Abstract
Background
Ultra-trail running races pose appreciable physiological challenges, particularly for glucose metabolism. Previous studies that yielded divergent results only measured glycaemia at isolated times.
Objectives
We aimed to explore the impact of an ultra-endurance race on continuously measured glycaemia and to understand potential physiological mechanisms, as well as the consequences for performance and behavioural alertness.
Methods
Fifty-five athletes (78% men, 43.7 ± 9.6 years) ran a 156-km ultra-trail race (six 26-km laps, total elevation 6000 m). Participants wore a masked continuous glucose monitoring sensor from the day before the race until 10 days post-race. Blood was taken at rest, during refuelling stops after each lap, and after 24-h recovery. Running intensity (% heart rate reserve), performance (lap times), psychological stress, and behavioural alertness were explored. Linear mixed models and logistic regressions were carried out.
Results
No higher risk of hypo- or hyperglycaemia was observed during the exercise phases of the race (i.e. excluding stops for scientific measurements and refuelling) compared with resting values. Laps comprising a greater proportion of time spent at maximal aerobic intensity were nevertheless associated with more time > 180 mg/dL (P = 0.021). A major risk of hyperglycaemia appeared during the 48-h post-race period compared with pre-race (P < 0.05), with 31.9% of the participants spending time with values > 180 mg/dL during recovery versus 5.5% during resting. Changes in circulating insulin, cortisol, and free fatty acids followed profiles comparable with those usually observed during traditional aerobic exercise. However, creatine phosphokinase, and to a lesser extent lactate dehydrogenase, increased exponentially during the race (P < 0.001) and remained high at 24-h post-race (P < 0.001; respectively 43.6 and 1.8 times higher vs. resting). Glycaemic metrics did not influence physical performance or behavioural alertness.
Conclusion
Ultra-endurance athletes were exposed to hyperglycaemia during the 48-h post-race period, possibly linked to muscle damage and inflammation. Strategies to mitigate muscle damage or subsequent inflammation before or after ultra-trail races could limit recovery hyperglycaemia and hence its related adverse health consequences.
Trial Registration Number
NCT05538442 2022–09-21 retrospectively registered.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
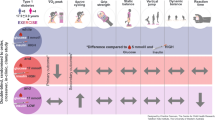
Laps including a greater proportion of time spent at maximal aerobic intensity were laps associated with greater risk of being hyperglycaemic. |
A significant risk of hyperglycaemia appears during recovery, up to 48 h post-race, possibly associated with muscle damage as a well-known factor in insulin resistance. |
Given the putative impact of repetitive ultra-trail-associated periods of hyperglycaemia, particularly on vascular health, we open up much-needed potential avenues for strategies to mitigate muscle damage or limit inflammation related to muscle damage. |
1 Introduction
Defined as sporting activities lasting > 6 h [1], ultra-endurance events have become increasingly popular. Many athletes compete in races lasting several days, throughout which taking breaks or naps is left to the athlete’s discretion and can significantly impact total race duration and performance. These races, known as single-stage races, therefore pose unique physiological challenges, mostly owing to their extreme duration, particularly with regard to energy substrate availability. Under such race conditions, muscle and hepatic glycogen stores are limited while glucose represents a fundamental substrate for muscle contraction, particularly for sustaining sufficient exercise intensity. Several teams of researchers have explored glycaemia around single-stage ultra-endurance events by measuring capillary or venous glucose levels at specific isolated times, i.e. mostly before and/or at the end of the race, or at the end of the stages of multistage races [2,3,4,5,6], 12 h to 16 days after the race [2, 4, 5], and, more rarely, at specific times during the race [4]. However, these one-off measures do not accurately reflect the physiological reality, with glycaemia change in direction rapidly reversing even in healthy individuals [7]. This may explain the divergence of the results from the abovementioned studies, in which there was either no change [3, 4] or an increase in glycaemia [2, 5, 6].
To our knowledge, only two studies [8, 9] have so far measured blood glucose continuously during ultra-endurance events, although the authors did not use masked glucose sensors, which may have influenced racing strategies (e.g. pacing and/or fuelling). Moreover, while the authors mentioned highly variable glucose concentrations (as averaged over the total duration of the race [8], or at hourly intervals [9], as well as minimum and maximum values during the race [8]) throughout the race and/or across the participants, they did not specifically determine time spent at low glucose levels (i.e. < 70 mg/dL) or in hyperglycaemia (> 140 mg/dL). Exploring these unusual glucose excursions is important given their putative deleterious impact on physical performance [10] and cognitive performance [11, 12], with possible consequences on behavioural alertness and hence the risk of injury. Whether the widely variable glycaemic values persist during recovery remains unstudied.
Finally, if ultra-endurance events do trigger significant glucose excursions (low glucose and/or hyperglycaemia), repeated participation in these events during an athlete’s sporting career may be presumed to impact vascular health [13,14,15,16,17,18]. It is therefore important to understand the factors involved. For example, muscle damage and sleep deprivation/restriction could alter insulin sensitivity [19, 20]; an increase in cortisol [6, 21] could contribute to an increase in glycaemia, with an insulin resistance/antagonizing effect described outside the context of exercise [22]. Ultimately, exercise intensity [23] and psychological strain [24] may also influence glycaemic excursions during the race.
The aim of this study was to assess the impact of an ultra-endurance race on glycaemia, continuously measured throughout the race and during the following 10 days, and to understand some physiological mechanisms as well as some confounding factors (sleep restriction, running intensity, initial psychological stress level). The aim was also to see whether extreme variations in glycaemia (low glucose, hyperglycaemia, glycaemic variability), if present, influence outcomes that are highly relevant for performance in such extreme sporting events, such as behavioural alertness and running time.
