Abstract
Introduction
The approval of orphan anticancer drugs is encouraged in Japan to meet high social demand. However, approval lag and its main component, submission lag, between the USA and Japan continues to be an issue for these drugs.
Objectives
We aimed to identify the main reasons for submission lags with orphan anticancer drugs, to compare these between orphan and non-orphan anticancer drugs, and to identify factors associated with the main reasons for submission lags for orphan anticancer drugs.
Methods
We investigated anticancer drugs approved in Japan between April 2004 and December 2017 using publicly available information. We used Pearson’s product moment correlation coefficient to identify correlations between submission lag and initiation lag or development-time lag, and we used the Mann–Whitney U test to compare contributors to submission lags for both orphan and non-orphan anticancer drugs. We used multiple regression analysis to identify potential factors associated with the main reasons for submission lags for orphan anticancer drugs at the indication level. Independent variables were selected using backward/forward stepwise selection according to the Akaike information criterion.
Results
In Japan, the number of approved indications for orphan anticancer drugs consistently increased between 2004–2007 and 2016–2017. The median submission lag for orphan anticancer drugs in 2016–2017 was 515.0 days [interquartile range (IQR) 182.0–999.0], and this lag was significantly correlated with the initiation lag (correlation coefficient 0.77, P < 0.001) but not with the development-time lag (correlation coefficient − 0.031; P = 0.82). The initiation lag was significantly longer for orphan than for non-orphan anticancer drugs [median 1428.0 (IQR 890.8–2655.8) vs. 1178.0 days (369.0–1874.0); P = 0.033]. Cytotoxic drugs were significantly associated with a longer initiation lag (coefficient 2011.8; P = 0.0023), whereas designation as a breakthrough therapy in the USA was significantly associated with a shorter initiation lag (coefficient − 1272.3; P = 0.020).
Conclusions
The initiation lag for orphan anticancer drugs was the main factor affecting submission lag and was longer than that for non-orphan drugs. Of the factors associated with initiation lags, designation as a breakthrough therapy (or the possibility of such a designation) in the USA may lead to earlier initiation of clinical development of an orphan anticancer drug in Japan. In turn, this may reduce the submission lag.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Approval and submission lags for orphan anticancer drugs still exist between the USA and Japan. |
Initiation lags were the main contributors to submission lags for orphan anticancer drugs and were longer for orphan than for non-orphan anticancer drugs. |
Designation as a breakthrough therapy (or the possibility of such a designation) in the USA is one factor that may lead to earlier initiation of clinical development of orphan anticancer drugs in Japan. |
1 Introduction
Drug lag, the time delay between approval of a specific drug in one country compared with another, is a recognized social issue in Japan. Delays in availability of new drugs because of approval lags negatively impact the population’s health [1]. The Ministry of Health, Labor and Welfare (MHLW) have released various guidelines in an attempt to reduce the approval lag in Japan, including guidance concerning ethnic factors in the acceptability of foreign clinical data, guidance for establishing safety in first-in-human studies, and basic principles for global clinical trials [2,3,4]. Various systems have also been established to promote drug development in Japan, including public knowledge-based applications, a committee (the Committee) to evaluate unapproved or off-label drugs for which medical need is high, the SAKIGAKE designation system, and the conditional early approval system [5,6,7,8,9].
Approval lags for anticancer drugs have historically attracted much attention because such drugs can be crucial for patients with serious diseases [10]. Among these, orphan anticancer drugs have attracted increasing attention in recent years because (1) rare cancers collectively account for 20% of cancers, (2) their mortality rate is higher than for common cancers, and (3) proper therapeutic management is not readily available [11, 12]. Despite this increased attention, drug development for rare cancers remains insufficient, primarily because the rarity of these diseases means the costs of drug development cannot be recovered by sales expected under normal market conditions [13]. Health authorities and regulatory agencies in the USA (1983) and Japan (1993) introduced orphan drug designation systems to stimulate research and development (R&D) of drugs for rare diseases, including rare cancers [13]. Orphan drug designation provides pharmaceutical companies with incentives, including financial support, to conduct R&D for rare diseases [14].
