Abstract
As part of its single technology appraisal process, the UK National Institute for Health and Care Excellence (NICE) invited the manufacturer of obinutuzumab (Roche) to submit evidence on its clinical and cost effectiveness when used in combination with bendamustine in patients with follicular lymphoma (FL) refractory to rituximab. The Evidence Review Group (ERG), the School of Health and Related Research Technology Appraisal Group at the University of Sheffield, produced a document summarising the key points from the company submission alongside a critical review. Efficacy for progression-free survival (PFS) and safety was positively demonstrated in the pivotal GADOLIN trial, which compared obinutuzumab in combination with bendamustine followed by obinutuzumab maintenance (O-Benda+O) against bendamustine monotherapy. Data on overall survival were immature. The company submitted a model-based economic analysis, including a patient access scheme. The ERG identified a number of limitations, in particular the absence of subgroup analysis and the approach used by the company to estimate overall survival (OS), which was more favourable to the intervention arm. The key uncertainty was the duration of the treatment effect on OS. This uncertainty is expected to be reduced when the final analysis of the GADOLIN trial is reported. Consequently, the NICE appraisal committee recommended O-Benda+O in the population covered by the marketing authorisation within the Cancer Drug Fund until NICE is able to review the guidance following publication of the final analysis of GADOLIN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obinutuzumab in combination with bendamustine appears to have an acceptable/manageable adverse event profile and is efficacious compared with bendamustine monotherapy in reducing the risk of progression in patients with follicular lymphoma refractory to rituximab. |
Data on overall survival from the GADOLIN trial were immature. |
Obinutuzumab in combination with bendamustine could be cost effective compared with bendamustine monotherapy in patients with follicular lymphoma refractory to rituximab, depending on the extent of survival gain. |
The final analysis of GADOLIN will reduce the uncertainty on the extent of overall survival gain and produce a more robust cost-effectiveness estimate. |
A key limitation was absence of subgroup analysis and the approach used to estimate overall survival. |
1 Introduction
The UK National Institute for Health and Care Excellence (NICE) is an independent organisation whose responsibilities include providing national guidance to the national health service (NHS) in England and Wales on health technologies [1]. The NICE single technology appraisal (STA) process usually covers new single health technologies within a single indication soon after they have received UK marketing authorisation.
Within this process, the company submits evidence on the clinical and cost effectiveness of the technology in the form of a written document alongside a mathematical economic model. The company submission (CS) is then reviewed by an external independent group, the evidence review group (ERG), with advice from clinical specialists. Findings from the ERG are summarised in the ERG report [2].
The NICE appraisal committee (AC) then consider the evidence submitted by the company and the ERG report alongside testimony from experts and other stakeholders. A final appraisal determination (FAD) is produced directly when the intervention is recommended without restriction. Otherwise, an appraisal consultation document (ACD) is initially produced, followed by an FAD if the recommendations from the NICE AC are restrictive or additional clarification/analyses are required from the company.
This paper presents a summary of the ERG report [2] and FAD [3] for the STA of obinutuzumab with bendamustine for treating follicular lymphoma (FL) refractory to rituximab. This paper also covers the subsequent development of the NICE guidance for the use of this drug in England. Full details of all relevant appraisal documents can be found on the NICE website [4].
2 Decision Problem
FL is an indolent non-Hodgkin lymphoma (NHL). It is the second most common NHL diagnosed in the USA and Western Europe, accounting for over 35% of all NHLs and 70% of indolent lymphomas. The diagnosis of FL is typically confirmed by surgical specimen/biopsy and histological report and reviewed by an expert haematologist. The treatment of FL is dependent on the stage of the disease, usually determined using the Ann Arbor system.
Indolent NHLs are chronic diseases characterised by repeated relapses requiring treatment and periods of disease progression. Standard therapeutic approaches focus on disease control. Patients with indolent lymphomas are usually considered incurable with standard therapeutic approaches.
The treatment pathway in FL is complex, and treatment options are limited after patients become refractory to rituximab [5]. Most patients needing treatment receive first-line induction treatment with a rituximab-containing regimen, followed by rituximab maintenance therapy. Second-line treatment for FL depends on the type of regimen used first-line and the timing of relapse following first-line treatment. Patients with FL who do not respond to first-line induction treatment with a rituximab-containing regimen are considered refractory and would typically receive bendamustine monotherapy. The choice of second-line treatment is less clear in patients who respond to first-line induction treatment with a rituximab-containing regimen but relapse during or within 6 months of completion of maintenance therapy. Typically, patients relapsing early within the maintenance phase would be considered refractory to rituximab and would be treated with bendamustine monotherapy. Patients who experience a relapse after some time on maintenance treatment with rituximab or at the end of maintenance treatment may not be considered refractory and may receive rituximab in combination with an alternative agent as second-line treatment.