2 Methods
This study was approved by the Ouest III Ethics Committee (no. 2021-A01765-36). Written consent was obtained from participants before their inclusion in the study.
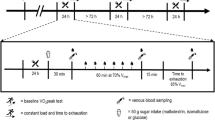
Fifty-five trained runners volunteered to take part in a scientific oriented ultra-trail race (‘Trail Scientifique de Clécy’ for research purposes), starting at 2.30 p.m. on 11 November 2021, and involving an elevation gain of 6000 m and a total distance of 156 km. The distance was divided into six identical laps of 26 km, interspersed with stops for scientific measurements organized in a precise order, as previously detailed in a methodological paper [25]. Stops included the possibility of naps (nap times were recorded when applicable, and any naps only took place after visiting the canteen) and taking refreshments. The runner had to be self-sufficient in terms of water and food between each refreshment point, i.e. replenishment only available at the end of each lap for food and water (at a canteen), and at each half-lap for water only. At the refreshment point canteen, runners were allowed to directly eat and/or to take food/snacks (provided by the organisers and/or by themselves) with them for consumption during the next lap. Runners were able to refill their water bottles halfway through each lap (i.e. at 13 km, from a water tap).
2.1 Continuous Interstitial Glucose Monitoring
The day before the race, a professional masked continuous glucose monitoring (CGM) sensor (Freestyle Libre Pro IQ, Abbott®; with a value every 15 min) was inserted on the back of participants’ arms. The CGM sensor was removed 10 days after the race. Glycaemic excursions and variability were calculated from CGM recordings over several specific periods (excluding the stops for scientific measurements and refuelling between each lap): (1) from the insertion of the CGM sensor the day before the start of the race (at 5.06 p.m. ± 48 min; minimum: 3.40 p.m. – maximum: 6.58 p.m.) up to 6.00 a.m. on the day of the race; (2) from the beginning to the end of each lap; (3) from the beginning to the end of the race (average of all laps); (4) during 2, 8, 12, 24, and 48 h after the end of the race; and (5) for the 10 days following the race, explored either using 24-h periods or distinguishing daytime (i.e. from 8.00 a.m. to 9.00 p.m. each day) from night time (i.e. from midnight to 6.00 a.m. each night).
Glycaemic excursions taken into consideration were the percentage of time spent in range (between 70 and 140 mg/dL), below range (< 70 mg/dL), and above range (> 140 mg/dL and > 180 mg/dL), low blood glucose index (LBGI), high blood glucose index (HBGI), and the highest and the lowest glycaemic values [26,27,28]. These thresholds were chosen in accordance with the typical CGM resting values in healthy non-diabetic populations of between 70 and 140 mg/dL during approximately 96% of time, with values > 180 mg/dL being uncommon [28]. Glycaemic variability was assessed through the coefficient of variation and the standard deviation (SD) of mean glucose values [27].
2.2 Food Intake
The day before, during, and the day after the race food intake data were collected. QuickTapSurvey, a data collection application, installed on digital tablets, was used to take photos and register answers to questions about food intake. For the day before and after the race, only questions were asked (no photos) (e.g. details about the quantity and quality of meals, snacks, and drink). During each scientific stop during the race a photo of food/snacks on each runner’s tray was taken before and after consumption. Moreover, at the end of each lap, a photo was taken of the empty packaging of what each runner had eaten during the lap. Questions were also asked in addition to taking photos (e.g. questions about unopened refreshments or whether any packaging was lost during the lap).
2.3 Running Intensity and Physical Performance
Heart rates (HRs), measured throughout the race with Hexoskin Pro Physiological Waistcoat (Carre Technologies Inc.), were used to calculate exercise intensities as percentages of heart rate reserve (%HRR) using the Karvonen formula: %HRR = (HR − resting HR)/(maximum HR − resting HR) × 100; with maximum HR theoretically calculated as HR = 208 − (0.7 × age) [29] and resting HR measured with the waistcoat during 5 min lying in bed before getting up on the day of the race. This further allowed us to calculate the percentage of time spent (during each lap) in three aerobic endurance exercise intensity ranges [30]: (1) light to moderate (up to 59% HRR), (2) vigorous (from 60 to 89% HRR), and (3) maximal (from 90 to 100% HRR). The time needed to complete each lap was recorded as an index of physical performance.
2.4 Initial Psychological Stress Level
The six-item (i.e. I feel/am: calm, tense, upset, relaxed, content, worried; graduated on a scale from 1, not at all, to 4, very much) short-form of the State-Trait Anxiety Inventory (STAI) [31] was self-completed on the morning of the race (7.13 a.m. ± 43 min).
2.5 Behavioural Alertness
2.5.1 Psychomotor Vigilance Task
On the day before the race, on the morning of the race, at the end of each lap, and at the end of the race, the runners performed a 5-min series response time test [32]. Outcomes considered were lapses of attention (as defined as the number of responses with a response time ≥ 500 ms) and the mean response time (Rt; mean calculated after excluding reaction times < 100 ms) [33].
