Abstract
Background
Concussion diagnosis and management is made through the clinical exam using assessment tools that include self-report symptomatology, postural control, and cognitive evaluations. The specific timing of concussion resolution varies between individuals. However, despite a lack of research in concussion recovery, it is widely accepted that the majority of young adults will recover in 7–10 days, with youth athletes taking longer.
Objectives
The purpose of this review is to directly compare the recovery duration among high school and collegiate athletes on symptom reports and cognitive assessments following concussion.
Data Sources
Data were collected from a literature search comprising high school or college athletes only. This included studies (n = 6) that reported symptom or cognitive performance recovery to the exact day.
Results
High school athletes self-reported symptom recovery at 15 days compared with 6 days in collegiate athletes. Both college and high school athletes showed cognitive recovery at similar rates of 5 and 7 days.
Limitations
This review only included articles that were directly related to concussed high school or college athletes. Additionally, athletes in the high school and college setting typically receive a battery of neurocognitive tests that may not be as sensitive or as comprehensive as a full neuropsychological exam.
Conclusion
The review finds that neurocognitive recovery rates are similar among high school and college athletes, while symptom reporting shows longer recovery time points in high school than in college.
Implications of Key Findings
An individualized and stepwise concussion management plan is important for proper concussion recovery regardless of age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
High school athletes report longer symptom recovery than college athletes following concussion. |
High school and college athletes show similar neurocognitive recovery rates following concussion. |
The heterogeneous nature of concussion necessitates an individualized concussion management strategy. |
1 Introduction
1.1 Rationale
Concussion is defined as “a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces” resulting in the rapid onset of symptoms and cognitive impairments [1]. Nearly 4 million concussions are thought to occur in the USA each year as a result of sport and physical activity [2]. Nearly 300,000 of these are among youth athletes aged 14–19 [3]. Concussion incidence is reported to vary by sport and level of participation [4–9], with collegiate athletes reporting greater concussion rates than high school athletes in identical sports; however, the percentage of concussions out of total injuries is higher in high school athletes than in college athletes [10].
The concussion diagnosis is a subjective process made through the clinical exam, but often supported using a number of measures that assess various domains of cerebral functioning [1, 11]. Most commonly, these assessments include self-report symptomatology, postural control, and cognitive evaluations. Concussion symptoms can be categorized into ‘cognitive’ (e.g., confusion, delayed verbal and motor responses), ‘somatic’ (e.g., headache, dizziness, balance disruption), ‘affective/emotional’ (e.g., emotional, irritable), and ‘sleep disturbances’ (e.g., trouble falling asleep, sleeping more or less than usual) [12], all of which may develop immediately or shortly after the time of injury [7]. Headache is the most commonly reported concussion symptom (93 %) [5, 13], followed by dizziness and confusion [4, 5, 14, 15].
The specific timing of concussion resolution varies between individuals, but a large sample of concussed collegiate athletes reported increased symptom reports until 1 week post-injury [16] and Lee et al. [17] reported symptom recovery in high school athletes at 7 days compared with 6 days in collegiate athletes. Erlanger et al. [18] found an average of 6 days until symptom resolution for both high school and college, and Zuckerman et al. [19] found high school athletes had symptom recovery within 8 days while college athletes took 6 days. Postural control has also been evaluated following injury, and deficits on computer-based measures show impaired balance function in collegiate athletes up to 3 days post-injury when compared with a pre-injury baseline [20–22], with similar results on clinical balance measures [16]. Balance testing directly comparing concussed high school and collegiate athletes showed no statistical difference in Balance Error Scoring System (BESS) performance between groups at days 1, 2, or 3 post-injury [23]. Lastly, cognitive function following concussion has been extensively evaluated among athletes using both pencil and paper and computer-based measures. Concussed collegiate athletes have been reported to return to pre-injury levels of function by 5 days [14, 16], 6 days [11, 16, 18], and 7 days post injury [16, 24, 25]. Less is known about high school athletes, who show larger declines in a number of cognitive domains immediately following injury [23], but the literature is unclear on the number of days until these athletes return to pre-injury levels. One often cited study found high school athletes performed worse than concussed collegiate athletes at 3 days post-injury on the Hopkins Verbal Learning Test, but the authors did not identify the average number of days until recovery following concussion for either athlete group [26].