Obinutuzumab is a type II anti-cluster of differentiation (CD)-20 antibody that targets the extracellular loop of the CD20 transmembrane antigen on the surface of non-malignant and malignant pre-B and mature B-lymphocytes but not on haematopoietic stem cells, pro-B cells, normal plasma cells or other normal tissue. Obinutuzumab is available as a liquid concentrate solution for infusion. Each pack contains one vial containing 1000 mg of obinutuzumab.
Obinutuzumab is indicated for the treatment of patients with FL refractory to rituximab and is given in combination with bendamustine as induction treatment (6 cycles of 28 days), followed by a maintenance phase with obinutuzumab monotherapy every 2 months for up to 2 years or until progression in patients who do not progress at the end of the induction phase.
3 Independent Evidence Review Group (ERG) Report
In accordance with the process for STAs, the ERG and NICE had the opportunity to seek clarification on specific points in the CS [6], in response to which the company provided additional information [7, 8]. The ERG also modified the company’s decision analytic model to produce an ERG base case and to assess the impact of alternative parameter values and assumptions on the model results. The evidence presented in the CS and the ERG’s review of that evidence is summarised here.
3.1 Clinical Evidence Provided by the Company
The CS [6] included a systematic review of the clinical effectiveness literature. The main supporting evidence was derived from GADOLIN [9,10,11,12,13,14]. This study was a company-sponsored, randomised, open-label, event-driven, multicentre study. It was designed to compare the efficacy and safety of induction therapy with obinutuzumab in combination with bendamustine followed by obinutuzumab maintenance therapy (O-benda+O) with bendamustine monotherapy as induction therapy in 413 patients (57.6% male; 87.4% Caucasian; mean age 62 years) with rituximab-refractory indolent NHL (81% had FL [population defined in the marketing authorisation of obinutuzumab] and 19% had non-FL).
3.1.1 Clinical Study Design
GADOLIN [9,10,11,12,13,14] was a phase III trial that consisted of three phases, including an induction phase (approximately 6 months), a maintenance/follow-up phase (2 years) and an extended follow-up phase (2 years). For the induction phase (six 28-day cycles, all treatments given intravenously), patients with rituximab-refractory indolent NHL (defined as a lack of response during treatment or progression within 6 months following the last dose of rituximab or a rituximab-containing regimen [including rituximab monotherapy as part of induction or maintenance treatment]) were randomly allocated to receive either obinutuzumab (1000 mg on days 1, 8 and 15 of cycle 1; and on day 1 only of cycles 2–6) in combination with bendamustine (90 mg/m2/day on days 1 and 2 for cycles 1–6; n = 204) or bendamustine monotherapy (120 mg/m2/day on days 1 and 2 for cycles 1–6; n = 209). Patients in the obinutuzumab plus bendamustine group without evidence of disease progression (i.e., patients with a complete response, partial response or stable disease) following induction received obinutuzumab maintenance therapy (1000 mg every 2 months) for up to 2 years. In contrast, as there was no equivalent maintenance phase in the bendamustine monotherapy group, these patients received no further active treatment (e.g. anti-lymphoma treatments) after completion of the bendamustine induction phase and therefore entered the follow-up phase of the study. Thereafter, all patients entered a 2-year extended follow-up phase. The primary outcome measure was progression-free survival (PFS), defined as the time from randomisation to first occurrence of progression or relapse, or death from any cause on study, as assessed by an independent review committee (IRC).
The data cut-off for the primary analysis of efficacy and futility was 1 September 2014 [6] and took place after 175 IRC-assessed PFS events had occurred. As the primary endpoint had been reached, an Independent Data Monitoring Committee recommended that the study be unblinded to the sponsor and fully analysed and the results made public. Additional post hoc exploratory analyses (updated analysis) were conducted based on an additional data cut-off of 1 May 2015 [6]. The overall median observation time (randomisation to last available assessment) for the FL population at the time of the updated clinical cut-off was 24.1 months in both groups. During the appraisal consultation period, the company provided revised updated data (with approximately 3 years of follow-up to April 2016) [7]. However, these data were marked academic-in-confidence and cannot be reproduced.