Several movements regarding anticancer drugs have motivated pharmaceutical companies to conduct R&D for orphan anticancer drugs. Progress in the development of molecular techniques has led to the discovery of tumor-specific molecular features. Therefore, molecular-targeted drugs can logically be applied to cancers with tumor-specific molecular features, including rare cancers [15]. Pharmaceutical companies have accordingly changed their business model from the “blockbuster” to the “category leader” model and their R&D targets from highly prevalent diseases to diseases with unmet medical needs, including rare cancers [16]. As a result, approvals for orphan anticancer drugs, including those for rare cancers, have increased in both the USA and Japan. In particular, in Japan, more indications have been approved for orphan than for non-orphan anticancer drugs in recent years [17, 18].
Despite these circumstances, to our knowledge, no studies have investigated drug lag and its components in orphan anticancer drugs in Japan, although several studies have examined these lags in anticancer drugs [10, 19, 20]. We previously reported that an approval lag for orphan anticancer drugs between the USA and Japan still exist in 2016–2017, which consists of submission lag and review-time lag. Of these, submission lag is the main factor and is longer than that for non-orphan drugs [21]. However, the main components and reasons for submission lags for orphan anticancer drugs have not been identified. In this study, we aimed to identify the main contributors to submission lags, between the lag in the start of clinical development (the initiation lag) and the lag in the development period (the development-time lag) for orphan anticancer drugs. We also aimed to compare the main contributors to submission lags for orphan and non-orphan anticancer drugs and to identify factors associated with the main component for orphan anticancer drugs.
In our previous study, we specifically investigated the approval lag for anticancer drugs between Japan and the USA because it is longer than that between Japan and the EU [10], and more new molecular entities are approved in the USA than in the EU [1]. Likewise, a study reported that, for anticancer drugs, the submission lag between Japan and the USA is longer than that between Japan and the EU [10]. Similar to our previous study, we specifically examined factors associated with submission lag and its components between the USA and Japan.
2 Methods
2.1 Samples
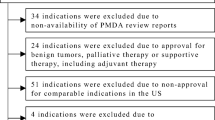
This study targeted all anticancer drugs for systemic therapies for malignant tumors approved in Japan as new active ingredients or for a new indication between April 2004 and December 2017, which included 129 anticancer drugs with 256 indications. The following indications were excluded: (1) those for which the Pharmaceuticals and Medical Devices Agency (PMDA) review reports were unavailable; (2) indications for benign tumors, palliative therapy or supportive therapy; (3) indications approved based on public knowledge-based applications; (4) indications that were not approved for comparable indications in the USA; (5) indications for which the new drug application (NDA) or biologics license application (BLA) dates in the USA were unavailable; and (6) indications for which the initial date of clinical development were unavailable. Consequently, we analyzed 123 indications of 76 anticancer drugs.
2.2 Data Collection
Data on anticancer drugs approved in Japan were obtained from the lists of approved products, review reports, package inserts, and summary of NDA dossier available from the PMDA website. Information related to the review of drugs by the US FDA was collected from approval letters, review reports, package inserts, and NDA and BLA approval reports available from the FDA website. We also obtained data on the initiation dates of clinical development from ClinicalTrials.gov, Japan Pharmaceutical Information Center Clinical Trials Information, published papers, and company websites.
The submission lag was calculated by subtracting the US NDA or BLA date from the NDA date in Japan. For the approval of new active ingredients, the initiation date of clinical development in the USA and Japan was defined as the US investigational new drug (IND) application date and the start date of the first clinical trial for the drug in Japan, respectively. For the approval of new indications, we defined the initiation date in the USA and Japan as the start date of the earliest registration trial for the target cancer in each country. If the initiation date could not be identified but the month and year could, clinical development was considered to have been initiated on the hypothetical initiation “day 1” of the identified month and year. Based on these definitions, the initiation lag was calculated by subtracting the US initiation date from that in Japan. We defined the development time as the period between the initiation date and the NDA or BLA date in the USA and the NDA date in Japan. We calculated the development-time lag by subtracting the development time in the USA from that in Japan. If several indications were approved in one NDA based on different pivotal studies, each indication was treated as a different NDA.
In Japan, the MHLW can designate drugs that satisfy the following criteria as orphan drugs after receiving applications for orphan drug designation: (1) the number of patients who may use the drug is < 50,000 in Japan, (2) the drugs are indicated for the treatment of serious diseases, including difficult-to-treat diseases with high medical needs, and (3) there is a theoretical rationale for use of the product for the target disease and the development plan is appropriate. We defined an orphan anticancer drug as an anticancer drug designated as an orphan drug in Japan [14].
