Abstract
Background
Pain is the most disabling characteristic of musculoskeletal disorders, and while exercise is promoted as an important treatment modality for chronic musculoskeletal conditions, the relative contribution of the specific effects of exercise training, placebo effects and non-specific effects such as natural history are not clear. The aim of this systematic review and meta-analysis was to determine the relative contribution of these factors to better understand the true effect of exercise training for reducing pain in chronic primary musculoskeletal pain conditions.
Design
Systematic review with meta-analysis
Data Sources
MEDLINE, CINAHL, SPORTDiscus, EMBASE and CENTRAL from inception to February 2021. Reference lists of prior systematic reviews.
Eligibility Criteria
Randomised controlled trials of interventions that used exercise training compared to placebo, true control or usual care in adults with chronic primary musculoskeletal pain. The review was registered prospectively with PROSPERO (CRD42019141096).
Results
We identified 79 eligible trials for quantitative analysis. Pairwise meta-analysis showed very low-quality evidence (GRADE criteria) that exercise training was not more effective than placebo (g [95% CI]: 0.94 [− 0.17, 2.06], P = 0.098, I2 = 92.46%, studies: n = 4). Exercise training was more effective than true, no intervention controls (g [95% CI]: 0.99 [0.66, 1.32], P < 0.001, I2 = 92.43%, studies: n = 42), usual care controls (g [95% CI]: 0.64 [0.44, 0.83], P < 0.001, I2 = 76.52%, studies: n = 33), and when all controls combined (g [95% CI]: 0.84 [0.64, 1.04], P < 0.001, I2 = 90.02%, studies: n = 79).
Conclusions
There is very low-quality evidence that exercise training is not more effective than non-exercise placebo treatments in chronic pain. Exercise training and the associated clinical encounter are more effective than true control or standard medical care for reductions in pain for adults with chronic musculoskeletal pain, with very low quality of evidence based on GRADE criteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Exercise training does not appear to be more effective than placebo interventions for reducing pain intensity when the placebo intervention consisted of sham electrotherapeutic interventions or oral supplementation (omega-3 plus calcium) in individuals with chronic pain. |
There are currently no published randomised controlled trials that have utilised an exercise-based placebo intervention in people with chronic primary musculoskeletal pain conditions. This is important because the relative effect of exercise training, contextual factors and natural history remains unknown. |
When considered together, exercise training and the associated clinical encounter was more effective than no treatment and standard medical care for reducing pain intensity in people with chronic primary musculoskeletal pain. |
1 Background
Chronic musculoskeletal pain affects approximately 20% of the global population [1], impacting work capacity and employment [2], quality of life [3], and mental and physical health [4, 5], and may cause social disadvantage [6]. Pain is the most disabling characteristic of musculoskeletal disorders [7], and is defined as a distressing experience associated with actual or potential tissue damage with sensory, emotional, cognitive and social components [8]. Chronic (persistent) pain is further defined as constant or recurrent pain for a period of time greater than 3 months [9]. Exercise training is a fundamental modality in various position stands and clinical guidelines to manage musculoskeletal pain and disability [10]; however, one study estimated that half (55.2%) of the effect of exercise on pain is related to the exercise prescription variables [11]. The specific mechanisms explaining the effect of exercise training on long-term reductions in pain is not clear [12].
The multidimensional nature of the chronic pain experience [8] may be influenced by factors other than an exercise training stimulus, such as the placebo effect. Previous evidence shows that the overall effect of exercise training on perception, mental health, cognition, and sports performance can be partly explained by placebo effects [13,14,15]. Placebo effects are elicited broadly via two main mechanisms: learning mechanisms (i.e., classical conditioning) and cognitive expectations [16]. In clinical practice, this is referred to as contextual factors that are associated with the experiences of the patient during the clinical encounter, shaped by positive context linked to the clinician’s characteristics (professional reputation, appearance, beliefs, behaviours); patient characteristics (expectations of treatment, beliefs, treatment preference, previous experience, type of injury, sex, age); treatment (diagnostic clarity, diagnostic method, intensity of therapy, observational learning, patient-centred approach, therapeutic touch); and the healthcare setting (environment, architecture, interior design) [17,18,19]. Positive contextual factors as part of a therapeutic encounter in clinical practice can reduce pain intensity [20]. The therapeutic encounter, and in particular the contextual factors surrounding the clinical encounter, are therefore important considerations in clinical practice. In particular, these factors seem to be extremely important during manual therapy where physiotherapists use context to boost placebo and simultaneously reduce nocebo effects [21]. Indeed, two recent Italian surveys showed that half of the physiotherapists working on musculoskeletal disorders are fully aware of frequently using “contextual factors” in their therapies [22], and more than half of the interviewed patients affected by musculoskeletal pain describe contextual factors as a treatment without a specific effect while still believing in its clinical effectiveness [23].
Previous meta-analyses [24, 25] have shown that the specific effect of treatment is modest when compared to the placebo response (i.e., combined relative effects of placebo effects and other non-specific effects such as Hawthorne effect [26] regression to the mean, natural history and spontaneous improvement [27]). One recent meta-analysis showed the placebo response represented approximately 75% of the overall effect on pain intensity reduction for a range of interventions (i.e., electrotherapeutic, pharmacological, non-pharmacological and surgical treatments) in patients with osteoarthritis [28]. Most notably, no exercise-based interventions were included in this review because of the perceived lack of randomised placebo-controlled exercise trials. The authors speculated that this was likely due to the difficulty in designing an adequate exercise placebo intervention. While this perspective is likely accurate given a subsequent meta-analysis [25] in osteoarthritis (knee) identified just one exercise-based placebo controlled study, it brings into question the scientific rigor surrounding the broad support of exercise training in many position statements and its use in the management of musculoskeletal pain conditions.
It is well accepted that psychological and social factors can moderate the pain experience for patients with chronic musculoskeletal pain [29]. To our knowledge, there are no meta-analyses to date that report the relative effect size of the placebo effect for exercise-based interventions in chronic musculoskeletal pain disorders. Therefore, the aim of this meta-analysis was to determine the relative contribution of the placebo effect, non-specific effects (i.e., natural history, regression to the mean, spontaneous improvement and the Hawthorne effect [26]), and exercise training for reducing pain in adults with chronic musculoskeletal pain. This is important for clinical practice to inform the relative contribution of exercise training and the placebo effects for enhancing patient outcomes [30, 31].
2 Methods
This systematic review and meta-analysis was completed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [32]. The review was registered prospectively with PROSPERO (CRD42019141096).