3.1.2 Clinical Study Results
3.1.2.1 Clinical Effectiveness
Based on analyses of the data available in May 2015 [6], treatment of the FL subgroup (i.e. the main target population in the CS; n = 335) with O-benda+O was associated with a statistically significant improvement in the risk of a PFS event as assessed by the IRC compared with bendamustine monotherapy (hazard ratio [HR] 0.47; 95% confidence interval [CI] 0.34–0.64; p < 0.0001, stratified log-rank test) resulting in an absolute increase in median IRC-assessed PFS of 15.4 months. For other secondary endpoints (based on IRC assessments) at the end of the induction period, there were no statistically significant differences in best overall response rates (p = 0.5098) or end-of treatment response rates (p = 0.6972) between treatment arms. However, the duration of response for patients who achieved a complete or partial response and disease-free survival for complete responders in the study were reported to be significantly longer in the O-benda+O group (median not reached) than in the bendamustine group (median 11.6 months for duration of response [p = not reported] and 13.0 months for disease-free survival [p = not reported]). An analysis of event-free survival found that statistically fewer patients with FL had an event with O-benda+O compared with bendamustine alone (p < 0.001). Although overall survival (OS) data were not mature at the time of the analysis, statistically fewer patients with FL in O-benda+O (18.3% [30/164]) had died compared with those in the bendamustine monotherapy group (28.1% [48/171]) at median follow-up of 24.1 months (p = 0.0379).
3.1.2.2 Safety
Adverse-event data were collected for all patients who had any component of obinutuzumab or bendamustine treatment in GADOLIN. At the last data-cut (1 May 2015), 98.8% of patients with FL in both trial arms had at least one adverse event (any grade). In the O-Benda+O arm (n = 164), 39.0% of patients had a serious treatment-related adverse event compared with 34.5% in the bendamustine monotherapy arm (n = 168). The most common serious adverse events were neutropenia (32.3 vs. 24.4%), infections (15.9 vs. 19.6%), thrombocytopenia (11.0 vs. 14.9%), infusion-related reactions (9.1 vs. 3.6%) and cardiac events (4.9 vs. 1.2%) [6].
3.1.2.3 Indirect Comparison and/or Multiple Treatment Comparison
As there was no connected network of evidence, the company was unable to make any indirect comparisons with other relevant interventions identified in the scope, e.g. chemotherapy regimens without rituximab (such as cyclophosphamide- or fludarabine-containing regimens or chlorambucil) or best supportive care.
3.2 Critique of the Clinical Evidence and Interpretation
3.2.1 Critique of Systematic Review
The systematic review process followed by the company was reasonably comprehensive. Despite minor limitations in the company’s search strategy, the ERG was confident that all relevant controlled studies of obinutuzumab in combination with bendamustine monotherapy for the treatment of rituximab-refractory FL were included in the CS, including data from ongoing or planned studies. However, the ERG was not confident that all relevant non-controlled studies had been identified and included in the CS, as details of the systematic review process (e.g. identification, selection, etc.) were lacking in the CS. The specified inclusion and exclusion criteria were mostly appropriate and generally reflected the decision problem set out in the final NICE scope [15]. The ERG considered the quality assessment tool used to appraise the included GADOLIN study was appropriate.
3.2.2 Critique of Clinical Evidence
Although the efficacy of obinutuzumab in combination with bendamustine compared with bendamustine monotherapy in GADOLIN appeared favourable, and the safety appeared acceptable, there were a number of limitations and uncertainties in the evidence base that warrant caution in its interpretation.
3.2.2.1 Limitations of the Randomized Controlled Trial
A key issue that may limit the robustness of the efficacy and safety data reported in the CS [6] related to the design of GADOLIN. In this open-label study, patients and investigators were all unblinded to the assigned treatment. Double-blinding protects against performance bias and measurement bias [16], and its absence in randomized controlled trials (RCTs) tends to result in larger treatment effects [17]. With many cytotoxic cancer drugs, the nature of the intervention precludes blinding (i.e. drug toxicities or manner of administration) for the practical and ethical reason that informed dose monitoring and adjustment is required. Although it is almost universally absent from oncology trials, blinded outcome assessment can enhance bias reduction [18].
