Abstract
Acute kidney injury (AKI) is a commonly reported adverse effect of administration of antimicrobials. While AKI can be associated with poorer outcomes, there is little information available to understand rates of AKI in children exposed to various antimicrobials. We performed a structured review using the PubMed and Embase databases. Articles were included if they provided an AKI definition in patients who were < 19 years of age receiving an antimicrobial and reported the frequency of AKI. Author-defined AKI rates were calculated for each study and mean pooled estimates for each antimicrobial were derived from among all study participants. Pooled estimates were also derived for those studies that reported AKI according to pRIFLE (pediatric risk, injury, failure, loss, end stage criteria), AKIN (acute kidney injury network), or KDIGO (kidney disease improving global outcomes) creatinine criteria. A total of 122 studies evaluating 28 antimicrobials met the inclusion criteria. Vancomycin was the most commonly studied drug: 11,514 courses across 44 included studies. Among the 27,285 antimicrobial exposures, the overall AKI rate was 13.2% (range 0–42.1% by drug), but the rate of AKI varied widely across studies (range 0–68.8%). Cidofovir (42.1%) and conventional amphotericin B (37.0%) had the highest pooled rates of author-defined AKI. Eighty-one studies used pRIFLE, AKIN, or KDIGO AKI criteria and the pooled rates of AKI were similar to author-defined AKI rates. In conclusion, antimicrobial-associated AKI is reported to occur frequently in children, but the rates of AKI varies widely across studies and drugs. Most published studies examined hospitalized patients and heterogeneity in study populations and in author definitions of AKI are barriers to a comparison of nephrotoxicity risk among antimicrobials in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Among published pediatric studies, acute kidney injury (AKI) complicated more than 10% of antimicrobial courses given to children, but there was substantial variation in this rate by drug. Individual drugs’ AKI rate estimates vary widely between different studies reflecting differing AKI definitions, patient populations, and study designs. |
Publication and ascertain bias may also influence the reporting and detection of AKI, respectively, for those drugs less often suspected to cause nephrotoxicity. |
More studies of antimicrobial-associated AKI are needed in children. Future studies should utilize standardized AKI definitions, and more detailed reporting of concomitant nephrotoxic medications to allow for better comparisons of AKI rates among drugs. |
1 Introduction
Acute kidney injury (AKI) is defined by declining renal function, traditionally signaled by increases in serum biomarkers, such as creatinine, and/or decreased urine output [1]. The pathophysiology and mechanisms of AKI are complex and frequently multifactorial but can involve pre-renal causes such as impaired perfusion of the kidneys, intra-renal causes such as pyelonephritis or myoglobin deposition, and post-renal causes such as urinary tract obstruction [1]. Drugs are among the most common causes of AKI exerting their harmful effects through a number of mechanisms including acute tubular injury, acute interstitial nephritis, and crystalline deposition [2]. Drug-related AKI is often denoted with the term nephrotoxicity, although the precise mechanisms of injury differ across agents [3].
Anti-infectives are among the most commonly implicated medications associated with AKI and are also among the most frequently prescribed medications in pediatrics [4,5,6]. Acute kidney injury impacts roughly 1 in 1000 children per year in the community and 6–50% of hospitalized children [7,8,9], and it is associated with a number of adverse outcomes including increased mortality, increased length of hospital and intensive care unit admission, and the subsequent development of chronic kidney disease [10]. Further, dosing of drugs can be challenging in patients with AKI, which may lead to a failure to achieve pharmacodynamic goals, an increased risk of non-renal drug toxicities, and the potential for sub-optimal outcomes [11]. Therefore, it is paramount that clinicians understand the risk of AKI associated with different antimicrobial medications so that they can make informed antibiotic decisions that account for not only the infection being treated, but the risk of toxicity.
However, drug-associated AKI is seldom solely due to drug toxicity. There are several identified risk factors for the development and severity of AKI related to the patient and their disease process, as well as the treatment prescribed. Patient-related factors, such as the presence of chronic pre-existing conditions including chronic kidney disease, diabetes mellitus, and heart disease, may make patients more susceptible to the nephrotoxic effects of drugs. Acute conditions such as sepsis, trauma, or hypovolemia may independently lead to AKI secondary to decrease renal blood flow/perfusion. These conditions are also more common in patients with underlying diseases, further compounding nephrotoxicity risks. Particularly when it comes to antimicrobial-associated AKI, patients and disease-related risk factors for AKI are often contributory, making it difficult to tease out the role of the medication. Yet, there are important factors related to the medications that are known to influence AKI risk: the agent, dose, and duration of use [12, 13]. In this review, we focus solely on the agent, but it is important to recognize nephrotoxicity is most often dose and duration dependent. Administration of higher dosages, as may be indicated for the treatment of more serious or drug-resistant infections, confers an increased risk of direct cellular injury compared with prophylactic or lower dosage regimens. Similarly, although it is hard to investigate the influence that treatment duration has on nephrotoxicity risk (i.e., whether toxicity is related to cumulative drug exposure), it is certain that longer durations of exposure provide an increased opportunity for nephrotoxicity to occur.