2.1 Search Strategy
Five online databases (MEDLINE, CINAHL, SPORTDiscus, EMBASE and CENTRAL) were electronically searched for research published from database inception to February 2021. The search-term strategy can be found in Supplementary Table S1 (Online Supplementary Material, OSM). The search strategy was developed based on the basis of current guidelines for the design of systematic reviews, our prior experience with systematic reviews, and input from content experts. The search had the following limits: MEDLINE (all adult: 19 + years; human), CINAHL (exclude MEDLINE records; human, randomised controlled trials; journal article; all adult), SPORTDiscus (Academic Journal), EMBASE (RCT; not MEDLINE; adult; article) and CENTRAL (trials). To locate additional references, we searched for previously published systematic reviews identified via the Cochrane Database of Systematic Reviews (search terms: placebo exercise; limits: none) and GoogleScholar (search terms: ‘systematic review’ placebo exercise; limits: previous 10 years). There were no additional restrictions for language or year of publication. All results of the search were screened by PJO to exclude duplicates. Independent screening of the titles and abstracts of the remaining studies was completed by JB, CTM, PJO and CAT against the predetermined inclusion and exclusion criteria. The full-text articles were independently assessed against the inclusion and exclusion criteria by four reviewers (CTM, PJO, JB and CAT). Any disagreements were adjudicated by CTM and discussed with co-authors as necessary. Excluded papers at the full-text stage were reassessed against the inclusion and exclusion criteria by KS.
2.2 Inclusion and Exclusion Criteria
To be included, studies were required to be published in a peer-reviewed journal (i.e., grey literature excluded) and be a randomised controlled trial that compared an exercise training intervention to either a non-intervention control group or to a placebo group. All other inclusion criteria followed the Participants, Interventions, Comparators, Outcomes and Study design (PICOS) framework [33].
2.2.1 Participants
Study participants were required to be adults (≥ 18 years) with any chronic primary musculoskeletal pain. Chronic pain was defined as pain duration at baseline ≥ 3 months [34]. There were no restrictions on sex or race. The exclusion criteria consisted of conditions that are not primary musculoskeletal pain in nature or are a result of structural compromise such as fracture, severe scoliosis, osteoporosis and bone pain secondary to metastases. Primary inflammatory conditions such as gout, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis, juvenile arthritis or secondary pain complaints associated with visceral pain presenting as musculoskeletal pain such as Crohn’s disease and other gastrointestinal disorders, angina, vascular insufficiency, asthma or other breathing-related conditions were also excluded.
2.2.2 Interventions
Interventions consisting of exercise training alone, without the addition of any other treatments (e.g., massage, ultrasound or hot and cold therapy, or education) were included.
2.2.3 Comparators
Comparator control groups consisted of: true control (wait-list control or no treatment control), usual care (standard medical care excluding physical therapies, education, psychotherapies or surgery), or placebo control. A placebo control intervention was defined as any intervention defined as a placebo or sham intervention by the study authors [35].
2.2.4 Outcomes
Any general or disease-specific measure of pain such as a visual analogue or numeric pain scale for pain intensity or Short Form (SF)-36 bodily pain subscale was included.
2.2.5 Study Design
Studies were included if the design was a parallel-arm (individual- or cluster-designed) randomised controlled trial.
2.3 Data Extraction
After duplicate removal (PJO), data screening was completed using Covidence (https://www.covidence.org/). Data extraction was completed in duplicate by four independent assessors (PJO, CTM, CAT and JB). Extracted information included relevant publication information (i.e., author, title, year, journal), study design, number of participants, participant characteristics (e.g., age and sex), intervention details (e.g., duration, type) and outcome measure (pain). Extracted outcome data were pre- and post-intervention mean and standard deviation (SD) for pain intensity. Data presented as median (interquartile range) or alternate measures of spread/variance were converted to mean and SD using established formulae. Where post-intervention SD was unavailable, pre-intervention SD was utilised based on Cochrane guidelines [36, 37]. In all instances where studies were potentially included, yet where data required for meta-analysis were not available, authors were contacted a minimum of three times over a 4-week period to request the information and final decision for inclusion. Similarity between extracted data from the independent assessors was evaluated through Covidence. Any discrepancies were reviewed by KS and CTM against the original paper. This method was piloted on the first ten studies chosen at random prior to commencing data extraction.
2.4 Risk of Bias Assessment and Quality Assessment
The Cochrane Collaboration Risk of Bias Tool was used to examine potential selection bias (random sequence generation and allocation concealment), performance bias (blinding of patients and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting) and other bias [36]. This assessment was completed independently by CAT and JB. Studies were classified as having a low, high or unclear (when reporting was not adequate to rate a specific domain) risk for each type of bias. Any disagreements on the risk of bias were adjudicated by CTM. In addition, to assess the quality of the evidence from the meta-analysis, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used [38].
2.5 Statistical Analysis
Pairwise random-effects meta-analysis was conducted in Stata 16.0 (Stata Corp, College Station, TX, USA). As all outcomes of interest were continuous, yet possibly subject to small sample bias, Hedges’ g, rather than Cohen’s d, was used as the standardised mean difference effect estimate [39]. In line with Cochrane guidelines, individual study groups were pooled when a study investigated multiple groups defined as exercise training to avoid overlapping samples [40]. The main analysis investigated pooled exercise training versus placebo comparators on pain intensity. Secondary (sub-group) analyses were performed for: (1) exercise training versus any comparator (i.e., placebo, true or usual-care control), (2) exercise training versus true control, and (3) exercise training versus usual-care control. Heterogeneity was assessed for all pairwise comparisons via the I2 statistic [40] and publication bias via visual inspection of funnel plots [see Figs. S1–S4 (OSM)] in addition to calculating the P-value of Egger’s test. Sensitivity analyses included: excluding each individual study from the main analysis [pooled exercise training vs. placebo comparators on pain intensity, Fig. S5 (OSM)] and omitting Mengshoel et al. [41] [Fig. S6 (OSM)] due to conversion of median (minimum, maximum) to mean (SD) in all applicable analyses. An alpha level of 0.05 was taken for statistical significance. The Stata code and data are included in Table S2 (OSM).
3 Results
3.1 Study Selection
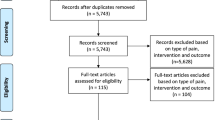
A summary of the systematic review process is presented in Fig. 1 according to the PRISMA guidelines. There were 5,263 studies screened against the inclusion and exclusion criteria for title and abstract after removal of duplicates. A total of 426 studies were included in the full-text screening with another 345 subsequently being assessed as ineligible for inclusion [Table S3 (OSM): Detailed reasons for exclusion at full-text stage]. Three studies were identified as potentially eligible, concerning which authors were contacted; however, all were ineligible due to essential pain data being unavailable from the authors [42, 43]. The authors of one study [44] provided raw pain data for analysis and was eligible for inclusion. A total of 79 studies were eligible for meta-analysis. Data were presented as median, minimum, and maximum in one study [41], 95% confidence intervals (CIs) in four studies [45,46,47,48], and standard error of the mean in four studies [49,50,51,52], and were converted to mean (SD).
3.2 Study Characteristics
The details of each included study (n = 79; participants: n = 4843) [41, 44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122] are shown in Table 1. The sample size of the individual groups within the included studies ranged from seven to 125 participants and mean age ranged from 20 to 76 years. The length of the intervention ranged from 2 to 104 weeks. Of the included studies, 23 investigated adults with fibromyalgia [41, 44, 46, 47, 52,53,54,55,56,57,58,59,60,61,62,63,64,65, 111, 117, 119,120,121]; 18 with knee or hip osteoarthritis [45, 49, 50, 66,67,68,69,70,71,72,73,74,75,76,77,78, 114, 115]; three with chronic patellofemoral pain [79,80,81]; one with chronic hip pain in young adults [82]; one with older adults with chronic lower extremity or low back pain [83]; 23 with chronic low back pain [51, 84,85,86,87,88,89,90, 92,93,94,95,96,97,98,99,100,101, 110, 112, 113, 116, 118]; seven with chronic neck or shoulder pain [48, 102,103,104,105,106,107]; one study with chronic Achilles tendinopathy pain [108]; and one study with chronic osteoarthritis of the hand [109].