2.5.2 Sleepiness
On the day before the race, on the morning of the race, at the end of each lap, and at the end of the race, a measure of the subjective level of sleepiness using the Karolinska Sleepiness Scale (KSS) [34, 35] was carried out. The KSS is a validated 9-point Likert scale [36] that is sensitive to fluctuations (1 = extremely alert, 2 = very alert, 3 = alert, 4 = rather alert, 5 = neither alert nor sleepy, 6 = some signs of sleepiness, 7 = sleepy, but no effort to keep awake, 8 = sleepy, some effort to keep awake, and 9 = very sleepy, great effort keeping awake, fighting sleep) assessing the runners’ state of fatigue during the immediately preceding 5 min.
2.6 Blood Biology
Blood samples were collected from the median cubital vein, in a sitting position, on the morning before the start of the race (after breakfast), at the end of each lap, at the end of the race (after visiting the refreshment canteen), and 24 h after the end of the race. They were centrifuged (immediately at 855 g, 4 °C, 5 min for containers with anticoagulants—heparin or citrate—and after 1 h at ambient temperature at 1520 g, 4 °C, 13 min for free-additive containers), and resulting supernatant was removed and frozen (− 80 °C) pending future analyses of glucose, hormones regulating glycaemia (insulin, cortisol), metabolites (free fatty acids, ß-hydroxybutyrate), and markers of muscle damage (creatine phosphokinase [CPK], lactate dehydrogenase [LDH]). The assays used for all these molecules are given in the electronic supplemental material (ESM), Supplementary Table 1.
2.7 Statistical Analysis
Statistical analyses were carried out using the IBM SPSS version 27.0 (IBM Corp., Armonk, New York, USA). Results are reported as means ± SD, unless otherwise indicated. We used linear mixed models and binary/multinomial logistic regressions with a random intercept for the participants (see details of the models in the ESM, Supplementary Table 2). For all the models, residuals were Gaussian and the results expressed as the mean estimation ‘e’. P ≤ 0.05 were considered statistically significant.
3 Results
The participants’ characteristics are presented in Table 1. Thirty-four males (79.1%) and seven females (58.3%) finished the race. The best performance was 17:11:02 (scientific experiment time deducted), and the last finisher came in at 35:55:21. For the 41 finishers, stops for scientific measurements between laps lasted about 64 ± 13 min, and 31 of them did not take any naps throughout the duration of the race. Stops for meals and snacks between laps lasted approximately 17 ± 3 min. Outdoor temperatures varied from 2.5 to 13.6 °C and humidity levels from 65.2 to 95.9%. The lowest and highest altitudes were 36 m and 254 m, respectively. The elevation profile is available in Supplementary Fig. 1 (see the ESM). Concerning nutrition, at the end of the race, the food intake data based on time-stamped photos collected on the application was unfortunately lost due to connection issues. All runners answered the ‘yes/no’ questions, but very few responded correctly to the open-ended questions (between 3.6 and 5.5% depending on the questions). The answers to the ‘yes/no’ questions show that 24% of the runners ate unpacked snacks and 94.5% lost the packaging of certain food items during the tour (mean of the six laps).
3.1 Glycaemic Excursions During the Race
Supplementary Fig. 2 in the ESM shows changes in glycaemia from CGM sensor during the race for each finisher. Overall, glycaemic spikes seem to occur during stops for scientific measurements or just after, probably corresponding to meals and/or snacks taken during these stops. It should be noted that outside these breaks during the race, HBGI was higher than at pre-race rest, while the risk of time spent > 140 or 180 mg/dL was not significantly increased (Tables 2, 3). The probability of spending time at low glucose values was lower during exercise than at rest (Tables 2, 3).
HBGI as well as the risk of time spent above 180 mg/dL were higher during a lap when the proportion of time spent at maximal aerobic exercise intensity during the lap was also higher (for HBGI, e = + 0.013, P = 0.006; for time > 180 mg/dL, e = + 0.041, P = 0.021; Supplementary Table 3 in the ESM). Hyperglycaemic indices and proportions of time spent within exercise intensity bands during the six laps are displayed in Fig. 1. Glycaemic excursions during the race (excluding stops between laps) were not influenced by initial psychological stress level, nap time, participants’ characteristics, or finisher status (Supplementary Table 3).
High blood glucose metrics and percentage of time spent in three exercise intensity ranges throughout the race in finishers. Data are expressed as mean ± standard error. A, B, and C High blood glucose metrics. Note that the y-axis scales of the graphs are different in order to make them easier to read. D Percentage of time spent in three exercise intensity ranges. The three aerobic exercise intensity ranges were light to moderate (up to 59% HRR), vigorous (from 60 to 89% HRR), and maximal (from 90 to 100% HRR). %HRR was calculated, using the Karvonen formula: (HR − resting HR)/(HRmax − resting HR) × 100; with HRmax theoretically calculated as HR = 208 − (0.7 × age) and resting HR measured with the Hexoskin Pro Physiological Waistcoat (Carre Technologies Inc) during the 5 min lying in bed before getting up the day of the race. %HRR percentage of HRR, HBGI high blood glucose index, HR heart rate, HRmax maximum HR, HRR heart rate reserve
Physical performance (time at each lap) and behavioural alertness (Rt, lapses of attention, KSS) at the end of each lap were not significantly influenced by glycaemic variability and time spent at low or high glucose values during the laps. Ultra-trail race completion time was not significantly associated with glycaemic metrics (percentage time spent < 70 mg/dL and > 140 and > 180 mg/dL throughout the race, excluding stops between laps), nor with the changes in performance in the psychomotor vigilance task from lap 1 to lap 6 (Spearman correlations).
Rt, lapses of attention, and KSS were not significantly influenced by lap time, lap number, and nap time (data not shown). Rt was significantly impaired in cases of higher initial psychological stress level (STAI) (e ≥ 0.259, P ≤ 0.03).