In the past, there was no singular definition for pediatric AKI and, thus, comparing rates reported across studies was challenging. However, over the last 15 years, efforts have been made to standardize AKI definitions, resulting in the development of several commonly used criteria including pRIFLE (pediatric risk, injury, failure, loss, end stage criteria), AKIN (acute kidney injury network), and KDIGO (kidney disease improving global outcomes) [14,15,16]. These definitions focus on changes in kidney function manifesting as changes in creatinine and urine output, rather than more novel kidney damage biomarkers that may indicate glomerular or tubular cell injury. Increased awareness of the clinical significance of AKI has also led to the implementation of protocols designed to detect and mitigate AKI in hospitalized children [17]. However, there are a dearth of studies directly comparing AKI rates among different antimicrobials to inform prescribing decisions.
The goals of this study were to summarize the rates of antimicrobial-associated AKI reported across drugs in order to gauge the relative nephrotoxic potential of different antimicrobials in children. We also sought to assess the variability in AKI rates across studies for a given drug and the heterogeneity in AKI definitions used.
2 Methods
2.1 Data Source and Search
The PubMed database was queried for articles published on/after the year 2000 using the following search terms: (English[Language]) AND (((antibiotic OR antiviral OR antifungal OR antimicrobial OR anti-infective OR anti-bacterial) AND (pediatr* OR paediatr* OR child* OR infant OR neonat*)) AND (renal OR kidney) AND (nephrotox* OR injur* OR toxic* OR safet*)). The query took place on 18 November, 2022. The Embase database was also searched on the same day with the terms: (antibiotic OR antiviral OR antifungal OR antimicrobial OR ‘anti infective’ OR ‘anti bacterial’) AND (pediatr* OR paediatr* OR child* OR infant OR neonat*) AND (renal OR kidney) AND (nephrotox* OR injur* OR toxic* OR safet*) AND [english]/lim.
2.2 Study Selection and Data Extraction
Abstracts were collated and a study was selected for a full review if it included: (a) hospitalized pediatric patients (< 19 years of age); (b) evaluated a systemic (i.e., enteral, intravenous, or intramuscularly administered) antimicrobial; and (c) reported on AKI or nephrotoxicity. Non-English articles, case reports, review articles, correspondences, and pre-clinical studies were excluded. For both screening and data extraction, each study was reviewed by a single author (TJ, MH, or KD).
Studies were included for complete data collection if: (a) the authors reported the AKI/nephrotoxicity definition clearly; (b) exposure to antimicrobial agent(s) was delineated; and (c) the number of children sustaining AKI/nephrotoxicity could be accurately determined. If an article included both pediatric and adult patients, the pediatric population was included in the analysis only if AKI in children could be specifically identified from the data reported, otherwise these studies were also excluded. If questions around appropriateness for inclusion occurred, the article was reviewed by all three primary reviewers (TJ, MH, KD) and a consensus was reached.
For each study, we recorded the type of study (e.g., clinical trial, retrospective cohort study), the study setting (e.g., intensive care unit, hospital ward), and study population details (e.g., age range, indication of drug [treatment, prophylaxis, not specified]). We collected all information about the criteria used to define AKI/nephrotoxicity in each study. The primary criteria reported in the manuscript were considered “author-defined AKI.” We abstracted the number of exposures to each antimicrobial agent and the number of courses that resulted in author-defined AKI while receiving that agent. If children were receiving combination therapies (i.e., multiple antimicrobials), we recorded AKI separately for each agent, as possible. When concomitant antimicrobials were not uniquely described (e.g., a study of amphotericin where concurrent administration of antibacterials was not individually reported), we only recorded AKI for the primary drug under study. This approach was taken because most studies evaluated a specific antimicrobial agent but did not separately report AKI development with all possible combinations of concomitant antimicrobial use.
In addition, we abstracted the number of antimicrobial courses that were reported to have led to AKI according to KDIGO, AKIN, or pRIFLE criteria for each drug [14,15,16]; these three definitions use comparable creatinine-based criteria and so AKI rates with each can be more directly combined. This was done to try to create a more standardized AKI definition for a comparison of AKI rates across studies. Studies that specifically used one of these three classification schemes, or whose author-defined AKI definition consisted of a ≥50% increase in serum creatinine or a decrease in serum creatinine-based estimated glomerular filtration rate of ≥ 25%, were considered for this subanalysis.