3.3 Risk of Bias Within Individual Studies
A summary of the risk of bias assessment is shown in Fig. 2, and for each study is shown in Tables S4–S6 (OSM). Overall, no studies were rated as being at low risk of bias, and all were rated as being at high risk of bias (Fig. 2). The primary reason for a high risk of bias was the lack of participant blinding (performance bias; 96.2% high risk) across the majority of studies. Additionally, participants were the outcome assessor for pain in all studies, (detection bias; 100% high risk), which was subjectively reported in the majority of cases via a numeric pain-rating scale or a visual analogue scale. Attrition bias (87.3%), selective reporting bias (88.9%), selection bias: sequence generation (68.4%) and selection bias: allocation concealment (60.8%) were predominately rated as low, with some studies rated as being unclear due to a lack of clear reporting.
Percentage of studies examining the effect of exercise training for reducing pain with low risk, some concerns (unclear) and high risk of bias for each aspect of the Cochrane Risk of Bias Tool (revised version). See Tables S3-S5 (Online Supplementary Material) for the assessment for each individual study. a exercise versus placebo; b exercise training versus all controls; c exercise training versus true control; d exercise training versus usual care
3.4 Quantitative Analysis
Four studies were eligible for quantitative analysis comparing the effect of exercise interventions to placebo intervention [60, 69, 80, 85]. There were insufficient placebo-controlled trials from which to perform a quantitative synthesis of the existing evidence to directly determine the effect of natural history, placebo and exercise. A pairwise meta-analysis comparing exercise training to placebo showed that exercise was not more effective for reducing pain when compared to a placebo intervention (g [95% CI]: 0.94 [− 0.17, 2.06], P = 0.098, I2 = 92.46%, studies: n = 4, participants: n = 253; Fig. 3, Table 2). There was no evidence of publication bias within the comparison (Egger’s P = 0.250; Table 2). The overall quality of evidence was rated as very low based on the GRADE criteria. Exploratory meta-regression for the primary outcome data using mean sample baseline pain intensity (studies: n = 4) showed this was unlikely to be a source of heterogeneity [Table S7 (OSM)].
Forest plot for the meta-analysis investigating the effectiveness of exercise training versus placebo comparators for reducing musculoskeletal pain. Horizontal lines represent standardised mean difference (Hedges’ g) and 95% confidence intervals. The size of the box represents the weight of each study. The diamond represents the overall estimated effect. INT intervention group, CON control group
When pooling all exercise interventions and comparing to all control comparator groups (true control, usual-care control, placebo control), exercise was more effective than control for reducing pain (g [95% CI]: 0.84 [0.64, 1.04], P < 0.001, I2 = 90.02%, studies: n = 79, participants: n = 4843; Fig. 4, Table 2). There was strong evidence of publication bias within the comparison (Egger’s P < 0.001; Table 2). The overall GRADE quality of evidence was considered very low. The authors of two studies [110, 114] reporting larger than anticipated effect sizes (Fig. 4) were contacted to confirm accuracy of reported data, but no response was received. Sensitivity analysis omitting Mengshoel et al. [41] due to conversion of median (minimum, maximum) to mean (SD) did not change the results [Fig. S6 (OSM)].
Forest plot for the meta-analysis investigating the effectiveness of exercise training versus all control comparators for reducing musculoskeletal pain. Horizontal lines represent standardised mean difference (Hedges’ g) and 95% confidence intervals. The size of the box represents the weight of each study. The diamond represents the overall estimated effect. INT intervention group, CON control group
Sub-group analysis for the effect of exercise interventions compared to true control (do-nothing control, wait-list control) comparators showed that exercise was more effective than true control for reductions in musculoskeletal pain (g [95% CI]: 0.99 [0.66, 1.32], P < 0.001, I2 = 92.43%, studies: n = 42, participants: n = 2361; Fig. 5, Table 2), however, there was strong evidence of publication bias within the comparison (Egger’s P < 0.001; Table 2). The overall GRADE quality of evidence was considered very low. Sensitivity analysis omitting Mengshoel et al. [41] due to conversion of median (minimum, maximum) to mean (SD) did not change the results [Fig. S6 (OSM)].
Forest plot for the meta-analysis investigating the effectiveness of exercise training versus true control comparators for reducing musculoskeletal pain. Horizontal lines represent standardised mean difference (Hedges’ g) and 95% confidence intervals. The size of the box represents the weight of each study. The diamond represents the overall estimated effect. INT intervention group, CON control group
When comparing the effect of exercise against usual-care control comparator (general practitioner standard care but not physical therapies) groups, exercise was more effective than usual care for reducing musculoskeletal pain (g [95% CI]: 0.64 [0.44, 0.83], P < 0.001, I2 = 76.52%, studies: n = 33, participants: n = 2229; Fig. 6, Table 2), and there was strong evidence of publication bias within the comparison (Egger’s P < 0.001; Table 2).
Forest plot for the meta-analysis investigating the effectiveness of exercise training versus usual-care control comparators for reducing musculoskeletal pain. Horizontal lines represent standardised mean difference (Hedges’ g) and 95% confidence intervals. The size of the box represents the weight of each study. The diamond represents the overall estimated effect. INT intervention group, CON control group
3.5 Protocol Deviations Compared with PROSPERO Registration
We initially aimed to conduct a network meta-analysis if data permitted, yet the lack of placebo trials precluded such analyses. Among these few trials (n = 4) there was marked heterogeneity (I2 = 92.46%), which we investigated via meta-regression using mean total sample baseline pain intensity [Table S7 (OSM)]. Given these meta-regressions were post hoc and included less than ten trials, we contend results are purely exploratory in nature.
4 Discussion
The primary aim of this systematic review and meta-analysis was to investigate the relative effect of exercise training, placebo effects and non-specific effects on pain in chronic primary musculoskeletal pain conditions. Only four randomised controlled trials that compared an exercise intervention to placebo were identified: one each in osteoarthritis [69], chronic low back pain [85], chronic patellofemoral pain [80] and fibromyalgia [60]. None of these studies used a placebo exercise-training protocol. In light of the lack of exercise-based placebo-controlled trials, the determination of effect size for placebo effects relative to non-specific effects and exercise was not feasible. Pairwise meta-analysis comparing exercise interventions to non-exercise placebo control showed there was no statistically significant evidence for exercise training to be more effective than placebo for reductions in pain intensity; however, the quality of evidence was rated as very low against the GRADE criteria. There was strong evidence of an approximately large effect size for exercise training when compared to all controls (pooled); however, there was also strong evidence of publication bias and the quality of evidence was rated as very low when assessed against the GRADE criteria.