All data generated or analyzed during this study are included in this article and in the Electronic Supplementary Material (ESM) (Table S1).
2.3 Data Analysis
First, we investigated time trends for submission lags for orphan anticancer drugs. Then, we identified the main components of the submission lag and compared initiation lags between orphan and non-orphan anticancer drugs. We also analyzed factors associated with initiation lags for orphan anticancer drugs. We identified correlations between submission lags and initiation lags or development-time lags according to the Pearson’s product moment correlation coefficient and used the Mann–Whitney U test to compare the initiation lag and development-time lag between orphan and non-orphan anticancer drugs. We used multiple regression analysis to identify potential factors associated with initiation lags at the indication level. Independent variables were selected using backward/forward stepwise selection according to Akaike’s information criterion. All statistical analyses were performed using EZR software [22] version 1.36, with a significance level of α = 0.05.
2.4 Independent Variables in the Multiple Regression Analysis
Given that initiation lags result from delays in starting clinical development in Japan, we hypothesized that potential factors affecting initiation lags would include company characteristics, R&D strategy, drug characteristics, regulatory status in the USA, and year, for which we examined eight independent variables in the multiple regression analysis.
For company characteristics, we selected company nationality as an independent variable. In general, pharmaceutical companies tend to prioritize development/launch in geographical regions that are culturally/linguistically close to their headquarters. This may be reflected in findings [23] that Japanese companies have a shorter submission lag than non-Japanese companies in Japan.
For R&D strategy, three independent variables were selected: external collaboration, bridging strategy, and global clinical trial. When the applicants for a drug differed between Japan and the USA, the R&D strategy was defined as an external collaboration [23]. We previously demonstrated that external collaboration was associated with longer submission lags for orphan anticancer drugs. We speculate that these longer submission lags may be affected by cases of external collaboration in the development of orphan anticancer drugs that start long after US approval of the drug [21]. Among the development strategies, we considered bridging strategy, global clinical trial, and independent development in Japan, with bridging strategy defined as one that extrapolates pivotal foreign studies as the main clinical data in a data package in Japan. A previous study found that a bridging strategy was associated with a longer initiation lag for anticancer drugs in Japan [19]. In contrast, global clinical trials were reportedly associated with shorter submission lags [24].
For drug characteristics, we considered four types of drugs: cytotoxic drugs, hormonal drugs/antagonists, molecular-targeted drugs, and other anticancer drugs. We defined a molecular-targeted drug as one known to target a specific molecule, in accordance with a previous study [25]. Among these drug characteristics, we used cytotoxic drug as an independent variable because cytotoxic drugs were previously associated with an initiation lag for anticancer drugs in Japan [19].
For regulatory status in the USA, we selected “breakthrough therapy designation by the FDA” and “accelerated approval by the FDA” because these comprise FDA programs intended to facilitate and expedite the development of new drugs [26]. We also previously found that the percentage of orphan anticancer drugs designated as a breakthrough therapy whose clinical development was started in Japan before US approval was higher than that for drugs without such a designation [21]. Moreover, a previous study [27] reported that initiation lags between the USA and Japan for anticancer drugs with breakthrough therapy designations were shorter than those for anticancer drugs without such designations.
Given that practices and strategies for clinical development and marketing applications in Japan may have changed during the 13-year period of this study, approval year was set as an independent variable. We divided the approval year into 2004–2011 and 2012–2017 because a trend towards decreasing submission lags was first observed in 2012–2013.
3 Results
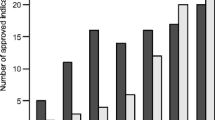
Figure 1 presents our sampling procedure. We analyzed 76 anticancer drugs with 123 indications approved in Japan from April 2004 to December 2017 (56 indications for orphan diseases, 67 for non-orphan conditions). Figure 2 shows the time trends for submission lags for orphan anticancer drugs between 2004 and 2017. The number of approved indications for orphan anticancer drugs in Japan increased consistently from 2004–2007 to 2016–2017. The submission lag increased between 2004–2007 and 2010–2011, varied greatly in 2012–2013, and declined thereafter. The median submission lag in 2016–2017 was 515.0 days [interquartile range (IQR) 182.0–999.0].