Venous glycaemia followed a pattern comparable to CGM (Fig. 2).
Venous blood glucose before the race, at the end of each lap after visiting the refreshment station, and 24 h after the race. Throughout the race, glucose values increased (e = + 4.733; P < 0.001) and remained high at 24 h of recovery compared with resting values (e = + 5.526; P < 0.001). Data expressed as mean ± standard error. A Finishers (n = 41). B Non-finishers; the data from one male participant who stopped the race at the end of lap 2 and one female who stopped the race at the end of lap 1 are not shown. Race stopped at the end of lap 5, n = 4; at the end of lap 4, n = 5; at the end of lap 3, n = 3
3.2 Glycaemic Excursions During Recovery
Except for the first 2 h of recovery, hyperglycaemia was pronounced during recovery (over 48 h) compared with values before and during the race (Tables 2, 3). For example, during the pre-race resting period, only three out of the 55 participants (5.5% of the sample) spent some time > 180 mg/dL (i.e. 2.9%, 1.5%, and 3.0% of time). Conversely, during the first 48 h of recovery, 17 participants (31.9% of the sample) spent time > 180 mg/dL (mean for the 17 participants 4.5 ± 5.0 [SD]%, minimum: 0.07% – maximum: 16.7%). The risk of hyperglycaemia then gradually decreased for 4 days post-exercise without any difference between daytime and night-time (i.e. time spent > 140 mg/dL: e = − 0.628, P < 0.001; time spent > 180 mg/dL: e = − 0.855, P < 0.001; HBGI: e = − 0.282, P < 0.001; highest glucose value: e = − 9.295, P < 0.001) (Supplementary Fig. 3B in the ESM). Conversely, for recovery, the probability for low glucose levels increased again progressively (Table 2) up to 4 days post-exercise (i.e. LBGI: e = 0.219, P = 0.005; lowest glucose value: e = − 3.683, P < 0.001) (Supplementary Fig. 3A).
Values for glycaemic metrics at rest, during the race, and during recovery are available in Table 3. Overall, there was a wide interindividual variability of time spent in hyperglycaemia during and after the race.
3.3 Metabolic and Hormonal Outcomes
Plasma and serum hormones, metabolites, and markers of muscle damage at rest, at the end of each lap, and after 24 h of recovery are shown in Fig. 3 for race finishers and in Supplementary Fig. 4 (in the ESM) for non-finishers (no significant differences between finishers and non-finishers).
Plasma and serum hormones, metabolites and markers of muscle damage at rest, at the end of each lap, and after 24 h of recovery in finishers. Data expressed as mean ± standard error. A Insulin. During the race, the insulin concentration tended to decrease (e = − 2.384; P = 0.080). Between the end of the race and 24 h of recovery, the insulin concentration increased (e = + 86.104; P < 0.001), with values after 24 h of recovery being higher than values at rest (e = + 6.137; P = 0.013). B Cortisol. During the race, the cortisol concentration increased (e = + 22.770; P < 0.001). Between the end of the race and 24 h of recovery cortisol decreased (e = − 235,042; P < 0.001), ultimately reaching values lower than at rest (e = 8.749; P = 0.003). C Free fatty acids (FFA). During the race, the FFA concentration increased (e = + 0.088; P < 0.001). Between the end of the race and 24 h of recovery FFA concentration decreased (e = − 0.926; P < 0.001). D ß-hydroxybutyrate. During the race, ß-hydroxybutyrate increased (e = + 0.164; P < 0.001). Between the end of the race and 24 h of recovery, ß-hydroxybutyrate decreased (e = − 1.086; P < 0.001), with values at 24 h of recovery still higher than at rest (e = + 0.011; P = 0.012). E Creatine phosphokinase (CPK). F Lactate dehydrogenase (LDH). During the race, CPK and LDH increased (CPK: e = 1638.908; P < 0.001; LDH: e = + 42.690; P < 0.001). They decreased between the end of the race and 24 h of recovery (CPK: e = − 4026.023; P < 0.001; LDH: e = − 88.658; P < 0.001), but values at 24 h of recovery remained higher than at rest (CPK: e = + 684.767; P < 0.001; LDH: e = + 20.574; P < 0.001)
The insulin concentration tended to decrease during the race (P = 0.080), and even more markedly during the last lap, while it then increased after 24 h of recovery, reaching values higher than at rest (P = 0.013). Free fatty acids and ß-hydroxybutyrate increased during the race (P < 0.001), and even more so during the last lap, followed by a decrease during the 24-h recovery phase (P < 0.001). Cortisol increased during the race (P < 0.001) and then decreased during the 24-h recovery phase (P < 0.001), ultimately reaching values lower than at rest (P = 0.003). However, CPK, and to a lesser extent LDH, increased exponentially during the race (P < 0.001) and remained high 24 h post-race (P < 0.001).
4 Discussion
Glycaemic metrics did not differ between race finishers and non-finishers, suggesting that abnormal variations in glycaemia did not occur when participants stopped prematurely. For the first time, this study shows that ultra-endurance races significantly trigger hyperglycaemia during the first 48 h of recovery. During the race, while the stops for scientific measurements and refreshments were logically associated with major increases in glycaemia, the exercise periods (i.e. during each lap, excluding the stops for scientific measurements and refuelling between each lap) did not significantly induce hyperglycaemia. However, the laps with more time spent ≥ 90% HRR were associated with a greater probability of spending time in hyperglycaemia during the lap.