Where possible, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews were followed for this study [18]. The risk of bias for individual studies was not assessed; however, given the number of studies evaluated and the goals of this review, we aimed to minimize bias by collecting data systematically and applying specific definitions for AKI. This review was not registered on PROSPERO.
2.3 Outcomes
The primary outcome of this study was the pooled author-defined AKI rate for each antimicrobial. This was determined by first identifying the number of children that met each study’s definition of AKI among all children who received that drug. If patients in a study had multiple drug exposures (e.g., vancomycin and piperacillin/tazobactam), AKI rates were calculated for each drug separately. A pooled AKI rate for each drug was then calculated by summating all antimicrobial courses of a given drug across all studies and dividing the number leading to AKI by all of those treated with that agent. To summarize the nephrotoxic potential of agents, drugs were classified as having a low, medium, or high risk of AKI if the pooled author-defined AKI estimate was < 10%, 10–20%, or > 20%, respectively. This was only done for drugs that had AKI rates reported in two or more studies.
Secondary analyses generated pooled AKI rates per drug using KDIGO/AKIN/pRIFLE criteria. As above, pooled AKI rates were generated and the range of AKI rates across studies were recorded. Only studies that used one of these definitions were included in this analysis.
Modified forest plots were generated to display the pooled AKI rates across drugs. Only drugs that were evaluated in multiple studies were included in these plots. Rather than plotting confidence intervals around the pooled estimate of AKI per drug, the minimum and maximum AKI rates (i.e., range) reported among the included studies were used. The minimum and maximum AKI rates reported across studies were used to calculate an absolute variability in AKI rates for each drug that was the subject of multiple publications. Separate plots were constructed using author-defined AKI and KDIGO/AKIN/pRIFLE AKI rates.
3 Results
3.1 Literature Search
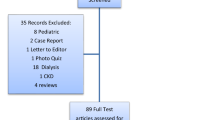
The initial search terms yielded 7935 unique articles of which 1052 were selected for a full review. One hundred and twenty-two articles met study inclusion criteria and were included in the final analysis (Fig. 1).
3.2 Study Characteristics
Among the 122 studies that met inclusion criteria, AKI/nephrotoxicity was reported for 28 unique antimicrobials. The most commonly studied drugs were vancomycin (n = 44 studies, 11,514 courses), colistin (n = 19 studies, 1222 courses), gentamicin (n = 17 studies, 3429 courses), piperacillin/tazobactam (n = 12 studies, 2517 courses), and liposomal amphotericin B (n = 8 studies, 438 patients). Ten drugs (ampicillin, cefotaxime, ceftazidime, clindamycin, fluconazole, foscarnet, ganciclovir, imipenem, linezolid, valganciclovir) were evaluated in only one included study, while 18 antimicrobials were evaluated in at least two included studies.
Across all included studies, there were a total of 27,285 antimicrobial courses (one or more doses) given. The total number of courses assessed for a particular antimicrobial ranged from 4 (foscarnet) to 11,514 (vancomycin) with a mean of 974. Of the 122 studies, 81 (66%) used pRIFLE, AKIN, or KDIGO criteria. The remaining 41 studies used other author-defined AKI definitions. Study characteristics including patient populations, setting, and the author-defined AKI definition used are shown in Table 1 of the Electronic Supplementary Material (ESM).
3.3 Author-Defined AKI
The pooled rate of author-defined AKI was 13.2% across all patients who received at least one antimicrobial. Among the 18 drugs that were evaluated in multiple included studies, the pooled AKI rate varied from 1.5% (ceftriaxone, two studies) to 42.1% (cidofovir, three studies); Fig. 2. For the ten drugs that were evaluated in only a single included study, the author-defined AKI rates were: ampicillin (n = 44/313; 14.1%), cefotaxime (n = 13/32; 40.6%), ceftazidime (n = 17/295; 5.8%), clindamycin (n = 0/100, 0%), fluconazole (n = 46/262, 17.6%), foscarnet (n = 1/4, 25%), ganciclovir (n = 4/30, 13.3%), imipenem (n = 24/268, 9.0%), linezolid (n = 1/179, 0.6%), and valganciclovir (n = 44/163, 27.0%).