Time trend in the submission lag for orphan anticancer drugs between 2004 and 2017. The bold horizontal line in each box shows the median. The line at the upper edge of each box shows the 75th percentile and that at the lower edge shows the 25th percentile. The upper limit of the vertical line is the maximum value within the 75th percentile plus 1.5 times the interquartile range and that at the lower limit is the minimum value within the 25th percentile minus 1.5 times the interquartile range. The plotted points are outliers
We then investigated correlations between submission lags for orphan anticancer drugs and its components: initiation lag and development-time lag. Figures 3 and 4 present scatter plots of the submission and initiation lags and the submission and development-time lags for orphan anticancer drugs, respectively. Submission lag was significantly correlated with initiation lag (correlation coefficient 0.77; P < 0.001) but not with development-time lag (correlation coefficient − 0.031; P = 0.82), suggesting that initiation lags are the main contributors to submission lags for orphan anticancer drugs.
3.1 Comparison of Initiation Lags between Orphan and Non-Orphan Anticancer Drugs
Based on our findings that initiation lags are the main reasons for submission lags for orphan anticancer drugs, we compared initiation lags between orphan and non-orphan anticancer drugs. We also compared the development-time lag between orphan and non-orphan anticancer drugs as a supplemental analysis. Table 1 presents the characteristics of the analyzed orphan and non-orphan anticancer drugs, and Fig. 5 shows a comparison of initiation lags between orphan and non-orphan anticancer drugs. Initiation lags were significantly longer for orphan than non-orphan anticancer drugs [median 1428.0 (IQR 890.8–2655.8) vs. 1178.0 days (369.0–1874.0); P = 0.033]. In contrast, there was no significant difference in the development-time lag between orphan and non-orphan anticancer drugs [median − 591.5 (IQR − 1489.8 to − 188.3) vs. − 773.0 days (IQR − 1257.5 to − 169.5); P = 0.83].
Comparison of the initiation lag between orphan and non-orphan anticancer drugs. The bold vertical line in each box shows the median. The line at the right edge of each box shows the 75th percentile and that at the left edge shows the 25th percentile. The right limit of the horizontal line is the maximum value within the 75th percentile plus 1.5 times the interquartile range and that at the left limit is the minimum value within the 25th percentile minus 1.5 times the interquartile range. The plotted points are outliers
3.2 Factors Associated with Initiation Lags for Orphan Anticancer Drugs
To investigate factors associated with initiation lags for orphan anticancer drugs in more detail, we performed a multiple regression analysis. Table 2 shows the initiation lags for independent variables used for the analysis of orphan anticancer drugs. Among the drug characteristics, the initiation lag was longer for cytotoxic drugs than for other orphan anticancer drugs, including molecular-targeted drugs. Orphan anticancer drugs designated as a breakthrough therapy by the FDA had a shorter initiation lag than drugs without such a designation.
Table 3 presents the factors associated with initiation lags for orphan anticancer drugs at the indication level. Cytotoxic drugs were significantly associated with a longer initiation lag (coefficient 2011.8; P = 0.0023), whereas breakthrough therapy designation by the FDA was significantly associated with a shorter initiation lag (coefficient − 1272.3; P = 0.020).
To identify factors associated with correlations between cytotoxic drugs and initiation lags, we conducted a supplemental analysis of the development status of cytotoxic drugs and other orphan anticancer drugs in Japan at the time of approval in the USA. As shown in Table 4, the percentage of cytotoxic drugs whose clinical development had not started in Japan at the time of approval in the USA was higher than that of other orphan anticancer drugs (62.5% vs. 31.2%).
Table 5 compares the development status in Japan at the time of US approval between orphan anticancer drugs with and without breakthrough therapy designations. The percentage of orphan anticancer drugs designated as breakthrough therapies whose clinical development had not started in Japan at the time of US approval was lower than those without such a designation (8.3% vs. 43.2%).
Table 6 compares the types of orphan anticancer drugs developed by Japanese and non-Japanese pharmaceutical companies: a higher percentage of cytotoxic drugs were developed by Japanese than by non-Japanese pharmaceutical companies (38.5% vs. 7.0%).
4 Discussion
We demonstrated that submission lags for orphan anticancer drugs in 2016–2017 were around 1.5 years and that initiation lags were the main reason for this. We also revealed that initiation lags were longer for orphan than for non-orphan anticancer drugs.