4.1 Putative Mechanisms of Hyperglycaemia Around the Ultra-Trail Race
Exercise intensity appears to be a clear contributing factor to hyperglycaemia during the race time of such long duration events: the higher the percentage of time spent at maximal aerobic exercise intensity, the more the runners displayed high glucose values during the lap, apart from stops between laps. In the example of an athlete who has a 20% probability of spending time above 180 mg/dL during a lap, if this athlete increases their time spent at maximal aerobic exercise intensity during the lap by 30%, they will then have a 34% (compared to 20%) probability of spending some time in hyperglycaemia. It can be presumed that higher exercise intensity was associated with activation of the sympathetic nervous system and increased epinephrine levels, resulting in greater hepatic glucose production [23, 37]. Unfortunately, we were unable to measure catecholamines during the race. However, we showed that circulating insulin tended to decrease during the race, which could reflect the inhibition of pancreatic insulin production by sympathetic nervous system activation [23]. It is worth noting that the increase in insulin concentrations at the end of exercise and at the start of recovery observed in our study has already been demonstrated after shorter, more intense aerobic exercise and is known to be only transient [23]. This insulin ‘rebound’ could explain why the hyperglycaemia phenomenon was transiently delayed during the first 2 h of recovery, while during the subsequent period of recovery (up to 48 h), it reappeared and was even more pronounced than during the race. Cortisol may also contribute to higher glucose values during the race [22, 38]. However, this cannot explain recovery hyperglycaemia because it decreased very sharply during recovery, with kinetics different from those of glucose, and reaching even lower values than at rest after 24 h of recovery. It should also be noted that the cortisol profile was unique, with an early decrease from the end of lap 5 in 41.5% of finishers (data not shown). Cortisol is related to circadian rhythm, but its analysis in relation to circadian rhythm is difficult, with highly contradictory results among studies involving sleep deprivation [20, 39].
As for free fatty acids, they logically increased throughout the race concomitantly with the decrease in insulin and the increase in cortisol, which are anti- and pro-lipolytic [23, 38, 40]. While the increase in free fatty acids availability might be a contributing factor to skeletal muscle insulin resistance [41] during the race, free fatty acid concentrations decreased to baseline levels after 24 h of recovery, thus discarding the hypothesis of their involvement in post-race hyperglycaemia.
On the contrary, circulating CPK and LDH (which reflect skeletal muscle enzymes leakage), after a sharp increase throughout the race, appeared to remain very high after 24 h of recovery. This makes muscle damage a prime candidate for post-exercise insulin resistance in such extreme sporting events, possibly through the inflammation associated with the acute-phase immunological response to muscle-cell disruption [19, 42,43,44,45,46].
Lastly, we cannot exclude the possibility that diet also influenced post-race glycaemic metrics, all the more since current nutritional guidelines recommend athletes ingest carbohydrates as soon as possible after competition [47]. It should also be remembered that ultra-endurance events may deregulate nutrient absorption and therefore endocrine responses [48], which makes it even more difficult to understand post-exercise diet and glycaemia interactions.
4.2 Clinical Implications
Physical (i.e. the time taken to complete a lap) or cognitive (more specifically behavioural alertness) performance did not appear to be influenced by low or high glucose values during the race in our study. However, the major hyperglycaemic risk observed during the 2 days of recovery could have significant long-term implications for athletes’ vascular health. Acute hyperglycaemia (i.e. 200 mg/dL), implemented for just 4 h, has indeed been shown to impair endothelial function in non-diabetic individuals [13]. This could hypothetically prove particularly critical in the long-term, in cases of repeated ultra-endurance race participation, when we consider the risk of endothelial dysfunction [14], of arterial stiffness [49], and of coronary artery calcification evidenced in lifelong athletes [15,16,17,18], especially in those participating in long-distance races [49, 50]. Of note, prevalence of coronary artery calcification is known to be increased in non-diabetic individuals with higher glycated haemoglobin, as a reflection of chronic hyperglycaemia [51]. Moreover, the possible aggravating role of hyperglycaemia could also be assumed from the study of Muller et al. [52] showing that a higher initial fasting glycaemia was associated with a greater increase in carotid intima media thickness during a follow-up period of 3.8 years in runners participating in long-distance competitions (including half marathons, marathons, and ultra-marathons). Longitudinal follow-up studies in ultra-endurance athletes will be needed to explore whether repeated post-exercise hyperglycaemia actually increases the risk of subclinical vascular dysfunctions and whether the latter can be outweighed by their higher aerobic fitness. In the general population, higher cardiorespiratory fitness indeed protects against cardiovascular disease events at all coronary artery calcification levels [53].
4.3 Limitations
Some limitations of the present study need to be mentioned. The stops for scientific measurements were made compulsory for the runners, while during single-stage ultra-trail races, the athletes can choose to either stop or not at the refreshment zones. It should be noted that, during the inter-lap stops for scientific measurements, the order in which the tests were run was sometimes changed if too many runners arrived simultaneously for the same test. The refuelling visit to the canteen was always the first stage in the process, but the blood sampling, which was initially planned just after the canteen, was sometimes postponed to a later point during the stop. For better standardisation, further studies should give special attention to the timing of blood collection compared to refuelling and exercising periods.