This systematic review highlights that exercise training does not appear to be more effective than placebo interventions when the placebo intervention consisted of sham electrotherapeutic interventions or oral dietary supplementation in individuals with chronic pain. Additionally, there was a lack of placebo-controlled clinical exercise training trials in chronic musculoskeletal pain conditions from which to determine the relative effects of exercise training, placebo effects and non-specific effects. Previous evidence showed that subjective outcomes, such as pain, are particularly influenced by placebo effects [123], and therefore it is critical to understand the relative contribution of exercise and the placebo effects associated with the clinical encounter. A recent meta-analysis evaluating the effect of various interventions on pain for individuals with osteoarthritis showed that the placebo response (placebo effects and non-specific effects such as natural history, regression to the mean, Hawthorne effect) contributed to 75% of the effect on pain [28]. Another meta-analysis showed that placebo interventions were more effective for reducing pain than no treatment for individuals with fibromyalgia [124]. The limitation with our meta-analysis and these previous meta-analyses is that no exercise-based placebo trials were identified in the published literature. This is important to recognise because the effect size of a placebo intervention on pain can be moderated by the type of placebo administered [125,126,127], and that matching (ensuring that the active treatment is indistinguishable from the placebo) is an important component of placebo study design [128]. Although a placebo response is likely involved in all interventions for pain as part of a clinical encounter [129], the magnitude of effect for placebo as part of an exercise-based intervention for individuals experiencing chronic musculoskeletal pain remains unknown. A better understanding of the direct effect of exercise and that of the placebo response is important as it will enable the direction of research either toward optimising exercise prescription variables or to enhance the contextual factors associated with the clinical encounter for individuals experiencing chronic pain. It is therefore critical that future randomised clinical trials consider including an exercise treatment arm, no-treatment control arm and placebo arm into the design to determine the true effect of exercise, placebo effects and non-specific effects for the management of chronic primary musculoskeletal pain. Due to the amount of funding, time, participants and personnel associated with conducting a three-arm clinical trial, it may be more pragmatic for researchers to consider a trial design that includes a treatment arm and placebo arm alone. These trials can be used to calculate and report the overall treatment response, the proportional contextual effect (inclusive of natural history and regression to the mean), and therefore the specific treatment effect [130]. The overall treatment effect (change in baseline in the active intervention) should be reported in addition to the proportional contextual effect by means of the improvement of the placebo group from baseline divided by the improvement in the active treatment group [130]. It must be emphasised that the design of the placebo intervention as well as the active intervention should be considered. As noted earlier, the type of placebo intervention selected should be indistinguishable from the active treatment. [128, 131] Importantly, the therapeutic ritual, environment, treatment provider and contact time should also be identical [131, 132], as far as practicable, and documented as part of a treatment procedural manual, often referred to as manualised treatments in psychotherapy literature [133]. These design elements are easily achievable for pharmacotherapy trials where the active or sham ingredient is encased within an indistinguishable pill, or the use of sham electrotherapeutic devices such as detuned ultrasound or shortwave diathermy where the visual and audible characteristics resemble the active devices. The design of an exercise-based placebo arm requires additional consideration due to the complexity of the treatment and necessary clinical interaction. A clear understanding of the hypothesised mechanisms of action for exercise on pain that is relative to the specific condition is important. For exercise training interventions, this may involve the manipulation of intensity or load, while maintaining the same exercise type, duration, frequency, method of progression and the surrounding contextual factors of the environment, therapeutic ritual, therapeutic alliance, clinician and language used [131].
Further analyses evaluated effectiveness trials comparing exercise interventions to usual care (i.e., standard medical care) and to no-treatment controls (i.e., true control). We found statistically strong evidence that exercise training was more effective than usual care and no treatment; however, the quality of evidence is very low according to the GRADE criteria. The exact nature and intensity of the usual-care treatment offered or permitted outside of study participation was not well reported in most studies, and therefore may confound observed effects. Most studies reported that standard care consisted of patient-initiated medical management consisting of pharmacotherapy and general education provided by healthcare professionals outside the study research group. Contextual factors as part of a therapeutic encounter in standard clinical practice [20] are consistent with placebo effects reported in clinical trials [126], and therefore a control arm consisting of standard care may provide some insight into the effect of the therapeutic encounter in the absence of a true placebo control. However, none of the studies included in this meta-analysis reported the specific nature and intensity of treatment provided to the usual-care control group and none attempted to standardise this form of treatment. There is inherent variation in treatment received for participants in such effectiveness trials, and this adds to external validity [134]; however, the use of a usual-care control group outside the confines of the study design prevents meaningful interpretation of the specific effects of exercise on pain in this context.
The findings from our meta-analysis are consistent with previous meta-analyses in fibromyalgia [135, 136], osteoarthritis [137, 138], chronic low back pain [139] and chronic neck pain [140], which all report that exercise provides greater reductions in self-reported pain when compared to usual care and true controls. Therefore, the results presented in our current systematic review and meta-analysis support that the experience and act of engaging in an exercise treatment intervention and its associated interactions appears to be more effective than not receiving any care or receiving standard medical care in individuals with chronic musculoskeletal pain. Our meta-analyses suggested a high level of heterogeneity between included studies (i.e., each meta-analysis was graded down via GRADE). We explored this heterogeneity in the primary meta-analysis (placebo) using mean total sample baseline pain intensity (i.e., scale 0–100 points; four studies). For pain intensity, the regression coefficient was 0.064 (95% CI: − 0.002, 0.131; P = 0.057) and R2 was 51.3%; hence, baseline pain intensity did not appear to moderate pain intensity, and approximately half of the between-study variance was explained by pain. The test statistic for residual homogeneity (Qres) was 14.91 (P < 0.001); thus, the null hypothesis of no residual heterogeneity was rejected. The I2 value suggested that approximately 86% of residual variation was due to heterogeneity that may be explained by other covariates. Overall, this means that baseline pain intensity is unlikely to be a source of heterogeneity.
The primary strength of this meta-analysis is that it was completed using wide search criteria for inclusion of studies specifically noting chronicity of primary musculoskeletal pain. This systematic review and meta-analysis has highlighted major limitations in the exercise training literature with regard to adequately reporting study methods and a lack of rigorous study designs that control for placebo effects or include post hoc secondary analysis that considers factors that have been shown to moderate placebo response in pain trials. Additional analyses via causal mediation analysis [141] may help to identify possible mechanisms to explain how complex interventions such as exercise work [142], when well-designed placebo controlled trials are not feasible. Importantly, an approach such as this to estimate the natural direct effect (specific effect of treatment without the contextual factors) requires pre-planning to ensure the hypothesised contextual factors are defined and measured [143]. Although possible, caution should be exercised due to the number of possible contextual factors at play and the inherent challenges when including multiple mediators [144]. The major limitation of our current study was the inability to fully answer the primary research question to determine the relative effects of specific exercise training, placebo effects and non-specific effects (e.g., via determining proportional placebo effects [24]). We anticipated a low number of placebo-controlled trials; however, the decision to include any chronic primary musculoskeletal condition into the analysis was made to increase the number of trials available for analysis. Unfortunately, this a priori decision did not overcome the inherent lack of studies available.