Another issue that may have limited the robustness of the efficacy and safety data reported in the CS [6] related to the subgroup analysis of participants in GADOLIN with FL that was refractory to rituximab or a rituximab-containing regimen (the population defined in the marketing authorisation of obinutuzumab). The study was not powered for this subgroup analysis, and the protection for unknown confounders provided by randomization may have been lost. In addition to the known limitations of subgroup analyses [19], further limitations of the subgroup include the slight imbalances of relevant prognostic factors (such as disease stage), small sample sizes and lack of statistical power to detect a clinically relevant difference between study groups. As a result, these results should be treated with caution. In addition, GADOLIN was designed to assess PFS benefit after induction and maintenance treatment as a whole, so the relative contribution of each treatment phase was difficult to assess.
3.2.2.2 Uncertainties Generated by the Evidence
The main uncertainty in the evidence base related to the lack of any head-to-head RCTs comparing obinutuzumab in combination with bendamustine with other relevant interventions such as chemotherapy regimens without rituximab (other than bendamustine monotherapy) or best supportive care for the treatment of rituximab-refractory FL. In addition, GADOLIN included a mixed population of three distinct subgroups of patients with FL: patients refractory to induction treatment with rituximab monotherapy, patients refractory to induction treatment with rituximab-chemotherapy and patients refractory during or within 6 months of completing maintenance treatment with rituximab maintenance therapy. In the UK, rituximab monotherapy is rarely used as induction treatment, and patients who relapse during or within 6 months of completing maintenance treatment with rituximab (after responding to rituximab in combination with chemotherapy) would typically be re-treated with rituximab in combination with alternative chemotherapies (and would not be considered ‘truly’ refractory). As a result, in the UK, bendamustine monotherapy would mostly be considered an appropriate comparator in patients refractory to induction treatment with rituximab-chemotherapy (where the chemotherapy used is not bendamustine). Although a few centres from the UK were included within the pivotal study, the subgroup populations of the GADOLIN trial are not an absolute reflection of the population with FL in the UK. Furthermore, as noted by Hamlin [20], in current practice rituximab plus bendamustine is increasingly being used as a first-line treatment regimen; as a result, the relevance of GADOLIN to the UK is unclear, particularly in patients previously exposed to bendamustine.
3.3 Cost-Effectiveness Evidence Submitted by The Company
As part of its submission to NICE, the company submitted a model-based health economic analysis in Microsoft® Excel. The analysis was undertaken from the perspective of the UK national health service (NHS) and Personal Social Services (PSS) over a lifetime horizon. All costs and health outcomes were discounted at a rate of 3.5% per annum [1].
The economic analysis was based on the clinical-effectiveness evidence for the FL subgroup of the population enrolled in GADOLIN [9,10,11,12,13,14] and assessed the cost effectiveness of O-benda+O versus bendamustine monotherapy in adults with FL who did not respond or who progressed during or within 6 months of completing treatment with rituximab.
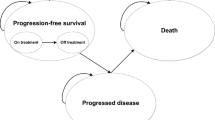
The company base-case model had three main health states; progression-free (separated into on- and off-treatment phase); progressed disease; and death. The model adopted a semi-Markov approach whereby OS is estimated indirectly from PFS and post-progression survival (PPS). This approach was justified by the company owing to the immaturity of the OS data in GADOLIN and the indolent nature of the condition.
Parametric survival functions (Weibull in the base case) were fitted to PFS separately for each arm of GADOLIN [9,10,11,12,13,14]. Patients leaving the progression-free state who had not died before progression moved to the progressed disease state. Tunnel states were used to allow the probability of death in the progressed disease state to be dependent on time since progression. Parametric survival functions were fitted to post-progression data pooled across both arms of GADOLIN. The Kaplan–Meier survival function for time-to-off-treatment from GADOLIN was used to estimate the duration of treatment.
The utility values in the base case were taken from a published UK study [21]. A sensitivity analysis was conducted using utility values from GADOLIN. Resource use associated with the management of FL (visits to the haematologist, diagnostic/laboratory tests, examinations and computed tomography scans, management of adverse events) was derived from UK guidelines [5] and previous evaluations of treatments for FL [22] uplifted to 2015 values when appropriate.
The company provided an initial confidential patient access scheme (PAS) offering obinutuzumab at a discount price to the NHS. The company’s deterministic base-case incremental cost-effectiveness ratio (ICER) was confidential. However, the company’s base-case ICER did not vary greatly in the majority of the sensitivity and scenario analyses presented by the company, with the exception of changes in the survival functions used for PFS and PPS.
3.4 Critique of the Cost-Effectiveness Evidence and Interpretation
The ERG critically appraised the company’s health economic analysis and the model upon which the analysis was based. As part of its critical appraisal, the ERG checked the calculations in the company’s economic model to identify any programming errors and/or inconsistencies. No major programming errors were identified in the company’s model during this process.