In addition, it was unfortunately impossible to interpret diet data, which highlights the complexity of recording food intake during such field events. The quality of responses to open-ended questions (e.g. about the quality and quantity of food) being very poor, in further studies it would be advisable to prefer ‘yes/no’ questions associated with time-stamped meal/snack photos. It is possible to store the latter in the device, but this would involve a rigorous and painstaking procedure. For example, to avoid confusion between participants, a label identifying each participant could be placed on the meal tray before taking the photo. Otherwise, photos could be sent to an online data collection application, provided that the internet connection is sufficient. In the current study, glycaemic spikes during the ultra-trail race were particularly evident only during the post-lap refreshments, and not specifically during exercise. Interpretations of these spikes would definitely require correct data collection about the nature, quantity, and timing of food intake [54, 55]. In addition, having access to food intake data may also help to understand metabolic and hormonal changes throughout ultra-endurance races. In the current study, circulating insulin dropped during the last lap in parallel to a concomitant sharp increase in free fatty acids and ß-hydroxybutyrate. It could be hypothesised that athletes ate less carbohydrates at the last refreshment point, possibly due to simple carbohydrates aversion [56] or because they knew that the end of the race was near. This would trigger a further decrease in insulin secretion, which would stimulate lipolysis and ß-hydroxybutyrate formation from fatty acids. Further studies should also investigate whether post-race nutritional strategies [57, 58] could influence recovery hyperglycaemia.
Lastly, the effect of sleep deprivation was indirectly estimated using the nap time, which is the time when runners were lying down. However, we do not have information on the exact time spent asleep during nap periods.
5 Conclusion
While no major abnormal glycaemic excursions occurred during the ultra-endurance race, a significant hyperglycaemic risk was particularly present during recovery, up to 48 h after the race, possibly related to muscle damage. Further studies should explore changes in pro- and anti-inflammatory cytokines as intermediates between muscle damage and insulin resistance. Ultimately, developing strategies to mitigate muscle damage (e.g. passive stretching performed 2 weeks before) or for limiting inflammation resulting from muscle damage (e.g. curcuma supplements, massage, cryotherapy) [59,60,61,62,63] before or after ultra-endurance races might be crucial not only for performance but also for vascular health.
References
Zaryski C, Smith DJ. Training principles and issues for ultra-endurance athletes. Curr Sports Med Rep. 2005;4(3):165–70.
Hoppel F, Calabria E, Pesta D, Kantner-Rumplmair W, Gnaiger E, Burtscher M. Physiological and pathophysiological responses to ultramarathon running in non-elite runners. Front Physiol. 2019;10:1300.
Ramos-Campo DJ, Avila-Gandia V, Alacid F, Soto-Mendez F, Alcaraz PE, Lopez-Roman FJ, et al. Muscle damage, physiological changes, and energy balance in ultra-endurance mountain-event athletes. Appl Physiol Nutr Metab. 2016;41(8):872–8.
Waskiewicz Z, Klapcinska B, Sadowska-Krepa E, Czuba M, Kempa K, Kimsa E, et al. Acute metabolic responses to a 24-h ultra-marathon race in male amateur runners. Eur J Appl Physiol. 2012;112(5):1679–88.
Millet GY, Tomazin K, Verges S, Vincent C, Bonnefoy R, Boisson RC, et al. Neuromuscular consequences of an extreme mountain ultra-marathon. PLoS One. 2011;6(2): e17059.
Vassalle C, Piaggi P, Weltman N, Prontera C, Garbella E, Menicucci D, et al. Innovative approach to interpret the variability of biomarkers after ultra-endurance exercise: the multifactorial analysis. Biomark Med. 2014;8(6):881–91.
DuBose SN, Li Z, Sherr JL, Beck RW, Tamborlane WV, Shah VN. Effect of exercise and meals on continuous glucose monitor data in healthy individuals without diabetes. J Diabetes Sci Technol. 2021;15(3):593–9.
Ishihara K, Uchiyama N, Kizaki S, Mori E, Nonaka T, Oneda H. Application of continuous glucose monitoring for assessment of individual carbohydrate requirement during ultramarathon race. Nutrients. 2020;12(4):1121.
Kinrade EJ, Galloway SDR. Dietary observations of ultra-endurance runners in preparation for and during a continuous 24-h event. Front Physiol. 2021;12: 765888.
Yardley JE, Colberg SR. Update on management of type 1 diabetes and type 2 diabetes in athletes. Curr Sports Med Rep. 2017;16(1):38–44.
Evans ML, Pernet A, Lomas J, Jones J, Amiel SA. Delay in onset of awareness of acute hypoglycemia and of restoration of cognitive performance during recovery. Diabetes Care. 2000;23(7):893–7.
Heller S, Houwing N, Kragh N, Ploug UJ, Nikolajsen A, Alleman CJ. Investigating the evidence of the real-life impact of acute hyperglycaemia. Diabetes Ther. 2015;6(3):389–93.
Joy NG, Perkins JM, Mikeladze M, Younk L, Tate DB, Davis SN. Comparative effects of acute hypoglycemia and hyperglycemia on pro-atherothrombotic biomarkers and endothelial function in non-diabetic humans. J Diabetes Complications. 2016;30(7):1275–81.
Goel R, Majeed F, Vogel R, Corretti MC, Weir M, Mangano C, et al. Exercise-induced hypertension, endothelial dysfunction, and coronary artery disease in a marathon runner. Am J Cardiol. 2007;99(5):743–4.
De Bosscher R, Dausin C, Claus P, Bogaert J, Dymarkowski S, Goetschalckx K, et al. Lifelong endurance exercise and its relation with coronary atherosclerosis. Eur Heart J. 2023;44(26):2388–99.
Dores H, de Araujo GP, Monge J, Costa R, Tata L, Malhotra A, et al. Subclinical coronary artery disease in veteran athletes: is a new preparticipation methodology required? Br J Sports Med. 2020;54(6):349–53.