We identified two factors associated with initiation lags for orphan anticancer drugs between Japan and the USA: cytotoxic drug and “breakthrough therapy designation by the FDA.” Although submission lags for orphan anticancer drugs have been decreasing since 2012, approval year was not significantly associated with initiation lags. We speculate that the association between cytotoxic drugs and initiation lags may be linked to the higher percentage of cytotoxic drugs for which clinical development had not started in Japan at the time of US approval compared with other orphan anticancer drugs. Our supplemental analysis supports this hypothesis. This may be because most cytotoxic drugs were clinically developed in the USA by pharmaceutical companies that were small or did not have an affiliate in Japan and likely had no intention of developing the drugs in Japan because of insufficient budget or lack of experience with clinical development in Japan. The very long period between US approval and the start of clinical development in Japan for cytotoxic drugs may be another major reason. For example, clofarabine, a cytotoxic orphan anticancer drug approved in Japan in March 2013 for the treatment of relapsed or refractory acute lymphoblastic leukemia, had a long initiation lag of 3008 days. Clinical development for clofarabine was started by Southern Research Institute and Bioenvision, Inc. in the USA in November 2001. Genzyme Corporation (Genzyme), which acquired the licensing rights to clofarabine, submitted the NDA in March 2004 and received approval in December 2004, at which time clinical development of clofarabine in Japan had not started. In October 2005, the Committee evaluated that clinical development should start early in Japan, and Genzyme subsequently started a phase I study in Japan in February 2010 and submitted the NDA in June 2012 [28,29,30].
We speculate that the Committee’s evaluation was the trigger for initiating the clinical development of clofarabine in Japan. In the data we analyzed, the Committee evaluated 50% of all cytotoxic orphan anticancer drugs. The Committee’s remit is to address issues associated with unapproved drugs or the off-label use of drugs that are approved in other developed counties but not in Japan. The Committee evaluates whether a proposed drug or treatment meets a high medical need by reviewing the formal petitions submitted by patient advocacy groups, academic societies, and pharmaceutical companies. The MHLW determines pathways for regulatory approval according to the Committee’s evaluation [7]. The hope is that the Committee will facilitate a reduction in not only the number of unapproved orphan anticancer drugs but also initiation lags for orphan anticancer drugs.
The breakthrough therapy designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. The criteria for breakthrough therapy designation requires preliminary clinical evidence demonstrating that the drug may substantially improve at least one clinically significant endpoint over available therapies. The FDA generally expects evidence derived from phase I or II studies [26]. The start date for the clinical development of a drug in Japan for which development has already started in the USA is at the discretion of the pharmaceutical company. A pharmaceutical company’s decision to develop a drug in Japan depends on various factors, such as the company’s experience in clinical development in Japan, the availability of a budget for the development, and the drug’s marketability in Japan. A high success rate for the development of drugs may also be a major factor in the decision [27]. Accordingly, pharmaceutical companies may start clinical development earlier for orphan anticancer drugs with a breakthrough therapy designation or the possibility of such a designation in Japan than for drugs without this designation. This may explain the higher percentage of orphan anticancer drugs with breakthrough therapy designations for which clinical development had started in Japan at the time of US approval compared with drugs without such a designation. Initiation lags for anticancer drugs with breakthrough therapy designation were previously reported [27] to be shorter than for anticancer drugs without this designation, which supports our results. In 2015, the MHLW and the PMDA established the SAKIGAKE designation system, which promotes R&D and early clinical research/trials in Japan with the aim of enabling early practical application of innovative medical products with significant prospective efficacy by prioritized consultations, substantial pre-application consultations, priority reviews, review partners, and substantial post-marketing safety measures. The system’s concept is similar to that of the breakthrough therapy designation in the USA; however, it contains unique designation criteria that requires planning an application for approval first in Japan or simultaneously in Japan and other countries [8]. The SAKIGAKE designation system is expected to contribute to a reduction of initiation lags for orphan anticancer drugs in Japan.