A key concern from the ERG was that the CS failed to provide a subgroup analysis according to whether patients were refractory to induction treatment with rituximab-chemotherapy or were refractory during or within 6 months of completing maintenance treatment with rituximab monotherapy. The ERG considered this to be an important limitation, as the population included in GADOLIN [9,10,11,12,13,14] was broader than the population that would be considered refractory in the UK and that would be eligible for bendamustine monotherapy.
The ERG also raised concerns regarding the generalisability of patients recruited in the trial to the UK setting, given that bendamustine in combination with rituximab is widely used in first-line treatment, therefore limiting the subsequent use of bendamustine in combination or not in later lines of treatment. GADOLIN [9,10,11,12,13,14] also included a proportion of patients who were refractory to rituximab monotherapy, which is rarely used in the UK.
The ERG further considered that the method used to estimate OS within the company model may have biased the cost-effectiveness estimates in favour of O-benda+O as it underestimated survival in the bendamustine arm when compared with the Kaplan–Meier data from GADOLIN.
The representation of the treatment pathway in the company’s model was also considered to be overly simplistic. The treatment pathway in FL is complex and may depend on the previous line of treatment, the time of relapse, and the patient’s characteristics, amongst other factors. For instance, allogeneic stem cell transplant was not considered despite being used in the UK in patients who are fit enough and who are in their second or subsequent remission.
There was also uncertainty about the most appropriate parametric extrapolation for PFS and PPS and the assumption of constant pre-progression mortality. Utility value point estimates used in the economic model were also uncertain. The ERG further considered that utility values needed to be adjusted for age-related declines in utility.
Finally, the ERG was concerned that the model did not adequately reflect subsequent lines of therapy for this population, particularly as a significant proportion were assumed to go on to rituximab-containing regimens, which seems unlikely in a rituximab refractory population.
3.5 Additional Work Undertaken by the ERG
The ERG undertook a number of analyses that informed its preferred base case. The main changes informing the ERG’s preferred base case were:
-
the use of an alternative approach (a partitioned survival approach) to estimate OS
-
adjustment of utility by age
-
assuming a lower cost for subsequent treatments in post-progression
-
using the cost for generic bendamustine
-
amendment to drug administration costs and
-
corrections of minor errors identified by the ERG.
In the ERG’s partitioned survival approach, the parametric survival functions fitted to the Kaplan–Meier survival functions were used to estimate parametric OS survival functions for both arms. However, rather than assuming a lifetime treatment effect, the OS survival function fitted to the O-benda+O arm was only applied up to the last event (31 months) with the hazard of death predicted by the bendamustine monotherapy arm applied thereafter. The ERG considered it reasonable not to assume a lifetime treatment effect given that the treatment duration was limited to 2.5 years (including both induction and maintenance therapy) and the OS data were still immature.
In addition to the ERG’s preferred base case conducted within the whole GADOLIN FL [9,10,11,12,13,14] population, the ERG undertook an exploratory analysis for the subgroup of patients with FL refractory to rituximab-chemotherapy induction. The ERG conducted this analysis because this was the subgroup most likely to be deemed ‘truly’ refractory to rituximab and to be offered bendamustine monotherapy in England. Nevertheless, despite the relevance of this subgroup, the ERG cautioned that this was a non-randomised subgroup and that the analysis relied on a smaller sample size.
3.6 Conclusion of the ERG Report
The ERG concluded that the efficacy (PFS) of obinutuzumab in combination with bendamustine was positively demonstrated (compared with bendamustine monotherapy) in GADOLIN [9,10,11,12,13,14] and that its safety profile was acceptable.
However, GADOLIN [9,10,11,12,13,14] had some issues with generalisability. In particular, the ERG did not consider the subgroup of patients with FL refractory to induction treatment with rituximab monotherapy to be relevant to UK clinical practice as induction treatment with rituximab monotherapy is rarely used in the UK. Similarly, the ERG did not consider bendamustine monotherapy to be an appropriate comparator in patients with FL who relapsed during or within 6 months of completing maintenance treatment with rituximab monotherapy (following successful induction with rituximab-chemotherapy).