5 Conclusion
There is very low-quality evidence that exercise training is not more effective than non-exercise placebo treatments in chronic pain. Exercise training and the associated clinical encounter are more effective than true control or standard medical care for reductions in pain for adults with chronic musculoskeletal pain, with very low quality of evidence based on GRADE criteria. The development of clinical practice guidelines heavily relies on evidence from randomised clinical trials; however, in our systematic review and meta-analysis there were insufficient placebo-controlled randomised clinical trials to confirm the specific therapeutic effects of exercise training, placebo effects or non-specific effects on pain reduction. To better inform clinical decision making and clinical practice guidelines, future randomised controlled trials should consider the role of placebo effects and the impact of exercise training on chronic musculoskeletal pain.
Change history
19 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40279-021-01578-8
References
Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11(1):770.
Smith BH, Elliott AM, Chambers WA, Smith WC, Hannaford PC, Penny K. The impact of chronic pain in the community. Fam Pract. 2001;18(3):292–9.
Majlesi J. Patients with chronic musculoskeletal pain of 3–6-month duration already have low levels of health-related quality of life and physical activity. Curr Pain Headache Rep. 2019. https://doi.org/10.1007/s11916-11019-10817-11916.
Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity. Arch Intern Med. 2003;163:2433–45.
Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–8.
Blyth FM, March LM, Brnabic AJM, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89(2):127–34.
Dieppe P. Chronic musculoskeletal pain. BMJ. 2013;346:bmj.f3146.
Williams AC, Craig KD. Updating the definition of pain. Pain. 2016;157(11):2420–3.
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160(1):19–27.
Cunningham NR, Kashikar-Zuck S. Nonpharmacological treatment of pain in rheumatic diseases and other musculoskeletal pain conditions. Curr Rheum Rep. 2013;15(2):1–8.
Polaski AM, Phelps AL, Kostek MC, Szucs KA, Kolber BJ. Exercise-induced hypoalgesia: a meta-analysis of exercise dosing for the treatment of chronic pain. PLoS ONE. 2019;14(1):e0210418.
Sullivan AB, Scheman J, Venesy D, Davin S. The role of exercise and types of exercise in the rehabilitation of chronic pain: specific or nonspecific benefits. Curr Pain Headache Rep. 2012;16(2):153–61.
Lindheimer JB, Szabo A, Raglin JS, Beedie C. Advancing the understanding of placebo effects in psychological outcomes of exercise: lessons learned and future directions. Eur J Sport Sci. 2020;20(3):326–37.
Lindheimer JB, O’Connor PJ, Dishman RK. Quantifying the placebo effect in psychological outcomes of exercise training: a meta-analysis of randomized trials. Sports Med. 2015;45(5):693–711.
Bérdi M, Köteles F, Szabó A, Bárdos G. Placebo effects in sport and exercise: a meta-analysis. Eur J Ment Health. 2011;6(2):196–212.
Carlino E, Guerra G, Piedimonte A. Placebo effects: from pain to motor performance. Neurosci Lett. 2016;632:224–30.
Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16(7):403–18.
Testa M, Rossettini G. Enhance placebo, avoid nocebo: how contextual factors affect physiotherapy outcomes. Man Ther. 2016;24:65–74.
Howe LC, Leibowitz KA, Crum AJ. When your doctor “gets it” and “gets you”: the critical role of competence and warmth in the patient-provider interaction. Front Psychiatry. 2019. https://doi.org/10.3389/fpsyt.2019.00475.
Doherty M, Dieppe P. The, “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartil. 2009;17(10):1255–62.
Rossettini G, Camerone EM, Carlino E, Benedetti F, Testa M. Context matters: the psychoneurobiological determinants of placebo, nocebo and context-related effects in physiotherapy. Arch Physiother. 2020;10(1):1–12.
Rossettini G, Palese A, Geri T, Fiorio M, Colloca L, Testa M. Physical therapists’ perspectives on using contextual factors in clinical practice: findings from an italian national survey. PLoS ONE. 2018;13(11):e0208159.
Rossettini G, Palese A, Geri T, Mirandola M, Tortella F, Testa M. The knowledge of contextual factors as triggers of placebo and nocebo effects in patients with musculoskeletal pain: findings from a national survey. Front Psychiatry. 2019;10:478.
Whiteside N, Sarmanova A, Chen X, Zou K, Abdullah N, Doherty M, et al. Proportion of contextual effects in the treatment of fibromyalgia—a meta-analysis of randomised controlled trials. Clin Rheumatol. 2018;37(5):1375–82.
Chen AT, Shrestha S, Collins JE, Sullivan JK, Losina E, Katz JN. Estimating contextual effect in nonpharmacological therapies for pain in knee osteoarthritis: a systematic analytic review. Osteoarthritis Cartil. 2020;28(9):1154–69.
McCambridge J, Witton J, Elbourne DR. Systematic review of the hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267–77.
Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ. 2020;370:m1668.
Zou K, Wong J, Abdullah N, Chen X, Smith T, Doherty M, et al. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2016;75(11):1964–70.
Tagliaferri SD, Miller CT, Owen PJ, Mitchell UH, Brisby H, Fitzgibbon B, et al. Domains of chronic low back pain and assessing treatment effectiveness: a clinical perspective. Pain Pract. 2019;20(2):211–25.
Rossettini G, Carlino E, Testa M. Clinical relevance of contextual factors as triggers of placebo and nocebo effects in musculoskeletal pain. BMC Musculoskelet Dis. 2018;19(1):27.
Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and hawthorne effects. Lancet Neurol. 2016;15(7):736–47.
Moher D, Liberati A, Tetzlaff J, Altman D, Group P. preferred reporting items for systematic reviews and meta-analyses: the prisma statement. BMJ. 2009;2009:1–8.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for icd-11. Pain. 2015;156(6):1003.
Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med. 2004;256(2):91–100.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.0 2019. http://www.training.cochrane.org/handbook. Accessed 13 Dec 2020.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. Grade guidelines: 1. Introduction—grade evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. New York: Wiley; 2011.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1. 2020. http://www.training.cochrane.org/handbook.
Mengshoel AM, Komnas HB, Forre O. The effects of 20 weeks of physical fitness training in female patients with fibromyalgia. Clin Exp Rheumatol. 1992;10:345–9.
Sañudo B, Galiano D, Carrasco L, Blagojevic M, de Hoyo M, Saxton J. Aerobic exercise versus combined exercise therapy in women with fibromyalgia syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91(12):1838–43.
Gowans S, DeHueck A, Voss S, Silaj A, Abbey S, Reynolds W. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Care Res. 2001;45(6):519–29.
Etnier JL, Karper WB, Gapin JI, Barella LA, Chang YK, Murphy KJ. Exercise, fibromyalgia, and fibrofog: a pilot study. J Phys Activ Health. 2009;6:239–46.
Abbott JH, Robertson MC, Chapple C, Pinto D, Wright AA, Leon de lan Barra S, et al. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee: a randomized controlled trial 1: clinical effectiveness. Osteoarthritis Cartil. 2013;21(4):525–34.
Da Costa D, Abrahamowicz M, Lowensteyn I, Bernatsky S, Dritsa M, Fitzcharles MA, et al. A randomized clinical trial of an individualized home-based exercise programme for women with fibromyalgia. Rheumatology. 2005;44(11):1422–7.
Gusi N, Tomas-Carus P, Häkkinen A, Häkkinen K, Ortega-Alonso A. Exercise in waist-high warm water decreases pain and improves health-related quality of life and strength in the lower extremities in women with fibromyalgia. Arthritis Rheumatol. 2006;55(1):66–73.
