, Cavaretta R, Créange A, Marche P, Perron H. Correlation between disease severity and in vitro cytokine production mediated by MSRV (multiple sclerosis associated retroviral element) envelope protein in patients with multiple sclerosis. J Neuroimmunol. 2005;160:195–203.
Perron H, Dougier-Reynaud HL, Lomparski C, Popa I, Firouzi R, Bertrand JB, Marusic S, Portoukalian J, Jouvin-Marche E, Villiers CL, Touraine JL, Marche PN. Human endogenous retrovirus protein activates innate immunity and promotes experimental allergic encephalomyelitis in mice. PLoS One. 2013;8:e80128.
Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol. 2006;176:7636–44.
Saresella M, Rolland A, Marventano I, Cavarretta R, Caputo D, Marche P, Perron H, Clerici M. Multiple sclerosis–associated retroviral agent (MSRV)-stimulated cytokine production in patients with relapsing–remitting multiple sclerosis. Mult Scler. 2009;15:443–7.
Firouzi R, Rolland A, Michel M, Jouvin-Marche E, Hauw JJ, Malcus-Vocanson C, Lazarini F, Gebuhrer L, Seigneurin JM, Touraine JL, Sanhadji K, Marche PN, Perron H. Multiple sclerosis–associated retrovirus particles cause T lymphocyte-dependent death with brain hemorrhage in humanized SCID mice model. J Neurovirol. 2003;9:79–93.
Zhang J, Vandevyver C, Stinissen P, Mertens N, van den Berg-Loonen E, Raus J. Activation and clonal expansion of human myelin basic protein-reactive T cells by bacterial superantigens. J Autoimmun. 1995;8:615–32.
Arad G, Levy R, Hillman D, Kaempfer R. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat Med. 2000;6:414–21.
Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108:1097–104.
Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87:385–95.
Delogu LG, Deidda S, Delitala G, Manetti R. Infectious diseases and autoimmunity. J Infect Dev Ctries. 2011;5:679–87.
Kremer D, Schichel T, Förster M, Tzekova N, Bernard C, van der Valk P, van Horssen J, Hartung HP, Perron H, Küry P. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann Neurol. 2013;74:721–32.
Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–36.
Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–16.
Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927.
Brück W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–5.
Aharoni R, Herschkovitz A, Eilam R, Blumberg-Hazan M, Sela M, Bruck W, Arnon R. Demyelination arrest and remyelination induced by glatiramer acetate treatment of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:11358–63.
Keough MB, Yong VW. Remyelination therapy for multiple sclerosis. Neurotherapeutics. 2013;10:44–54.
Antony JM, van Marle G, Opii W, Butterfield DA, Mallet F, Yong VW, Wallace JL, Deacon RM, Warren K, Power C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7:1088–95.
Perron H, Lazarini F, Ruprecht K, Péchoux-Longin C, Seilhean D, Sazdovitch V, Créange A, Battail-Poirot N, Sibaï G, Santoro L, Jolivet M, Darlix JL, Rieckmann P, Arzberger T, Hauw JJ, Lassmann H. Human endogenous retrovirus (HERV)-W ENV and GAG proteins: physiological expression in human brain and pathophysiological modulation in multiple sclerosis lesions. J Neurovirol. 2005;11:23–33.
Perron H, Germi R, Bernard C, Garcia-Montojo M, Deluen C, Farinelli L, Faucard R, Veas F, Stefas I, Fabriek BO, Van-Horssen J, Van-der-Valk P, Gerdil C, Mancuso R, Saresella M, Clerici M, Marcel S, Creange A, Cavaretta R, Caputo D, Arru G, Morand P, Lang AB, Sotgiu S, Ruprecht K, Rieckmann P, Villoslada P, Chofflon M, Boucraut J, Pelletier J, Hartung HP. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler. 2012;18:1721–36.
Perron H, Van Horssen J. HERV-W Env protein is strongly upregulated in inflammatory multiple sclerosis lesions [poster no. P355]. ECTRIMS Congress; Copenhagen; 2013.
Curtin F, Perron H, Kromminga A, Porchet H, Lang AB. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs. 2015;7:265–75.
Kremer D, Förster M, Schichel T, Göttle P, Hartung HP, Perron H, Küry P. The neutralizing antibody GNbAC1 abrogates HERV-W envelope protein-mediated oligodendroglial maturation blockade. Mult Scler. 2015;21:1200–3.
Piaton G, Williams A, Seilhean D, Lubetzki C. Remyelination in multiple sclerosis. Prog Brain Res. 2009;175:453–64.
Bonora M, De Marchi E, Patergnani S, Suski JM, Celsi F, Bononi A, Giorgi C, Marchi S, Rimessi A, Duszyński J, Pozzan T, Wieckowski MR, Pinton P. Tumor necrosis factor-α impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 2014;21:1198–208.