The survival data on which the cost-effectiveness estimates were based were immature, and this increased the uncertainty associated with the ICERs. A key concern from the ERG was the company’s approach to estimating OS. The ERG considered that the method used to estimate OS within the company model may have biased the cost-effectiveness estimates in favour of O-benda+O as it underestimated survival in the bendamustine arm when compared with the Kaplan–Meier survival function from GADOLIN. The ERG’s alternative approach provided a better fit to the observed OS data in the bendamustine arm without significantly altering the estimates for OS in the O-benda+O arm.
ICERs were confidential but the ERG’s alternative approach to model OS increased the ICER.
3.7 Additional Evidence Submitted by the Company in Response to the Appraisal Consultation Document and Comments from the ERG
Following preliminary guidance in the ACD, the company submitted additional evidence to support the use of obinutuzumab in patients with FL that is refractory to rituximab [7].
The company’s response to the ACD included both longer-term follow-up data from GADOLIN and a revised economic model. The company also proposed a revised PAS.
The company provided updated clinical effectiveness and safety results from GADOLIN using a data cut-off of 1 April 2016, but these results were academic in confidence and cannot be reproduced here.
The revised economic model submitted after the ACD used a partitioned survival approach as recommended by the ERG, but the survival functions for OS were fitted to the updated data, and the OS survival function for O-benda+O was applied up to the longest observed follow-up period (5.5 years) rather than the last observed event (4.0 years). Different durations of treatment effect (4.0 years, 7 years and lifetime) were explored in sensitivity analyses. The following changes were also made:
-
Use of PFS assessed by investigator (second of secondary endpoint) instead of PFS assessed by IRC based on latest data-cut from GADOLIN
-
Updated data on time on treatment and adverse events based on latest data-cut from the GADOLIN trial
-
Use of ERG’s preferred assumptions (see Sect. 3.5)
-
Use of separate utility values for patients on and off treatment in the progression-free state based on data from GADOLIN.
The ERG considered that the updated analysis of GADOLIN confirmed the benefits of O-benda-O versus bendamustine monotherapy.
However, a key concern from the ERG was not addressed in that results were only presented for the whole subgroup of patients with FL from GADOLIN rather than the subgroup refractory to rituximab-chemotherapy induction.
The ERG was generally satisfied with the approach taken by the company in the revised economic model but noted the assumption made by the company about the duration of the treatment effect. The ERG also identified a small inconsistency that was subsequently corrected.
4 Key Methodological Issues
The key methodological issue in this appraisal was the approach to modelling OS given the immaturity of the GADOLIN trial data. The company used a semi-Markov model whereby OS was estimated indirectly from PFS and PPS. However, this approach provided a poor fit to the bendamustine monotherapy arm. Whilst the ERG considered the use of a semi-Markov approach to be generally acceptable, the ERG highlighted that the choice of modelling approach should be guided by the quality of the data available and the face validity of the model.
In light of this, the ERG suggested an alternative modelling approach; the partitioned survival model. The ERG considered that a partitioned survival approach provided a more realistic OS survival functions for both arms
When using the partitioned survival model, a decision must be made as to whether patients are assumed to follow the parametric survival function that has been fitted to the trial data for their whole lifetime, which is equivalent to assuming a life-long treatment effect, or whether the treatment effect observed in the trial is assumed to end at some point. In this case, the ICERs were very sensitive to the duration of treatment effect assumed when extrapolating OS, suggesting that the collection of further evidence to quantify long-term OS would be necessary.
5 National Institute for Health and Care Excellence Guidance
In July 2017, on the basis of the evidence available, the NICE AC produced the following final guidance to the NHS in England (TA472) [3]. Obinutuzumab in combination with bendamustine followed by obinutuzumab maintenance is recommended for use within the Cancer Drugs Fund as an option for treating FL that did not respond or progressed during or up to 6 months after treatment with rituximab or a rituximab-containing regimen, only if the conditions in the managed access agreement for obinutuzumab are followed [3].
The committee discussed the population included in GADOLIN and concluded that the most relevant population was patients with disease that is refractory to induction with rituximab-chemotherapy or who relapse early during rituximab maintenance. Clinical experts echoed the view from the ERG that induction treatment with rituximab monotherapy was not the current standard of care in England, although the committee heard that this may change following the recent publication of NICE’s guidance on NHL. The committee agreed that the population who might be offered obinutuzumab in combination with bendamustine was potentially broader than those patients with rituximab-chemotherapy-refractory disease and therefore would not limit its consideration to this subgroup.