Rendant D, Pach D, Lüdtke R, Reisshauer A, Mietzner A, Willich SN, et al. Qigong versus exercise versus no therapy for patients with chronic neck pain. Spine. 2011;36(6):419–27.
Cheung C, Wyman JF, Resnick B, Savik K. Yoga for managing knee osteoarthritis in older women: a pilot randomized controlled trial. BMC. 2014;14(160):1–11.
Lund H, Weile U, Christensen R, Rostock B, Downey A, Bartels EM, et al. A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J Rehabil Med. 2008;40(2):137–44.
Williams K, Abildso C, Steinberg L, Doyle E, Epstein B, Smith D, et al. Evaluation of the effectiveness and efficacy of iyengaryoga therapy on chronic low back pain. Spine. 2009;34(19):2066–76.
Wong A, Figueroa A, Sanchez-Gonzalez MA, Son W-M, Chernykh O, Park S-Y. Effectiveness of tai chi on cardiac autonomic function and symptomatology in women with fibromyalgia: a randomized controlled trial. J Aging Phys Act. 2018;26(2):214–21.
Kayo AH, Peccin MS, Sanches CM, Trevisani VFM. Effectiveness of physical activity in reducing pain in patients with fibromyalgia: a blinded randomized clinical trial. Rheumatol Int. 2011;32(8):2285–92.
Collado-Mateo D, Dominguez-Muñoz FJ, Adsuar JC, Garcia-Gordillo MA, Gusi N. Effects of exergames on quality of life, pain, and disease effect in women with fibromyalgia: a randomized controlled trial. Arch Phys Med Rehabil. 2017;98(9):1725–31.
Baptista AS, Villela AL, Jones A, Natour J. Effectiveness of dance in patients with fibromyalgia: a randomised, single-blind, controlled study. Clin Exp Rheumatol. 2012;30:S18–23.
García-Martínez AM, De Paz JA, Márquez S. Effects of an exercise programme on self-esteem, self-concept and quality of life in women with fibromyalgia: a randomized controlled trial. Rheumatol Int. 2011;32(7):1869–76.
Häkkinen A, Häkkinen K, Hannonen P, Alen M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: comparison with healthy women. Ann Rheumatol. 2001;60:21–6.
Sañudo B, Galiano D, Carrasco L, de Hoyo M, McVeigh JG. Effects of a prolonged exercise program on key health outcomes in women with fibromyalgia: a randomized controlled trial. J Rehabill Med. 2011;43(6):521–6.
Schachter CL, Busch AJ, Peloso PM, Sheppard MS. Effects of short versus long bouts of aerobic exercise in sedentary women with fibromyalgia: a randomized controlled trial. Phys Ther. 2003;83(4):340–58.
Sencan S, Ak S, Karan A, Muslumanoglu L, Ozcan E, Berker E. A study to compare the therapeutic efficacy of aerobic exercise and paroxetine in fibromyalgia syndrome. J Back Musculoskelet Rehabil. 2004;17(2):57–61.
Tomás-Carus P, Gusi N, Häkkinen A, Häkkinen K, Leal A, Ortega-Alonso A. Eight months of physical training in warm water improves physical and mental health in women with fibromyalgia: a randomized controlled trial. J Rehabil Med. 2008;40(4):248–52.
Tomás-Carus P, Gusi N, Hakkinen A, Hakkinen K, Raimundo A, Ortega-Alonso A. Improvements of muscle strength predicted benefits in HRQOL and postural balance in women with fibromyalgia: an 8-month randomized controlled trial. Rheumatol. 2009;48(9):1147–51.
Tomás-Carús P, Gusi N, Leal A, García Y, Ortega-Alonso A. The fibromyalgia treatment with physical exercise in warm water reduces the impact of the disease on female patients’ physical and mental health. Reumatol Clin. 2007;3(1):33–7.
Assumpção A, Matsutani LA, Yuan SL, Santo AS, Sauer J, Mango P, et al. Muscle stretching exercises and resistance training in fibromyalgia: which is better? A three-arm randomized controlled trial. Eu J Phys Rehabil Med. 2018;54(5):663–70.
King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. The effects of exercise and education, individually or combined, in women with fibromyalgia. J Rheumatol. 2002;29(12):2620–7.
Aglamis B, Toraman NF, Yaman H. The effect of a 12-week supervised multicomponent exercise program on knee OA in Turkish women. J Back Muscoloskelet Rehabil. 2008;21:121–8.
An B, Dai K, Zhu Z, Wang Y, Hao Y, Tang T, et al. Baduanjin alleviates the symptoms of knee osteoarthritis. J Altern Complement Med. 2008;14(2):167–74.
Borjesson M, Robertson E. Physiotherapy in knee osteoarthrosis: effect on pain and walking. Physiother Res Int. 1996;1(2):89–97.
Cheing GL, Hui-Chan CW. Does four weeks of tens and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain? Cllin Rehabil. 2002;16:749–60.
French HP, Cusack T, Brennan A, Caffrey A, Conroy R, Cuddy V, et al. Exercise and manual physiotherapy arthritis research trial (EMPART) for osteoarthritis of the hip: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2013;94(2):302–14.
Jan M-H, Lin J-J, Liau J-J, Lin Y-F, Lin D-H. Investigation of clinical effects of high- and low-resistance training for patients with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2008;88(4):427–36.
Lim BW, Hinman RS, Wrigley TV, Sharma L, Bennell KL. Does knee malalignment mediate the effects of quadriceps strengthening on knee adduction moment, pain, and function in medial knee osteoarthritis? A randomized controlled trial. Arthritis Rheumatol. 2008;59(7):943–51.
Lin D-H, Lin C-HJ, Lin Y-F, Jan M-H. Efficacy of 2 non-weight-bearing interventions, proprioception training versus strength training, for patients with knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther. 2009;39(6):450–7.
Rosedale R, Rastogi R, May S, Chesworth BM, Filice F, Willis S, et al. Efficacy of exercise intervention as determined by the mckenzie system of mechanical diagnosis and therapy for knee osteoarthritis: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44(3):173-A176.
Schilke JM, Johnson GO, Housh TJ, O’Dell JR. Effects of muscle-strength training on the functional status of patients with osteoarthritis of the knee joint. Nurs Res. 1996;45(2):68–72.
Wang T-J, Lee S-C, Liang S-Y, Tung H-H, Wu S-FV, Lin Y-P. Comparing the efficacy of aquatic exercises and land-based exercises for patients with knee osteoarthritis. J Clin Nurs. 2011;20(17–18):2609–22.
Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87(1):32–43.
Fransen M, Nairn L, Winstanley J, Lam P, Edmonds J. Physical activity for osteoarthritis management: a randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthr Rheumatol. 2007;57(3):407–14.
Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Garcia Lucareli PR, De Almeida Carvalho NA. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736–42.
Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and hip strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22–9.
Saad MC, Vasconcelos RAD, Mancinelli LVDO, Munno MSDB, Liporaci RF, Grossi DB. Is hip strengthening the best treatment option for females with patellofemoral pain? A randomized controlled trial of three different types of exercises. Braz J Phys Ther. 2018;22(5):408–16.