Lechner K, Spanier B, Lechner B, Scherr J. Your athlete-patient has a high coronary artery calcification score-’Heart of Stone’. What should you advise? Is exercise safe? Br J Sports Med. 2021;55(18):1001–2.
Scheer V, Tiller NB, Doutreleau S, Khodaee M, Knechtle B, Pasternak A, et al. Potential long-term health problems associated with ultra-endurance running: a narrative review. Sports Med. 2022;52(4):725–40.
Kirwan JP, Hickner RC, Yarasheski KE, Kohrt WM, Wiethop BV, Holloszy JO. Eccentric exercise induces transient insulin resistance in healthy individuals. J Appl Physiol (1985). 1992;72(6):2197–202.
Gonzalez-Ortiz M, Martinez-Abundis E, Balcazar-Munoz BR, Pascoe-Gonzalez S. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr Metab. 2000;13(2):80–3.
Baur DA, Bach CW, Hyder WJ, Ormsbee MJ. Fluid retention, muscle damage, and altered body composition at the Ultraman triathlon. Eur J Appl Physiol. 2016;116(3):447–58.
Bratusch-Marrain PR. Insulin-counteracting hormones: their impact on glucose metabolism. Diabetologia. 1983;24(2):74–9.
Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl 1):S271–83.
Rand JS, Kinnaird E, Baglioni A, Blackshaw J, Priest J. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med. 2002;16(2):123–32.
Mauvieux B, Hingrand C, Drigny J, Hodzic A, Baron P, Hurdiel R, et al. Study of the kinetics of the determinants of performance during a mountain ultramarathon: multidisciplinary protocol of the first trail Scientifique de Clecy 2021. JMIR Res Protoc. 2022;11(6): e38027.
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603.
Battelino T, Alexander CM, Amiel SA, Arreaza-Rubin G, Beck RW, Bergenstal RM, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2023;11(1):42–57.
Shah VN, DuBose SN, Li Z, Beck RW, Peters AL, Weinstock RS, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356–64.
Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–6.
Canadian Association for Global Health C. Mesure de l’intensité de l’activité physique. 2012.
Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31(3):301–6.
Wilkinson RT, Houghton D. Field test of arousal: a portable reaction timer with data storage. Hum Factors. 1982;24(4):487–93.
Hurdiel R, Peze T, Daugherty J, Girard J, Poussel M, Poletti L, et al. Combined effects of sleep deprivation and strenuous exercise on cognitive performances during The North Face(R) Ultra Trail du Mont Blanc(R) (UTMB(R)). J Sports Sci. 2015;33(7):670–4.
Miley AA, Kecklund G, Akerstedt T. Comparing two versions of the Karolinska Sleepiness Scale (KSS). Sleep Biol Rhythms. 2016;14(3):257–60.
Hurdiel RRS, Millet G, Mauvieux B, Pezé T, Elsworth-Edelsten C, Martin D, Zunquin G, Dupont G. Cognitive performance and self-reported sleepiness are modulated by time-of-day during a mountain ultramarathon. Res Sports Med. 2018;26(4):1–8.
Kaida K, Takahashi M, Akerstedt T, Nakata A, Otsuka Y, Haratani T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117(7):1574–81.
Zinker BA, Mohr T, Kelly P, Namdaran K, Bracy DP, Wasserman DH. Exercise-induced fall in insulin: mechanism of action at the liver and effects on muscle glucose metabolism. Am J Physiol. 1994;266(5 Pt 1):E683–9.
De Feo P, Perriello G, Torlone E, Ventura MM, Fanelli C, Santeusanio F, et al. Contribution of cortisol to glucose counterregulation in humans. Am J Physiol. 1989;257(1 Pt 1):E35-42.
Klumpers UM, Veltman DJ, van Tol MJ, Kloet RW, Boellaard R, Lammertsma AA, et al. Neurophysiological effects of sleep deprivation in healthy adults, a pilot study. PLoS One. 2015;10(1): e0116906.
Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38(12):1595–601.
Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–65.
Kirwan JP, del Aguila LF. Insulin signalling, exercise and cellular integrity. Biochem Soc Trans. 2003;31(Pt 6):1281–5.
Del Aguila LF, Krishnan RK, Ulbrecht JS, Farrell PA, Correll PH, Lang CH, et al. Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279(1):E206–12.
Aoi W, Naito Y, Yoshikawa T. Role of oxidative stress in impaired insulin signaling associated with exercise-induced muscle damage. Free Radic Biol Med. 2013;65:1265–72.
Nieman DC, Dumke CL, Henson DA, McAnulty SR, Gross SJ, Lind RH. Muscle damage is linked to cytokine changes following a 160-km race. Brain Behav Immun. 2005;19(5):398–403.
Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34(4):246–51.
Podlogar T, Wallis GA. New horizons in carbohydrate research and application for endurance athletes. Sports Med. 2022;52(Suppl 1):5–23.
Smith KA, Pugh JN, Duca FA, Close GL, Ormsbee MJ. Gastrointestinal pathophysiology during endurance exercise: endocrine, microbiome, and nutritional influences. Eur J Appl Physiol. 2021;121(10):2657–74.
Burr JF, Drury CT, Phillips AA, Ivey A, Ku J, Warburton DE. Long-term ultra-marathon running and arterial compliance. J Sci Med Sport. 2014;17(3):322–5.