Despite a lack of supporting evidence in the literature, consensus groups and professional medical organizations have indicated the majority of young adult athletes will recover in 7–10 days, with youth athletes taking longer [1, 11]. While numerous investigations have evaluated declines from baseline as a proxy for recovery duration (i.e., days), limited work has sought to define the natural history of concussion and the day on which high school and collegiate athletes typically return to pre-injury levels of functioning. Therefore, the purpose of this systematic review and meta-analysis is to directly compare the recovery duration among high school and collegiate athletes on symptom and cognitive assessments following sport concussion.
2 Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27].
2.1 Data Sources and Searches
An electronic search was conducted in PubMed from database inception to December 2013 using the following search strategy: (athletes or sports) and (concussion or mild traumatic brain injury) and (recovery or symptoms or cognition). Reference lists from retrieved articles and other related reviews also supplemented the article search.
2.2 Study Selection
Inclusion criteria were as follows: (1) the sample included high school- or college-aged athletes concussed during a sporting event, (2) the dependent variable was number of days to recovery, and (3) recovery was defined as return to an asymptomatic state as measured by self-reported symptoms or neurological tests taken at baseline.
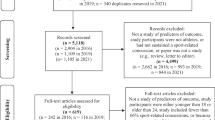
Exclusion criteria were as follows: (1) review articles, abstracts, case studies, and editorials, (2) studies involving imaging techniques, or (3) lack of data necessary for the calculation of effect size. Figure 1 presents a flowchart of study selection.
2.3 Data Extraction and Quality Assessment
Two authors (RMW, SPB) independently extracted data, and discrepancies were resolved by consensus judgment. Effect sizes were calculated by subtracting the number of days to recovery (i.e., asymptomatic relative to baseline symptoms or neurocognitive scores) from the day of injury (i.e., day zero) and dividing the difference by the recovery day standard deviation [28, 29]. Effect sizes were adjusted using Hedges’ small sample size bias correction [28]. Two-way (effects × raters) intraclass correlation coefficients (ICC) for absolute agreement were calculated to examine inter-rater reliability for effect sizes and moderators. The initial ICCs, based on ten effects, were ≥0.90.
Study quality was assessed using a 15-item scale addressing randomization, sample selection, quality of outcome measures, and statistical analysis [30]. Quality scores were not used as weights or moderators because of the potential disparity in results that depends on the specific quality scale employed [31].
2.4 Data Synthesis and Analysis
An SPSS macro (i.e., MeanES; SPSS version 22.0, IBM Inc., Armonk, NY, USA) was used to calculate the aggregated mean effect size delta (Δ), associated 95 % confidence interval (CI), and the sampling error variance according to a random effects model [32]. Random effects models were used to account for between-studies heterogeneity associated with both study-level sampling error and population variance [32]. Each effect was weighted by the inverse of its variance and re-estimated after the random effects variance component was added [28]. Heterogeneity and consistency were evaluated with the Q statistic and the I 2 statistic, respectively [33]. Heterogeneity also was examined relative to observed variance and was indicated if the sampling error accounted for less than 75 % of the observed variance [28]. Publication bias was addressed by inspection of a funnel plot [33] and quantified with rank correlation [34].
2.5 Primary Moderator Analysis
Three variables were selected based on logical, theoretical, or empirical relations to concussion recovery: age, concussion measure (i.e., self-reported symptoms or neurological tests), and age × measure interaction. Variable definitions can be found in Table 1.
Using an SPSS macro (MetaReg), primary moderator variables were included in random-effects, weighted least squares, multiple linear regression analyses with maximum-likelihood estimation [28, 32]. All models and analyses were adjusted for non-independence of multiple effects contributed by single studies [35]. Tests of the regression model (Q R) and its residual error (Q E) are reported. The Johnson–Neyman procedure was conducted to identify the critical point (i.e., age) in significant interactions of categorical and continuous variables in order to define significance regions [36]. That is, to establish the point at which age influences the recovery process. Regions of significance can help define the exact ages in which there is a statistically significant difference between self-reported and neurological concussion symptom recovery in high school- and college-aged athletes.
2.6 Secondary Moderators and Analysis
Secondary moderators were selected for descriptive, univariate analyses based on logical, theoretical, or prior empirical relation with concussion recovery. Variable definitions are provided in Table 1. Mean effect sizes and 95 % CIs were computed for continuous and categorical variables using a random effects model [32].