Harris-Hayes M, Czuppon S, Van Dillen LR, Steger-May K, Sahrmann S, Schootman M, et al. Movement-pattern training to improve function in people with chronic hip joint pain: a feasibility randomized clinical trial. J Orthop Sports Phys Ther. 2016;46(6):452–61.
Ferrell BA, Josephson KR, Pollan AM, Loy S, Ferrell BR. A randomized trial of walking versus physical methods for chronic pain management. Aging Clin Exp Res. 1997;9:99–105.
Cortell-Tormo JM, Sánchez PT, Chulvi-Medrano I, Tortosa-Martínez J, Manchado-López C, Llana-Belloch S, et al. Effects of functional resistance training on fitness and quality of life in females with chronic nonspecific low-back pain. J Back Musculoskelet Rehabil. 2018;31(1):95–105.
Costa LOP, Maher CG, Latimer J, Hodges PW, Herbert RD, Refshauge KM, et al. Motor control exercise for chronic low back pain: a randomized placebo-controlled trial. Phys Ther. 2009;89(12):1275–86.
Jackson JK, Shepherd TR, Kell RT. The influence of periodized resistance training on recreationally active males with chronic nonspecific low back pain. J Strength Cond Res. 2011;25(1):242–51.
Kell RT, Asmundson GJG. A comparison of two forms of periodized exercise rehabilitation programs in the management of chronic nonspecific low-back pain. J Strength Cond Res. 2009;23(2):513–23.
Kell RT, Risi AD, Barden JM. The response of persons with chronic nonspecific low back pain to three different volumes of periodized musculoskeletal rehabilitation. J Strength Cond Res. 2011;25(4):1052–64.
Kofotolis N, Eleftherios K. Effects of two 4-week proprioreceptive neuromuscular facilitation programs on muscle endurance, flexibility, and functional performance in women with chronic low back pain. Phys Ther. 2006;86(7):1001–12.
Masharawi Y, Nadaf N. The effect of non-weight bearing group-exercising on females with non-specific chronic low back pain: a randomized single blind controlled pilot study. J Back Musculoskelet Rehabil. 2013;26(4):353–9.
Moussouli M, Vlachopoulos SP, Kofotolis ND, Theodorakis Y, Malliou P, Kellis E. Effects of stabilization exercises on health-related quality of life in women with chronic low back pain. J Phys Act Health. 2014;11(7):1295–303.
Oh H-W, Lee M-G, Jang J-Y, Jin J-J, Cha J-Y, Jin Y-Y, et al. Time-effects of horse simulator exercise on psychophysiological responses in men with chronic low back pain. Isokinet Exerc Sci. 2014;22(2):153–63.
Segal-Snir Y, Lubetzky VA, Masharawi Y. Rotation exercise classes did not improve function in women with non-specific chronic low back pain: a randomized single blind controlled study. J Back Musculoskelet Rehabil. 2016;29(3):467–75.
Schinhan M, Neubauer B, Pieber K, Gruber M, Kainberger F, Castellucci C, et al. Climbing has a positive impact on low back pain: a prospective randomized controlled trial. Clin J Sport Med. 2016;26(3):199–205.
Cho H-Y, Kim E-H, Kim J. Effects of the core exercise program on pain and active range of motion in patients with chronic low back pain. J Phys Ther Sci. 2014;26:1237–40.
Gladwell V, Head S, Haggar M, Beneke R. Does a program of pilates improve chronic non-specific low back pain? J Sport Rehabil. 2006;15:338–50.
de Fonseca JL, Magini M, van Freitas TH. Laboratory gait analysis in patients with low back pain before and after a pilates intervention. J Sport Rehabil. 2009;18:269–82.
Steele J, Bruce-Low S, Smith D, Jessop D, Osborne N. A randomized controlled trial of the effects of isolated lumbar extension exercise on lumbar kinematic pattern variability during gait in chronic low back pain. PM&R. 2016;8(2):105–14.
Yoo JH, Kim SE, Lee MG, Jin JJ, Hong J, Choi YT, et al. The effect of horse simulator riding on visual analogue scale, body composition and trunk strength in the patients with chronic low back pain. Int J Clin Pract. 2014;68(8):941–9.
Shaughnessy M, Caulfield B. A pilot study to investigate the effect of lumbar stabilisation exercise training on functional ability and quality of life in patients with chronic low back pain. Int J Rehabil Res. 2004;27(4):297–301.
Zadro JR, Shirley D, Simic M, Mousavi SJ, Ceprnja D, Maka K, et al. Video-game–based exercises for older people with chronic low back pain: a randomized controlledtable trial (gameback). Phys Ther. 2019;99(1):14–27.
de Araujo CL, Jones A, Roger-Silva D, Ribeiro LHC, Natour J. Effectiveness of the pilates method in the treatment of chronic mechanical neck pain: a randomized controlled trial. Arch Phys Med Rehabil. 2018;99(9):1740–6.
Falla D, Lindstrøm R, Rechter L, Boudreau S, Petzke F. Effectiveness of an 8-week exercise programme on pain and specificity of neck muscle activity in patients with chronic neck pain: a randomized controlled study. Eur J Pain. 2013;17(10):1517–28.
von Trott P, Wiedemann AM, Lüdtke R, Reißhauer A, Willich SN, Witt CM. Qigong and exercise therapy for elderly patients with chronic neck pain (qibane): a randomized controlled study. J Pain. 2009;10(5):501–8.
Buttagat V, Taepa N, Suwannived N, Rattanachan N. Effects of scapular stabilization exercise on pain related parameters in patients with scapulocostal syndrome: a randomized controlled trial. J Bodyw Mov Ther. 2016;20(1):115–22.
Saeterbakken AH, Nordengen S, Andersen V, Fimland MS. Nordic walking and specific strength training for neck- and shoulder pain in office workers: a pilot-study. Eur J Phys Rehab Med. 2017;53(6):928–35.
Viljanen M, Malmivaara A, Uitti J, Rinne M, Palmroos P, Laippala P. Effectiveness of dynamic muscle training, relaxation training, or ordinary activity for chronic neck pain: randomised controlled trial. BMJ. 2003;327:1–5.
Horstmann T, Jud HM, Fröhlich V, Mündermann A, Grau S. Whole-body vibration versus eccentric training or a wait-and-see approach for chronic achilles tendinopathy: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43(11):794–803.
Osteras N, Hagen KB, Grotle M, Sand-Svartrud AL, Mowinckel P, Kjeken I. Limited effects of exercises in people with hand osteoarthritis: results from a randomized controlled trial. Osteoarthritis Cartil. 2014;22:1224–33.
Alikhajeh Y, Barabadi E, Rahimi GRM. A comparison of 6 weeks of aquatic exercise and kinesio taping in patients with chronic nonspecific low back pain. J Sport Rehabil. 2020;30(1):37–42.
Izquierdo-Alventosa R, Inglés M, Cortés-Amador S, Gimeno-Mallench L, Sempere-Rubio N, Chirivella J, et al. Comparative study of the effectiveness of a low-pressure hyperbaric oxygen treatment and physical exercise in women with fibromyalgia: randomized clinical trial. Ther Adv Musculoskelet Dis. 2020;12:1759720X20930493.