We did not find a shorter initiation lag for orphan anticancer drugs developed by Japanese pharmaceutical companies compared with non-Japanese pharmaceutical companies. One potential reason for this may be that many Japanese pharmaceutical companies have transferred their development basis from Japan to the USA [19]. As a result, the clinical development strategies of Japanese pharmaceutical companies may be similar to those of non-Japanese companies. Another potential reason is the development of a higher percentage of cytotoxic drugs, a factor associated with a longer initiation lag, by Japanese pharmaceutical companies, whereas non-Japanese companies mainly developed molecular-targeted drugs. This might be because Japanese companies have fallen behind in R&D for molecular-targeted drugs.
4.1 Limitations
Some limitations are associated with this study. First, we only evaluated drugs that were successfully approved in both Japan and the USA. Inclusion of drugs for which development failed or is ongoing in one country may result in longer or shorter submission and initiation lags depending on the country in which the drug development failed or the NDA or BLA was delayed. Second, we defined the date of initiation of clinical development in the USA and Japan as the US IND application date and the start date of the first clinical trial for the drug in Japan, respectively, because the clinical trial notification (CTN) submission date is not disclosed in Japan. In Japan, the first clinical trial for a drug cannot be started until a 30-day investigation of the CTN is complete, so the CTN is generally submitted more than 30 days before the actual initiation date. However, we speculate that these 30 days had little impact on the results of this study because the median initiation lag for orphan and non-orphan anticancer drugs was 1428.0 days and 1178.0 days, respectively, both much longer than 30 days. Third, the adjusted R2 in the multiple regression analysis was 0.3091, suggesting that the independent variables in the model could not sufficiently explain the initiation lag. However, despite these limitations, we are confident our analyzed data are sufficient for the purposes of this study.
5 Conclusions
The number of approved indications for orphan anticancer drugs in Japan increased consistently from 2004–2007 to 2016–2017. We demonstrated that the submission lag for orphan anticancer drugs in 2016–2017 was approximately 1.5 years, and the main contributor to this was initiation lag, which was longer than that for non-orphan drugs. Cytotoxic drugs had longer initiation lags, whereas breakthrough therapy designation by the FDA was associated with shorter initiation lags. Of the factors associated with initiation lags, breakthrough therapy designation by the FDA or the possibility of such a designation may lead to earlier initiation of clinical development for orphan anticancer drugs in Japan, which may in turn contribute to reducing the submission lag.
References
Tsuji K, Tsutani K. Approval of new drugs 1999–2007: comparison of the US, the EU and Japan situations. J Clin Pharm Ther. 2010;35(3):289–301.
Ministry of Health, Labour and Welfare. Ethnic factors in the acceptability of foreign clinical data. (in Japanese) 1998. https://www.pmda.go.jp/files/000156571.pdf Accessed 15 Aug 2018.
Ministry of Health, Labour and Welfare. Guidance for establishing safety in first-in-human studies during drug development. 2005. https://www.pmda.go.jp/files/000208201.pdf Accessed 15 Aug 2018.
Ministry of Health, Labour and Welfare. Basic principles on global clinical trials. 2007. https://www.pmda.go.jp/files/000157900.pdf Accessed 15 Aug 2018.
Ito Y, Narimatsu H, Fukui T, Fukao A, Yoshioka T. Critical review of ‘Public domain application’: a flexible drug approval system in Japan. Ann Oncol. 2013;24(5):1297–305.
Shimazawa R, Ikeda M. Japanese regulatory system for approval of off-label drug use: evaluation of safety and effectiveness in literature-based applications. Clin Ther. 2012;34(10):2104–16.
Ito T, Urushihara H, Matsushima Y, Nakajima K, Kurokawa T. Mode of regulatory applications of drugs used off-label reviewed by the evaluation committee on unapproved or off-labeled drugs with high medical needs. Jpn J Clin Pharmacol Ther. 2015;46(5):233–41 (in Japanese).
Ministry of Health, Labour and Welfare. Strategy of SAKIGAKE. 2014. https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/dl/140729-01-01.pdf Accessed 15 Aug 2018.
Ministry of Health, Labour and Welfare. Implementation of a conditional early approval system for pharmaceutical products. 2017. https://www.pmda.go.jp/files/000222439.pdf Accessed 15 Aug 2018.
Yonemori K, Hirakawa A, Ando M, Hirata T, Yunokawa M, Shimizu C, et al. The notorious “drug lag” for oncology drugs in Japan. Invest New Drugs. 2011;29(4):706–12.