3 Results
Six studies of 702 high school- and college-aged athletes were included in the meta-analysis and are presented along with their characteristics and study quality assessment in Tables 2 and 3 [17–19, 37–39]. A funnel plot was inspected and found to be roughly symmetrical (Fig. 2). The Begg’s rank correlation analysis was not statistically significant, suggesting an absence of publication bias [τ(15) = 0.30, p = 0.150].
3.1 Primary Moderator Analyses
During the recovery period, significant changes were noted for concussion symptoms (increase) and neurocognitive function (decline) (Δ = 1.14; 95 % 1.04–1.25; z = 21.16, p < 0.001). Distribution of the effects is presented in Fig. 3. The effect was heterogeneous [Q T(15) = 29.54, p = 0.001]. Sampling error accounted for 53.3 % of the observed variance. The effect was not consistent across studies (I 2 = 52.6 %; 95 % CI 36.4–64.7).
The overall multiple regression model of concussion recovery in high school- and college-aged athletes was significantly related to effect size [Q R(4) = 11.57; p = 0.021, R 2 = 0.39; Q E(11) = 17.97, p = 0.082]. The interaction of age and concussion measure (Fig. 4) was independently related to effect size (β = 0.132, z = 2.13, p = 0.001). The Johnson–Neyman procedure yielded a critical point for age at 17.8 years (β = 0.17, t = 2.18, p = 0.050). Further decomposition revealed (1) greater and more variable recovery times in high school than in college athletes based on self-reported concussion symptoms (15 vs. 6 days), and (2) more similar and less variable recovery times in high school and college athletes based on cognitive measures (7 vs. 5 days; Fig. 5).
3.2 Secondary Moderator Analyses
The number of effects (k), mean effect Δ, 95 % CI, p value, and I 2 for each level of each moderator are presented in Table 1.
4 Discussion
This meta-analytic review sought to define the natural history of concussion among injured high school and collegiate athletes by incorporating self-reported symptomatology, postural control, and cognitive recovery. The analysis indicated greater variability in self-reported concussion symptom recovery periods among high school athletes than among college-aged athletes. Symptom recovery is characterized as an athlete’s self-reported feeling of being asymptomatic. High school athletes self-reported symptom recovery at 15 days compared with 6 days in collegiate athletes. In addition, there was less variability and more similar time points on cognitive recovery measures when comparing high school and college athletes following concussion. High school athletes showed cognitive recovery at 7 days, similar to college athletes, who showed recovery within 5 days.
Self-reported symptoms are the most commonly used assessment tool implemented by clinicians for concussion diagnosis and injury management [1, 11, 16, 40]. Their use is supported by their strong reliability and sensitivity [24, 41] and backed by consensus groups and medical organizations [1, 11, 42, 43]. One investigation evaluated concussed collegiate (male football and female soccer) and high school (male football and male soccer) athletes compared with age-related matched controls on a variety of neuropsychological tests and self-report symptomatology at fixed post-injury time points (24 h, 3, 5, and 7 days) [26]. The subjects were administered a 20-item Likert scale post-concussion symptom checklist as well as the following neuropsychological tests: Hopkins verbal learning, Digit Span Test, Symbol Digit Modalities Test, Trail-making Test A/B, Controlled Oral Word Association Test, Brief Visual Memory Test Revised. Symptom reports among the concussed high school athletes were higher at 24 h, and days 3 and 5 compared with the matched controls, while collegiate athletes showed significant differences compared with matched controls only at 24 h and day 3. Direct comparisons between concussed collegiate and high school athletes were not completed. In addition, Field et al. [26] observed that both high school and collegiate athletes reported fewer self-reported symptoms at days 5 and 7 than at baseline. This paper suggested recovery in both ages in 7 days or less; however, without a direct comparison between the high school and collegiate athletes, it is not possible to conclude that the two groups recovered at different intervals.