Madadi-Shad M, Jafarnezhadgero AA, Sheikhalizade H, Dionisio VC. Effect of a corrective exercise program on gait kinetics and muscle activities in older adults with both low back pain and pronated feet: a double-blind, randomized controlled trial. Gait Posture. 2020;76:339–45.
Prado ÉRA, Meireles SM, Carvalho ACA, Mazoca MF, Neto ADMM, Da Silva RB, et al. Influence of isostretching on patients with chronic low back pain. A randomized controlled trial. Physiother Theory Pract. 2019;32(2):287–94.
Rezasoltani Z, Sanati E, Mofrad RK, Azizi S, Dadarkhah A, Najafi S. Randomized controlled trial of aquatic cycling for treatment of knee osteoarthritis in elderly people. Top Geriatr Rehabil. 2020;36(2):103–9.
Vincent KR, Vasilopoulos T, Montero C, Vincent HK. Eccentric and concentric resistance exercise comparison for knee osteoarthritis. Med Sci Sports Exerc. 2019;51(10):1977–86.
McIlveen B, Robertson VJ. A randomised controlled study of the outcome of hydrotherapy for subjects with low back or back and leg pain. Physiotherapy. 1998;84(1):17–26.
Nichols DS, Glenn TM. Effects of aerobic exercise on pain perception, affect, and level of disability in individuals with fibromyalgia. Phys Ther. 1994;74(4):327–32.
Turner JA, Clancy S, McQuade KJ, Cardenas DD. Effectiveness of behavioral therapy for chronic low back pain: a component analysis. J Consult Clin Psychol. 1990;58(5):573.
Valkeinen H, Alén M, Häkkinen A, Hannonen P, Kukkonen-Harjula K, Häkkinen K. Effects of concurrent strength and endurance training on physical fitness and symptoms in postmenopausal women with fibromyalgia: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89(9):1660–6.
Wigers SHR, Stiles T, Vogel P. Effects of aerobic exercise versus stress management treatment in fibromyalgia. Scand J Rheumatol. 1996;25(2):77–86.
McBeth J, Prescott G, Scotland G, Lovell K, Keeley P, Hannaford P, et al. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Arch Intern Med. 2012;172(1):48–57.
Keane LG. Comparing aquastretch with supervised land based stretching for chronic lower back pain. J Bodyw Mov Ther. 2017;21(2):297–305.
Hróbjartsson A, Copenhagen P. Placebo is better than no treatment for subjective continuous outcomes and for treatment of pain. N Engl J Med. 2001;344:1594–602.
Chen X, Zou K, Abdullah N, Whiteside N, Sarmanova A, Doherty M, et al. The placebo effect and its determinants in fibromyalgia: meta-analysis of randomised controlled trials. Clin Rheumatol. 2017;36(7):1623–30.
de Craen AJ, Tijssen JG, de Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol. 2000;247(3):183–8.
Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(12):1716–23.
Branthwaite A, Cooper P. Analgesic effects of branding in treatment of headaches. Br Med J (Clin Res Ed). 1981;282(6276):1576–8.
Bello S, Wei M, Hilden J, Hróbjartsson A. The matching quality of experimental and control interventions in blinded pharmacological randomised clinical trials: a methodological systematic review. BMC Med Res Methodol. 2016;16(1):18.
Friesen P. Placebos as a source of agency: evidence and implications. Front Psychiatry. 2019;10:721.
Zhang W, Doherty M. Efficacy paradox and proportional contextual effect (PCE). Clin Immunol. 2018;186:82–6.
Kamper SJ, Williams CM. The placebo effect: powerful, powerless or redundant? Br J Sports Med. 2013;47(1):6–9.
Rossettini G, Testa M. Manual therapy rcts: should we control placebo in placebo control? Eur J Phys Rehabil Med. 2018;54(3):500–1.
Rains JC, Penzien DB. Behavioral research and the double-blind placebo-controlled methodology: challenges in applying the biomedical standard to behavioral headache research. Headache. 2005;45(5):479–86.
Singal AG, Higgins PDR, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5(1):e45.
Sosa-Reina MD, Nunez-Nagy S, Gallego-Izquierdo T, Pecos-Martín D, Monserrat J, Álvarez-Mon M. Effectiveness of therapeutic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomized clinical trials. BioMed Res Int. 2017;2017:2356346.
Andrade A, Dominski FH, Sieczkowska SM. What we already know about the effects of exercise in patients with fibromyalgia: an umbrella review. Semin Arthritis Rheum. 2020;50(6):1465–80.
Zampogna B, Papalia R, Papalia GF, Campi S, Vasta S, Vorini F, et al. The role of physical activity as conservative treatment for hip and knee osteoarthritis in older people: a systematic review and meta-analysis. J Clin Med. 2020;9(4):1167.
Goh S-L, Persson MSM, Stocks J, Hou Y, Lin J, Hall MC, et al. Efficacy and potential determinants of exercise therapy in knee and hip osteoarthritis: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62(5):356–65.
Owen PJ, Miller CT, Mundell NL, Verswijveren SJ, Tagliaferri SD, Brisby H, et al. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br J Sports Med. 2019;54(21):1279–87.
Bertozzi L, Gardenghi I, Turoni F, Villafañe JH, Capra F, Guccione AA, et al. Effect of therapeutic exercise on pain and disability in the management of chronic nonspecific neck pain: systematic review and meta-analysis of randomized trials. Phys Ther. 2013;93(8):1026–36.
Imai K, Keele L, Tingley D, Yamamoto T. Causal mediation analysis using r. New York: Springer; 2010. p. 129–54.
Lee H, Herbert RD, Lamb SE, Moseley AM, McAuley JH. Investigating causal mechanisms in randomised controlled trials. Trials. 2019;20(1):524.
Cashin AG, McAuley JH, Lamb SE, Lee H. Disentangling contextual effects from musculoskeletal treatments. Osteoarthritis Cartilage. 2021;29(3):297–9.
Jérolon A, Baglietto L, Birmelé E, Alarcon F, Perduca V. Causal mediation analysis in presence of multiple mediators uncausally related. Int J Biostat. 2020. https://doi.org/10.1515/ijb-2019-0088.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was provided for this systematic review.
Conflict of interest
Clint Miller, Patrick Owen, Christian Than, Jake Ball, Kate Sadler, Alessandro Piedimonte, Fabrizio Benedetti and Daniel Belavy declare that they have no conflicts of interest relevant to the content of this review.
Data availability
The data extracted as part of this systematic review and used in subsequent analysis are made available in Table 1 and the Stata code and data are included in Table S2 (OSM).
Author contributions
Systematic review conception: CTM, PJO, DLB, AP. Screening: PJO, JB, CAT, KS. Extraction: JB, CAT, KS. Statistical analyses: PJO. Drafted manuscript: CTM. Edited and approved final manuscript: All.
Additional information
The original article has been updated: Due to Figures update.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miller, C.T., Owen, P.J., Than, C.A. et al. Attempting to Separate Placebo Effects from Exercise in Chronic Pain: A Systematic Review and Meta-analysis. Sports Med 52, 789–816 (2022). https://doi.org/10.1007/s40279-021-01526-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01526-6