Perron H, Bertrand JB, Faucard R, Bernard C, Von Horssen J, Firouzi R, Potoukalian J, Germi R, Garcia-Montojo M, Morand P, Marche P, Sanhadji K, Tourraine JL, Lang A, Curtin F. Novel humanized antibody therapy in multiple sclerosis targeting immunopathogenic protein from endogenous retroviral element while preserving hosts’ immune system [poster no. P470]. ECTRIMS Congress; Lyon; 2012.
Curtin F, Lang AB, Perron H, Laumonier M, Vidal V, Porchet HC, Hartung HP. GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis–associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin Ther. 2012;34:2268–78.
Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, Pasquier RD, Schluep M, Desmeules J, Lang AB, Perron H, Faucard R, Porchet H, Hartung HP, Kappos L, Lalive PH. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis–associated endogenous retrovirus in multiple sclerosis patients. Mult Scler. 2015;21:885–93.
Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, Pasquier RD, Schluep M, Desmeules J, Lang AB, Perron H, Faucard R, Porchet H, Hartung HP, Kappos L, Lalive PH. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis–associated endogenous retrovirus in multiple sclerosis patients—a 12-month extension. J Neuroimmunol. 2015;285:68–70.
Zimmermann M, Sanderson N, Rasenack M, Lalive P, Curtin F, Lang A, Kappos L, Derfuss T. Immunomonitoring of a phase II study testing the monoclonal antibody GNbAC1 in multiple sclerosis patients [poster no. P1011]. ECTRIMS Congress; Copenhagen; 2013.
Garson JA, Tuke PW, Giraud P, Paranhos-Baccala G, Perron H. Detection of virion-associated MSRV-RNA in serum of patients with multiple sclerosis. Lancet. 1998;351:33.
Arru G, Mameli G, Astone V, Serra C, Huang YM, Link H, Fainardi E, Castellazzi M, Granieri E, Fernandez M, Villoslada P, Fois ML, Sanna A, Rosati G, Dolei A, Sotgiu S. Multiple sclerosis and HERV-W/MSRV: a multicentric study. Int J Biomed Sci. 2007;3:292–7.
Dolei A, Serra C, Mameli G, Pugliatti M, Sechi G, Cirotto MC, Rosati G, Sotgiu S. Multiple sclerosis–associated retrovirus (MSRV) in Sardinian MS patients. Neurology. 2002;58:471–3.
Mameli G, Poddighe L, Astone V, Delogu G, Arru G, Sotgiu S, Serra C, Dolei A. Novel reliable real-time PCR for differential detection of MSRVenv and syncytin-1 in RNA and DNA from patients with multiple sclerosis. J Virol Methods. 2009;161:98–106.
Garcia-Montojo M, Dominguez-Mozo M, Arias-Leal A, Garcia-Martinez Á, De las Heras V, Casanova I, Faucard R, Gehin N, Madeira A, Arroyo R, Curtin F, Alvarez-Lafuente R, Perron H. The DNA copy number of human endogenous retrovirus-W (MSRV-type) is increased in multiple sclerosis patients and is influenced by gender and disease severity. PLoS One. 2013;8(1):e53623.
Sotgiu S, Serra C, Mameli G, Pugliatti M, Rosati G, Arru G, Dolei A. Multiple sclerosis–associated retrovirus and MS prognosis: an observational study. Neurology. 2002;59:1071–3.
Sotgiu S, Arru G, Mameli G, Serra C, Pugliatti M, Rosati G, Dolei A. Multiple sclerosis–associated retrovirus in early multiple sclerosis: a six-year follow-up of a Sardinian cohort. Mult Scler. 2006;12:698–703.
Sotgiu S, Mameli G, Serra C, Zarbo IR, Arru G, Dolei A. Multiple sclerosis–associated retrovirus and progressive disability of multiple sclerosis. Mult Scler. 2010;16:1248–51.
Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, Achiti I, Confavreux C, Coustans M, le Page E, Edan G, McDonnell GV, Hawkins S, Trojano M, Liguori M, Cocco E, Marrosu MG, Tesser F, Leone MA, Weber A, Zipp F, Miterski B, Epplen JT, Oturai A, Sørensen PS, Celius EG, Lara NT, Montalban X, Villoslada P, Silva AM, Marta M, Leite I, Dubois B, Rubio J, Butzkueven H, Kilpatrick T, Mycko MP, Selmaj KW, Rio ME, Sá M, Salemi G, Savettieri G, Hillert J, Compston DA. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–51.