Despite the heavy reliance on symptom reports by medical personnel for concussion assessment and management, they are inherently subjective. In addition to purposeful manipulation, as mentioned below, there are also circumstances where athletes are unaware of symptoms or misinterpret symptoms, which may not necessarily represent ‘manipulation’. For example, several studies suggest symptom-reporting issues and concussion under-reporting among athletes continues to be an issue for medical professionals and researchers [44–49]. General concussion awareness and concussion education efforts have increased, but research indicates that players continue to under-report concussions and symptoms alike to continue playing or return to play sooner. For example, McCrea et al. [44] reported that 52 % of concussed high school athletes would not report concussion-related symptoms to a medical professional, coach, or parent. Register-Mihalik et al. [45] found similar results, where high school athletes were significantly under-reporting concussions as well as continuing to play in their sport while experiencing concussion symptoms. In addition, Sefton et al. [50] reported that 80 % of concussed collegiate athletes will similarly not report concussion-related symptoms. The reasons for not reporting the injury are likely multifactorial. For example, one investigation reported that concussed collegiate athletes did not report their concussions because they did not realize their symptoms were concussion related [47, 48]. One possibility is that collegiate athletes may feel they have more to lose by reporting concussion symptoms and risking being withheld from play. High school athletes, on the other hand, may not perceive the loss of playing time to be as great and may be more honest in their reporting when approached by an adult about a medical condition.
In addition to athlete unawareness of symptoms or the deliberate hiding of known symptoms, the team environment established by coaches, parents, and teammates may influence symptom reports. Sullivan et al. [51] found that 83 % of high school parents were able to recognize, and had basic knowledge of, concussion symptoms and understood the significance and seriousness of a concussion. However, McLeod et al. [52] found that 26 % of youth coaches would let a symptomatic athlete return to play. The majority of coaches in this study were able to recognize basic signs and symptoms of concussion; those with previous coaching education were able to correctly identify concussion symptoms [52]. Sarmiento et al. [53] studied coaches and found that coaches typically face barriers from athletes and athletes’ parents who do not understand or discount the severity of the concussion. Factors such as these may influence both concussion identification at the time of injury and may be taken into account when interpreting ‘concussion recovery’ based only on symptom reports. Education for players, parents, and coaches is important and could increase concussion reporting and potentially decrease the number of athletes who choose to play while symptomatic.
In addition to symptom reports, the evaluation of cognitive function has been established as a vital component to concussion recovery [1, 11, 42, 43]. Cognitive function has been shown to decrease immediately following a concussion [54] and has been indexed with measures of reaction time, information processing, memory, and attention [14, 55–58]. Despite the commonly stated extended neurocognitive recovery time for concussed high school athletes [26], the findings from this review do not support this conclusion. Indeed, this analysis indicates that high school- and college-aged athletes demonstrate cognitive recovery within 5 and 7 days of injury, respectively. Iverson et al. [59] found similar results while studying amateur athletes at junior high, high school, and university settings and found that symptoms and performance decrements largely resolved within 5 days post-injury and fully resolved by 10 days [59]. Additionally, McCrea et al. [16] found cognitive recovery reaching baseline measures in concussed collegiate athletes at 5–7 days post-injury.
This review indicates that concussed high school and collegiate athletes have similar recovery rates for cognitive functioning (5 vs. 7 days), yet symptom recovery among high school athletes is longer than their collegiate counterparts (15 vs. 6 days). The difference in symptom and cognitive recovery reported here is likely explained by two factors. First, the biological response to injury among the younger high school athletes may result in a longer recovery period. This topic is beyond the scope of this review, but some literature has shown that the immature brain has several developmental vulnerabilities to traumatic brain injury (TBI). Preclinical studies indicate that, after TBI, the young brain is more susceptible to impaired neurotransmission and plasticity [60, 61] and that unmyelinated axons are more vulnerable to biomechanical insult [62]. In addition, college-level athletes are more physically gifted than their high school counterparts and may be self-selected as less likely to develop concussion symptoms.
A second potential explanation is the larger under-reporting of concussion symptoms by collegiate athletes for the reasons outlined above. This explanation is supported by previous research showing ongoing cognitive impairment among 40 % of concussed collegiate athletes who report being asymptomatic [41]. Other reports have shown that concussed athletes no longer reporting concussion-related symptoms perform worse on computer-based examinations of cognitive function than control athletes [63]. That is, based on the results reported here and in previous work, athletes over the age of 18 years (i.e., collegiate level) may be under-reporting post-concussion symptoms, resulting in a shortened symptom recovery time, yet the cognitive recovery times reported between the two groups is similar, as these tests cannot be manipulated as easily as symptom reports. Conversely, high school athletes may be influenced by parental concerns, and younger athletes may be more likely to attribute or misattribute symptoms to concussion.