Jafar O, Friedman J, Bogdanowicz I, Muneer A, Thompson PD, Ling J, et al. Assessment of coronary atherosclerosis using calcium scores in short- and long-distance runners. Mayo Clin Proc Innov Qual Outcomes. 2019;3(2):116–21.
Jung CH, Rhee EJ, Kim KJ, Kim BY, Park SE, Chang Y, et al. Relationship of glycated hemoglobin A1c, coronary artery calcification and insulin resistance in males without diabetes. Arch Med Res. 2015;46(1):71–7.
Muller J, Dahm V, Lorenz ES, Pressler A, Haller B, Grabs V, et al. Changes of intima-media thickness in marathon runners: a mid-term follow-up. Eur J Prev Cardiol. 2017;24(12):1336–42.
Radford NB, DeFina LF, Leonard D, Barlow CE, Willis BL, Gibbons LW, et al. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men: results from the cooper center longitudinal study. Circulation. 2018;137(18):1888–95.
Jeukendrup A. A step towards personalized sports nutrition: carbohydrate intake during exercise. Sports Med. 2014;44(Suppl 1):S25–33.
Ranchordas MK. Nutrition for adventure racing. Sports Med. 2012;42(11):915–27.
Tiller NB, Roberts JD, Beasley L, Chapman S, Pinto JM, Smith L, et al. International Society of Sports Nutrition Position Stand: nutritional considerations for single-stage ultra-marathon training and racing. J Int Soc Sports Nutr. 2019;16(1):50.
Kerksick CR. M. Supplements for Endurance Athletes. Strength Condition J. 2010;32:55–64.
Neubauer O, Reichhold S, Nics L, Hoelzl C, Valentini J, Stadlmayr B, et al. Antioxidant responses to an acute ultra-endurance exercise: impact on DNA stability and indications for an increased need for nutritive antioxidants in the early recovery phase. Br J Nutr. 2010;104(8):1129–38.
Tanabe Y, Fujii N, Suzuki K. Dietary supplementation for attenuating exercise-induced muscle damage and delayed-onset muscle soreness in humans. Nutrients. 2021;14(1):70.
Connolly DA, McHugh MP, Padilla-Zakour OI, Carlson L, Sayers SP. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br J Sports Med. 2006;40(8):679–83 (discussion 83).
Berger NJA, Best R, Best AW, Lane AM, Millet GY, Barwood M, et al. Limits of ultra: towards an interdisciplinary understanding of ultra-endurance running performance. Sports Med. 2023;54(1):73–93.
Bowtell J, Kelly V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med. 2019;49(Suppl 1):3–23.
Howatson G, von Someren KA. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2012;38(6):483–503.
Acknowledgements
The authors would like to thank the participants in this study. We thank J. Naturel and J. Lutun (Lille University) and V. Deprez and M-A Allain (Lille University Hospital) for their administrative support; J. Dereumetz (Lille University) for her help with data analyses; A. Bertrand (Statistical Methodology and Computing Service, UCLouvain, Belgium) for support with statistical analyses; and S. Platt (International Eyes SARL) for revising the English. We also thank A. Oliveira Alves and M. Osmont (Paris Cité University) for blood sampling and P. Pigny, F. Zerimech, and J. Demaret (Lille University Hospital) for blood analyses. Glycaemic analyses were carried out at the EURASPORT facility managed by S. Berthoin (Lille University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This protocol was partly financed by the RIN TREMPLIN APEEX (Adaptation Physiologiques En Environnements Extrêmes – 2020/2022) supported by the Normandy Region – France and the European Regional Development Fund (ERDF). This study was undertaken as part of the project run by the International Joint Laboratory REGALE-1 (Glycemic Regulation During Exercise in Type 1 Diabetes), bringing together the URePSSS (Lille University) and IRCM (Montréal) and involving an international ‘SPORT-1’ Chair for R. Rabasa-Lhoret (I-SITE ULNE, WILL grant). This study was supported in part by grants from the Type 1 Running Team Association. EH received a grant from the Institut Universitaire de France, and CP received grant assistance as a doctoral student from the Hauts-de-France Regional Authority.
Competing Interests
The authors declare no competing interests.
Ethics Approval
Approval institution, Ouest III Ethics Committee; ethics number, 2021-A01765-36; date of approval, 26 October 2021. Trial registration number, NCT05538442 2022-09-21, retrospectively registered. The study was performed in accordance with the ethical standards in the Declaration of Helsinki.
Consent to Participate
All participants were fully informed of the nature and possible risks of the experimental procedures before their written informed consent was obtained.
Consent to Publication
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
All authors read and approved the final version. EH and BM designed the experiments. FXG and RRL gave advice on some methodological points. CP, EL, EH, JCV, RH, PLD, SB, PN, VG, PB, QM, and RJ carried out the experiments and collected the data. PM performed blood analyses. EH and CP analysed the data. JH created algorithms for analyses of glycaemic excursions and variability. BM and CH recruited the participants, organized the protocol and the race, and submitted the protocol to the ethics committee. EH and CP wrote the manuscript. All authors were involved in reviewing the manuscript. EH has guaranteed the integrity of the study throughout its duration and, as such, had full access to data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Data Availability Statements
All data related to this article are available on http://www.data.gouv.fr/fr/ platform (https://doi.org/10.57745/EO2SBC).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parent, C., Mauvieux, B., Lespagnol, E. et al. Glycaemic Effects of a 156-km Ultra-trail Race in Athletes: An Observational Field Study. Sports Med 54, 2169–2184 (2024). https://doi.org/10.1007/s40279-024-02013-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-024-02013-4