Mameli G, Serra C, Astone V, Castellazzi M, Poddighe L, Fainardi E, Neri W, Granieri E, Dolei A. Inhibition of multiple-sclerosis-associated retrovirus as biomarker of interferon therapy. J Neurovirol. 2008;14:73–7.
Arru G, Leoni S, Pugliatti M, Mei A, Serra C, Delogu LG, Manetti R, Dolei A, Sotgiu S, Mameli G. Natalizumab inhibits the expression of human endogenous retroviruses of the W family in multiple sclerosis patients: a longitudinal cohort study. Mult Scler. 2014;20:174–82.
Committee for Medicinal Products for Human Use (CHMP). Reflection paper on methodological issues associated with pharmacogenomic biomarkers in relation to clinical development and patient selection. European Medicine Agency. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC50010948672.pdf. Accessed 15 Jul 2015.
US Food and Drug Administration. In vitro companion diagnostic devices: guidance for industry. US Food and Drug Administration. 2014. http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm262327.pdf. Accessed 15 Jul 2015.
Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. Euopean Medicines Agency. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdf. Accessed 15 Jul 2015.
Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89:225–40.
Perron H, Lang A. The human endogenous retrovirus link between genes and environment in multiple sclerosis and in multifactorial diseases associating neuroinflammation. Clin Rev Allergy Immunol. 2010;39:51–61.
Rommer PS, Zettl UK, Kieseier B, Hartung HP, Menge T, Frohman E, Greenberg BM, Hemmer B, Stüve O. Requirement for safety monitoring for approved multiple sclerosis therapies: an overview. Clin Exp Immunol. 2014;175:397–407.
Harris VK, Sadiq SA. Biomarkers of therapeutic response in multiple sclerosis: current status. Mol Diagn Ther. 2014;18:605–17.
Gotovac K, Hajnšek S, Pašić MB, Pivac N, Borovečki F. Personalized medicine in neurodegenerative diseases: how far away? Mol Diagn Ther. 2014;18:17–24.
Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C, Sarrazin S, LeGuen E, Houenou J, Delavest M, Moins-Teisserenc H, Bengoufa D, Yolken R, Madeira A, Garcia-Montojo M, Gehin N, Burgelin I, Ollagnier G, Bernard C, Dumaine A, Henrion A, Gombert A, Le Dudal K, Charron D, Krishnamoorthy R, Tamouza R, Leboyer M. Molecular characteristics of human endogenous retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry. 2012;2:e201.
Faucard R, Madeira A, Panaite PA, Lesage C, Gehin N, Burgelin I, Curtin F, Lang AB, Steck A, Perron H, Kuntzer T, Créange A. Multiple sclerosis associated retrovirus (MSRV) envelope expression in peripheral blood mononuclear cells is associated with CIDP [poster]. Saint-Malo: Peripheral Nerve Society Meeting; 2013.
Perl A, Nagy G, Koncz A, Gergely P, Fernandez D, Doherty E, Telarico T, Bonilla E, Phillips PE. Molecular mimicry and immunomodulation by the HRES-1 endogenous retrovirus in SLE. Autoimmunity. 2008;41:287–97.
Nelson PN, Roden D, Nevill A, Freimanis GL, Trela M, Ejtehadi HD, Bowman S, Axford J, Veitch AM, Tugnet N, Rylance PB. Rheumatoid arthritis is associated with IgG antibodies to human endogenous retrovirus gag matrix: a potential pathogenic mechanism of disease? J Rheumatol. 2014;41:1952–60.
Tugnet N, Rylance P, Roden D, Trela M, Nelson P. Human endogenous retroviruses (HERVs) and autoimmune rheumatic disease: is there a link? Open Rheumatol J. 2013;7:13–21.
Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol. 2011;69:141–51.
Sander DM, Szabo S, Gallaher WR, Deas JE, Thompson JJ, Cao Y, Luo-Zhang H, Liu LG, Colmegna I, Koehler J, Espinoza LR, Alexander SS, Hart DJ, Tom DM, Fermin CD, Jaspan JJ, Kulakosky PC, Tenenbaum SA, Wilson RB, Garry RF. Involvement of human intracisternal A-type retroviral particles in autoimmunity. Microsc Res Tech. 2005;68:222–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest disclosure
All authors (F. Curtin, H. Perron, R. Faucard, H. Porchet, and A. Lang) are employees and/or shareholders of GeNeuro SA, Switzerland, and received funding from GeNeuro SA for their work as employees.
Rights and permissions
About this article
Cite this article
Curtin, F., Perron, H., Faucard, R. et al. Treatment Against Human Endogenous Retrovirus: A Possible Personalized Medicine Approach for Multiple Sclerosis. Mol Diagn Ther 19, 255–265 (2015). https://doi.org/10.1007/s40291-015-0166-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-015-0166-z