4.1 Limitations
Although these findings are compelling, this review is not without limitations. The most significant limitation of the analysis includes the neurological data tied to eight effects from one study. However, despite a growing scientific interest in concussive injuries, the manuscripts included here were the only ones that directly identified the day of recovery among concussed high school and collegiate athletes. This limitation identifies the need for additional research to clarify the natural history of concussion using clinically based timepoints (e.g., immediate post-injury, asymptomatic, return to play, etc.) to better understand the difference between these two groups. In addition, we were unable to include any investigations to identify the recovery profile of concussed high school and collegiate athletes on measures of postural stability. Clinical and laboratory-based measures of postural control are widely accepted among the clinical community and implemented within scientific protocols [1, 11, 54], but their use to define the natural history of concussion is limited. Lastly, neuropsychological testing is a comprehensive exam that encompasses a larger cognitive spectrum than typically achieved in the high school and college settings. However, the neurocognitive battery of tests that athletes are given are relatively sensitive, but more sensitive measures (e.g. diffusion tensor imaging, and functional magnetic resonance imaging, and/or magnetic resonance spectroscopy) may help elucidate the differences in cognitive and symptom recovery reported here. Although there are known effects on concussion recovery based on gender [23, 64], assessment intervals [1, 11], and concussion history [1, 5, 11, 12], these were not the aim of this review. In addition, it is plausible that the culture surrounding different sports or the person who evaluated and treated the athlete may have influenced recovery times. It should also be noted that there are no age-specific data comparing concussion recovery between ages younger than high school with collegiate/young adult athletes; thus, conclusions for athletes younger than high school are currently mostly extrapolated. This also identifies a gap in knowledge on which future investigations should be focused.
5 Conclusion
In the final review, this analysis indicates that recovery from concussion-related symptoms among high school athletes is longer than among college athletes, yet cognitive recovery is similar between the two groups. Clinically, these findings do not change practice standards, as each athlete should be managed individually, regardless of age, but considerations for symptom recovery compared with cognitive recovery should be noted. Although not reviewed here, clinicians managing sport-related concussions should include measures of motor control (e.g., balance and gait) in their assessment and management strategy. The combined use of athlete-reported symptoms, neurocognitive function, and motor control has been shown to have large effect sizes immediately post-injury [54] and provide the highest sensitivity to injury [41].
Ultimately, the injured athlete’s clinical management and return-to-play process should be dictated by the clinical exam findings and supported by objective assessment tools from a multi-disciplinary management team. When available, pre-morbid measures of symptoms, postural control, and cognitive functioning are recommended by consensus and medical groups [1, 11, 43] with additional input from coaches, parents, school administrators, athletic trainers, and other healthcare professionals.
References
McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. Consensus statement on concussion in sport—the 4th international conference on concussion in sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–8.
Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehab. 2006;21(5):375–8.
Buzzini SR, Guskiewicz KM. Sports-related concussion in the young athlete. Curr Opin Pediatr. 2006;18:376–82.
Guskiewicz K, Weaver N, Padua D, Garrett W. Epidemiology of concussion in collegiate and high school football players. Am J Sport Med. 2000;28:643–50.
Guskiewicz K, McCrea M, Marshall S, Cantu R, Randolph C, Barr W. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA concussion study. J Am Med Assoc. 2003;290(19):2549–55.
Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. J Am Med Assoc. 1999;282(10):958–63.
Lovell MR, Collins MW, Iverson GL, Field M, Maroon JC, Cantu R, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296–301.
Pellman EJ, Lovell MR, Viano DC, Casson IR, Tucker AM. Concussion in professional football: neuropsychological testing–part 6. Neurosurg. 2004;55(6):1290–303 (discussion 303–5).
Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–9.
Gessel LM, Fields SK, Collins CL, Comstock R. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42:495–503.
Broglio SP, Cantu RC, Gioia GA, Guskiewicz KM, Kutcher J, Palm M, Valovich McLeod TC. National Athletic Trainers’ Association position statement: management of sport concussion. J Athl Train. 2014;49(2):245–65.
Herring SA, Cantu RC, Guskiewicz KM, Putukian M, Kibler WB, Bergfeld JA, et al. Concussion (mild traumatic brain injury) and the team physician: a consensus statement—2011 update. Med Sci Sports Exerc. 2011;43(12):2412–22.