The committee discussed the clinical effectiveness from GADOLIN and noted an improvement in PFS despite the lack of difference in response rate at the end of induction. The committee was unclear whether the improvement in PFS was attributable to the inclusion of a maintenance phase for obinutuzumab or a better type of response in the intervention arm (as shown using data on minimum residual disease) [3].
The committee discussed the relationship between PFS and OS but noted that, because the data were immature, the relationship was unclear. Longer follow-up data submitted by the company after the ACD were considered relevant by the committee [3], but the committee felt that the magnitude of any survival gain remained uncertain.
The committee considered the revised company base case, which used the alternative modelling approach suggested by the ERG and noted that the ICER was very sensitive to the assumption regarding the duration of treatment effect on OS. The committee noted that the ICERs were above what is normally considered a cost-effective use of NHS resources when the duration of treatment effect was assumed to be < 7 years. Given the large uncertainty, the committee concluded that, because the duration of treatment effect on OS was the main driver in the model, the cost effectiveness should be based on the final analysis of GADOLIN, which is expected to be reported in 2019 [3].
The committee concluded that O-Benda-O could not be recommended for routine use as the company and ERG base-case ICERs were above the level that could be accepted. However, given the possibility for O-Benda-O to be cost effective when more mature OS data become available, the committee discussed whether it could be recommended within the Cancer Drug Fund. Under this arrangement, “drugs that appear promising, but for which the evidence is not robust enough, may be given a conditional recommendation by NICE and made available to NHS through the Cancer Drugs Fund” [3].
The committee noted that scenario analyses conducted by the company suggested that O-Benda-O had the potential to be considered cost effective when the treatment duration effect on OS was increased alongside the revised PAS. The committee reiterated the immaturity of the data and noted that more mature data, which are expected in a few years, would produce a more robust cost-effectiveness estimate and resolve the uncertainty around the duration of treatment effect.
In conclusion, the committee decided to recommend O-Benda+O within the Cancer Drugs Fund for the population covered by the marketing authorisation until NICE is able to review the guidance based on the final analyses from GADOLIN [3].
6 Conclusions
Efficacy for PFS was demonstrated in GADOLIN. Data on OS were promising but immature. The cost-effectiveness estimate was largely dependent upon the duration of treatment effect on OS, and the committee concluded that the cost effectiveness should be based on the final analysis of GADOLIN but recommended obinutuzumab within the Cancer Drugs Fund for the population covered by the marketing authorisation until the final analyses from GADOLIN are available.
References
National Institute for Health and Care Excellence, Guide to the methods of technology appraisal. London UK, 2013: Available at: https://www.nice.org.uk/article/pmg9/ [Last accessed 07 March 2018].
Rafia R, P.A., Stevens J, Harnan S, Davis S, Clowes M, McMillan A, Sorour Y, Cutting R, Obinutuzumab in combination with bendamustine for treating rituximab-refractory follicular lymphoma: a single technology appraisal. School of Health and Related Research (ScHARR), 2016: Available at: https://www.nice.org.uk/guidance/ta472/documents/appraisal-consultation-document-2 [Last acessed 07 March 2018].
National Institute for Health and Care Excellence, Final appraisal determination: Obinutuzumab with bendamustine for treating follicular lymphoma refractory to rituximab. London UK, 2016: Available at: https://www.nice.org.uk/guidance/ta472/documents/final-appraisal-determination-document [Last accessed 07 March 2018].
National Institute for Health and Care Excellence, Obinutuzumab with bendamustine for treating follicular lymphoma refractory to rituximab; Technology appraisal guidance [TA472]. London UK, 2017: Available at: https://www.nice.org.uk/guidance/ta472/history [Last accessed 07 March 2018].
National Institute for Health and Care Excellence, Non-Hodgkin’s lymphoma: diagnosis and management: NICE guideline [NG52]. London UK, 2016: Available at: https://www.nice.org.uk/guidance/ng52 [Last accessed 07 March 2018].
Roche Products Limited, Gazyvaro▼ (obinutuzumab) in combination with bendamustine for treating rituximab-refractory follicular lymphoma. Company evidence submission to NICE, 2016: Available at: https://www.nice.org.uk/guidance/ta472/documents/appraisal-consultation-document-2 [Last acessed 07 March 2018].
Roche Products Limited, ID841 Gazyvaro (obinutuzumab) with bendamustine for treating follicular lymphoma refractory to rituximab - Response to ACD. 2016, National Institute for Health and Care Excellence: London UK. Available at: https://www.nice.org.uk/guidance/ta472/documents/appraisal-consultation-document-2 [Last acessed 07 March 2018].