Blay JY, Coindre JM, Ducimetiere F, Ray-Coquard I. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016;17(2):e62–9.
Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–511.
Heemstra HE, van Weely S, Buller HA, Leufkens HG, de Vrueh RL. Translation of rare disease research into orphan drug development: disease matters. Drug Discov Today. 2009;14(23–24):1166–73.
Ministry of Health, Labour and Welfare. Overview of orphan drug/medical device designation system. (in Japanese) 2015. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000068484.html Accessed 15 Aug 2018.
Boyd N, Dancey JE, Gilks CB, Huntsman DG. Rare cancers: a sea of opportunity. Lancet Oncol. 2016;17(2):e52–61.
Kumar Kakkar A, Dahiya N. The evolving drug development landscape: from blockbusters to niche busters in the orphan drug space. Drug Dev Res. 2014;75(4):231–4.
Gaddipati H, Liu K, Pariser A, Pazdur R. Rare cancer trial design: lessons from FDA approvals. Clin Cancer Res. 2012;18(19):5172–8.
Nakayama H, Tsukamoto K. Unique characteristics of regulatory approval and pivotal studies of orphan anticancer drugs in Japan. Invest New Drugs. 2018;36(4):702–8.
Maeda H, Kurokawa T. Recent trends for drug lag in clinical development of oncology drugs in Japan: does the oncology drug lag still exist in Japan? Int J Clin Oncol. 2015;20(6):1072–80.
Kawabata-Shoda E, Masuda S, Kimura H. Anticancer drug development from traditional cytotoxic to targeted therapies: evidence of shorter drug research and development time, and shorter drug lag in Japan. J Clin Pharm Ther. 2012;37(5):547–52.
Nakayama H, Matsumaru N, Tsukamoto K. The drug lag and associated factors for orphan anticancer drugs in Japan compared to the United States. Invest New Drugs. 2018. https://doi.org/10.1007/s10637-018-0612-y.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48(3):452–8.
Hirai Y, Kinoshita H, Kusama M, Yasuda K, Sugiyama Y, Ono S. Delays in new drug applications in Japan and industrial R&D strategies. Clin Pharmacol Ther. 2010;87(2):212–8.
Ueno T, Asahina Y, Tanaka A, Yamada H, Nakamura M, Uyama Y. Significant differences in drug lag in clinical development among various strategies used for regulatory submissions in Japan. Clin Pharmacol Ther. 2014;95(5):533–41.
Maeda H, Kurokawa T. Differences in maximum tolerated doses and approval doses of molecularly targeted oncology drug between Japan and Western countries. Invest New Drugs. 2014;32(4):661–9.
US Food and Drug Administration. Guidance for industry expedited programs for serious conditions—drugs and biologics. 2014. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM358301.pdf Accessed 15 Aug 2018.
Tanaka M, Matsumaru N, Tsukamoto K. Influence of breakthrough therapy designation in the United States on oncology drug development timelines in Japan. Pharm Med. 2018;32(3):201–7.
Pharmaceuticals and Medical Devices Agency. Review report for clofarabine. (in Japanese) 2013. http://www.pmda.go.jp/drugs/2013/P201300047/34053100_22500AMX00882000_A100_3.pdf Accessed 15 Aug 2018.
US Food and Drug Administration. Medical Review(s) for clofarabine. 2004. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-673_Clolar_medr.PDF. Accessed 15 Aug 2018.
Sanofi (Genzyme, a Sanofi Company). A study of clofarabine in Japanese patients with acute myeloid leukemia (AML). ClinicalTrials.gov. 2014.https://www.clinicaltrials.gov/ct2/show/NCT01090167Accessed 15 Aug 2018.
Acknowledgements
The authors express their gratitude to Makoto Tanaka for his useful suggestions and to Katsuya Nakano for his review of the study from a regulatory affairs viewpoint.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of Interest
Hiroki Nakayama is an employee of Astellas Pharma Inc. Naoki Matsumaru and Katsura Tsukamoto have no conflicts of interest that are directly relevant to the content of this article.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakayama, H., Matsumaru, N. & Tsukamoto, K. Delays in New Drug Applications and Associated Factors for Orphan Anticancer Drugs in Japan Compared with the USA. Pharm Med 32, 403–412 (2018). https://doi.org/10.1007/s40290-018-0257-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-018-0257-3