Meehan WP 3rd, Micheli LJ. Concussion results in deficits in neurocognitive functioning. Preface. Clin Sports Med. 2011;30(1):xvii–iii.
Macciocchi SN, Barth JT, Alves W, Rimel RW, Jane JA. Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery. 1996;39(3):510–4.
Ellemberg D, Leclerc S, Couture S, Daigle C. Prolonged neuropsychological impairments following a first concussion in female university soccer athletes. Clin J Sport Med. 2007;17(5):369–74.
McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. J Am Med Assoc. 2003;290(19):2556–63.
Lee YM, Odom MJ, Zuckerman SL, Solomon GS, Sills AK. Does age affect symptom recovery after sports-related concussion? A study of high school and college athletes. J Neurosurg Pediatr. 2013;12(6):537–44.
Erlanger D, Kaushik T, Cantu R, Barth JT, Broshek DK, Freeman JR, et al. Symptom-based assessment of the severity of a concussion. J Neurosurg. 2003;98(3):477–84.
Zuckerman SL, Kuhn A, Dewan MC, Morone PJ, Forbes JA, Solomon GS, et al. Structural brain injury in sports-related concussion. Neurosurgical Focus. 2012;33(6):E6:1–12.
Guskiewicz KM, Riemann BL, Perrin DH, Nashner LM. Alternative approaches to the assessment of mild head injury in athletes. Med Sci Sports Exerc. 1997;29(7 Suppl):S213–21.
Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263–73.
Peterson CL, Ferrara MS, Mrazik M, Piland S, Elliott R. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230–7.
Covassin T, Elbin RT, Larson E, Kontos A. Sex and age difference in depression and baseline sport-related concussion neurocognitive performance and symptoms. Clin J Sport Med. 2012;22:98–104.
McCrea M, Barr WB, Guskiewicz K, Randolph C, Marshall SW, Cantu R, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropyschol Soc. 2005;11(1):58–69.
Echemendia RJ, Putukian M, Mackin RS, Julian L, Shoss N. Neuropsychological test performance prior to and following sports-related mild traumatic brain injury. Clin J Sports Med. 2001;11(1):23–31.
Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes.J. Pediatrics. 2003;142(5):546–53.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals Intern Med. 2009;151(4):264–9, W64.
Hedges L, Olkin I. Methods for Meta-Analysis. New York: Academic Press; 1985.
Dunlop W, Cortina J, Vaslow J, Burke M. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–7.
Detsky AS, Naylor CD, Orourke K, Mcgeer AJ, Labbe KA. Incorporating variations in the quality of individual randomized trials into metaanalysis. J Clin Epidemiol. 1992;45(3):255–65.
Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. J Am Med Assoc. 1999;282(11):1054–60.
Lipsey M, Wilson DB. Practical meta-analysis. Newbury Park: Sage; 2001.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Gleser L, Olkin I. Stochastically dependent effect sizes. New York: Sage; 1994.
Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006. Win;31(4):437–48.
Brown NJ, Mannix RC, O’Brien MJ, Gostine D, Collins MW, Meehan WP 3rd. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133(2):e299–304.
Broglio SP, Macciocchi SN, Ferrara MS. Neurocognitive performance of concussed athletes when symptom free. J Athl Train. 2007;42(4):504–8.
Lau BC, Collins MW, Lovell MR. Cutoff scores in neurocognitive testing and symptom clusters that predict protracted recovery from concussions in high school athletes. Neurosurg. 2012;70(2):371–9 (discussion 9).
Notebaert AJ, Guskiewicz KM. Current trends in athletic training practice for concussion assessment and management. J Athl Train. 2005;40(4):320–5.
Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurg. 2007;60(6):1050–7 (discussion 7–8).
McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport—the 3rd international conference on concussion in sport held in Zurich, November 2008. J Inj Funct Rehab. 2009;1(5):406–20.
Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250–7.
McCrea M, Guskiewicz K, Marshall S. Acute effects and recovery time following concussion in collegiate football players. Sports Med Update. 2004;38:369–71.
Register-Mihalik JK, Guskiewicz KM, McLeod TC, Linnan LA, Mueller FO, Marshall SW. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Athl Train. 2013;48(5):645–53.