Roche Products Ltd, Gazyvaro▼ (obinutuzumab) in combination with bendamustine for treating rituximab-refractory follicular lymphoma: Response to clarification. 2016: Available at: https://www.nice.org.uk/guidance/ta472/documents/appraisal-consultation-document-2 [Last acessed 07 March 2018].
Cheson B. et al. GADOLIN: primary results from a phase III study of obinutuzumab plus bendamustine compared with bendamustine alone in patients with rituximab-refractory indolent non-Hodgkin lymphoma. In: 13th International Conference on Malignant Lymphoma, 2015: Abstract 123.
Sehn L, et al. GADOLIN: primary results from a phase III study of obinutuzumab plus bendamustine compared with bendamustine alone in patients with rituximab-refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2015;33:8502 (ASCO Annual Meeting).
Pott C. et al. Analysis of minimal residual disease in follicular lymphoma patients in GADOLIN, a Phase III study of obinutuzumab plus bendamustine versus bendamustine in relapsed/refractory indolent non-Hodgkin lymphoma. In: ASH 57th Annual Meeting and Exposition. 2015: Orlando, Florida. Abstract 3978
Cheson B et al Primary results of the health-related quality of life assessment from the Phase III GADOLIN study of obinutuzumab plus bendamustine compared with bendamustine alone in patients with rituximab-refractory, indolent non-Hodgkin lymphoma. In: ASH 57th Annual Meeting and Exposition. 2015: Orlando, Florida. Abstract 1523.
F. Hoffmann-La Roche Ltd, Primary Clinical Study Report—GAO4753g/GO01297—An open-label, multicenter, randomized, Phase III study to investigate the efficacy and safety of bendamustine compared with bendamustine + RO5072759 (GA101) in patients with rituximab-refractory, indolent non-Hodgkin’s lymphoma. Clinical Study Report—1051204. July 2015.
F. Hoffmann-La Roche Ltd, Update Clinical Study Report—GAO4753g/GO01297—An open-label, multicenter, randomized, Phase III study to investigate the efficacy and safety of bendamustine compared with bendamustine + RO5072759 (GA101) in patients with rituximabrefractory, indolent non-Hodgkin lymphoma. Report No. 1067639. December 2015.
National Institute for Health and Care Excellence, Final Scope: Obinutuzumab in combination with bendamustine for treating rituximabrefractory follicular lymphoma. London, UK, 2016: Avaialble at: https://www.nice.org.uk/guidance/ta472/documents/final-scope [Last accessed 07 March 2018].
Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. In: The Cochrane Collaboration. US: Wiley; 2011. http://handbook.cochrane.org.
Schulz KF, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12.
Juni P, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60.
Brookes S, et al. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess. 2001;5(33):1–56.
Hamlin PA. Obinutuzumab plus bendamustine in rituximab-refractory indolent lymphoma. Lancet Oncol. 2016. https://doi.org/10.1016/S1470-2045(16)30157-7.
Wild D, Pettengell R, Walker M, et al. Utility elicitation in patients with follicular lymphoma. Value Health. 2006;9(6):A294.
Papaioannou D, Rafia R, Rathbone J, Stevenson M, Woods HB, Stevens J. Rituximab for the first-line treatment of stage III–IV follicular lymphoma (review of technology appraisal no. 110): a systematic review and economic evaluation. Health Technol Assess. 2012;16(37):1–253, iii–iv.
Acknowledgements
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number 14/177/09 STA). See the HTA programme website for further project information (http://www.hta.ac.uk). This summary of the ERG report was compiled after NICE issued guidance. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of NICE or the Department of Health. The authors also wish to thank Dr. Andrew McMillan for providing clinical advice and commenting on draft materials during the project. This summary was externally reviewed by PharmacoEconomics.
Author information
Authors and Affiliations
Contributions
RR, AP, SD drafted the manuscript and take responsibility as guarantors of the content. JWS, SH, MC, YS and RC revised the manuscript for important intellectual content or provided comments. All authors have given their approval for the final version to be published.
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number 14/177/09 STA). See the HTA programme website for further project information (http://www.hta.ac.uk).
Conflicts of Interest
RR, AP, SD, JWS, SH, MC, YS and RC have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Rafia, R., Pandor, A., Davis, S. et al. Obinutuzumab with Bendamustine for Treating Follicular Lymphoma Refractory to Rituximab: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 36, 1143–1151 (2018). https://doi.org/10.1007/s40273-018-0645-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0645-2