Register-Mihalik JK, Linnan LA, Marshall SW, Valovich McLeod TC, Mueller FO, Guskiewicz KM. Using theory to understand high school aged athletes’ intentions to report sport-related concussion: Implications for concussion education initiatives. Brain injury (BI). 2013;27(7–8):878–86.
Delaney JS, Lacroix VJ, Gagne C, Antoniou J. Concussions among university football and soccer players: a pilot study. Clin J Sport Med. 2001;11(4):234–40.
Delaney JS, Lacroix VJ, Leclerc S, Johnston KM. Concussions among university football and soccer players. Clin J Sport Med. 2002;12(6):331–8.
Kaut KP, DePompei R, Kerr J, Congeni J. Reports of head injury and symptom knowledge among college athletes: implications for assessment and educational intervention. Clin J Sport Med. 2003;13(4):213–21.
Sefton J, Pirog K, Capitao A, Harackiewicz D, Cordova M. An examination of factors that influence knowledge and reporting of milf traumatic brain injuries in collegiate football (Abstract). J Athl Train. 2004;39(S52–S53).
Sullivan SJ, Bourne L, Choie S, Eastwood B, Isbister S, McCrory P, Gray A. Understanding of sport concussion by the parents of young rugby players: a pilot study. Clin J Sport Med. 2009;19(13):228–30.
McLeod TCV, Schwartz C, Bay RC. Sport-related concussion misunderstandings among youth coaches. Clin J Sports Med. 2007;17(2):140–2.
Sarmiento K, Mitchko J, Klein C, Wong S. Evaluation of the Centers for Disease Control and Prevention’s concussion initiative for high school coaches: “heads up: concussion in high school sports”. J Schol Health. 2010;80(3):112–8.
Broglio SP, Puetz TW. The effect of sport concussion on neurocognitive function, self-report symptoms and postural control : a meta-analysis. Sports Med. 2008;38(1):53–67.
Maddocks D, Saling M. Neuropsychological deficits following concussion. Brain injury (BI). 1996;10(2):99–103.
Collins MW, Grindel SH, Lovell MR, Dede DE, Moser DJ, Phalin BR, et al. Relationship between concussion and neuropsychological performance in college football players. J Am Med Assoc. 1999;282(10):964–70.
Eckner JT, Kutcher JS, Broglio SP, Richardson JK. Effect of sport-related concussion on clinically measured simple reaction time. Br J Sports Med. 2014;48(2):112–8.
Eckner JT, Kutcher JS, Richardson JK. Effect of concussion on clinically measured reaction time in 9 NCAA division I collegiate athletes: a preliminary study. J Inj Funct Rehab. 2011;3(3):212–8.
Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain injury (BI). 2006;20(3):245–52.
Fineman I, Giza C, Nahed B, Lee S, Hovda D. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J Neurotrauma. 2000;17(9):739–49.
Ip E, Giza C, Griesbach G, Hovda D. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J Neurotrauma. 2002;19(5):573–85.
Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Nerurol. 2005;196(1):126–37.
Fazio VC, Lovell MR, Pardini JE, Collins MW. The relation between post concussion symptoms and neurocognitive performance in concussed athletes. Neurorehabil. 2007;22(3):207–16.
Covassin T, Elbin RJ, Bleecker A, Lipchik A, Kontos AP. Are there differences in neurocognitive function and symptoms between male and female soccer players after concussions? Am J Sports Med. 2013;41(12):2890–5.
Funding
Dr. Giza is supported by the Department of Defense, the Joseph Drown Foundation, the National Collegiate Athletic Association (NCAA), the National Institutes of Health (NIH), Today’s and Tomorrow’s Children Fund, University of California, Los Angeles (UCLA) Brain Injury Research Center (BIRC), UCLA Steve Tisch BrainSPORT.
Conflict of interests
Richelle Williams declares no conflict. Tim Puetz declares no conflict. Steven Broglio declares no conflict. Chris Giza has received funding from many sources with interest in sports and concussions, and also serves on multiple advisory committees for sports organizations. For the purpose of this manuscript, these did not directly influence the creation of the study, but are included in an effort to have transparency and full disclosure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, R.M., Puetz, T.W., Giza, C.C. et al. Concussion Recovery Time Among High School and Collegiate Athletes: A Systematic Review and Meta-Analysis. Sports Med 45, 893–903 (2015). https://doi.org/10.1007/s40279-015-0325-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-015-0325-8