Forest plot of author-defined acute kidney injury (AKI) by drug. Only drugs that multiple studies evaluated were included in this figure (total number of studies is shown in parentheses). Black circles represent the proportion of AKI summarized across all studies for a given drug (e.g., 24 of 57 patients [42.1%] treated with cidofovir had author-defined AKI across three studies). The tails of bars reflect the minimum and maximum proportions of AKI among the studies included (e.g., 31.0% and 68.8%, respectively, for cidofovir). The dotted vertical line represents the proportion of AKI across all studies (i.e., number of patients with AKI across all studies divided by the number of patients included in all studies)
Of the 18 drugs evaluated in at least two studies, six were determined to be low risk for causing AKI (< 10% pooled AKI rate across studies): caspofungin, cefepime, ceftriaxone, meropenem, micafungin, and teicoplanin. Eight drugs were classified as medium risk (10–20% pooled AKI rate): acyclovir, amphotericin B (lipid formulation), amphotericin B (liposomal formulation), colistin, gentamicin, piperacillin/tazobactam, tobramycin, and vancomycin. Four were classified as high risk (> 20% pooled AKI rate): amikacin, conventional amphotericin B, cidofovir, and trimethoprim/sulfamethoxazole. In both studies evaluating trimethoprim/sulfamethoxazole, treatment was the indication for antimicrobial receipt.
For most drugs, there was substantial variability in the absolute rates of AKI reported across included studies, varying from 5.4% (across the two studies that evaluated caspofungin) to 59.6% (across the 44 studies that evaluated vancomycin). The mean absolute variability in AKI rates across the 18 drugs evaluated in multiple studies was 29.9%.
3.4 KDIGO/AKIN/pRIFLE AKI
Two thirds of included studies (n = 81) utilized pRIFLE, KDIGO, or AKIN AKI criteria. These studies spanned 22 unique antimicrobials and 22,958 antimicrobial exposures. The pooled AKI rate across all studies that evaluated AKI using one of these definitions was 13.7% (n = 3134). Fourteen drugs were evaluated in multiple studies using one of these AKI definitions (Fig. 3); the pooled AKI rate ranged from 5.5% (meropenem) to 53.6% (cidofovir). The variability in AKI using these definitions was similar to that for author-defined AKI (29.6%) and ranged from 7.8 to 57.4% across drugs.
Forest plot of acute kidney injury (AKI) using KDIGO, PRIFLE, or AKIN criteria. Only drugs that multiple studies evaluated were included in this figure (total number of studies is shown in parentheses). Black circles represent the proportion of AKI summarized across all studies for a given drug. The tails of bars reflect the minimum and maximum proportions of AKI among the studies included. The dotted vertical line represents the proportion of AKI across all studies
3.5 AKI Criteria Comparisons
For most drugs, the pooled AKI rates were lower in studies that utilized an author-defined AKI definitions other than KDIGO/AKIN/pRIFLE criteria. For instance, the pooled rate of AKI for amikacin was 41.3% across four studies that used KDIGO/AKIN/pRIFLE criteria versus 10.4% across four studies that used different author-defined criteria. Similarly, for conventional amphotericin B, the pooled rate of AKI was 47.5% across five studies that used KDIGO/AKIN/pRIFLE criteria and 24.0% across the two studies that did not. The pooled rate of AKI for cidofovir was 53.6% across two studies that used the KDIGO/AKIN/pRIFLE criteria versus 31.0% in the one study that did not. Cidofovir (53.6% [two studies] vs 31.0% [one study]), liposomal amphotericin B (11.8% [five studies] vs 7.5% [three studies]), acyclovir (12.3% [six studies] vs 2.2% [one study]), gentamicin (12.7% [11 studies] vs 4.9% [six studies]), and colistin (17.3% [12 studies] vs 9.1% [seven studies]) all had higher rates of AKI reported among studies that utilized KDIGO/AKIN/pRIFLE criteria. The pooled AKI rates for vancomycin were similar (13.2% vs 11.0%) regardless of the definition used, as they were for cefepime (7.2% [six studies] vs 7.9% [one study]). Meanwhile, piperacillin/tazobactam (15.9% [11 studies] vs 25.9% [one study]) had higher pooled AKI rates among studies that did not use KDIGO/AKIN/pRIFLE criteria.
4 Discussion
In this review, which included 122 studies and 27,285 antimicrobial courses in hospitalized children, we found that AKI during antimicrobial treatment was common. Across all of the studies, which included numerous classes of drugs and varying patient populations, more than one in eight children developed AKI while receiving antimicrobial treatment. In total, there were 12 drugs that qualified for our definition of medium-risk or high-risk agents, associated with AKI development in more than 10% and 20% of pediatric recipients, respectively. While we cannot ascribe the AKI to receipt of antimicrobials directly, as AKI in children being treated for infections is often multifactorial, we believe that the findings of our review are noteworthy.
First, while most of the drugs in the medium-risk and high-risk groups are agents traditionally felt to be nephrotoxic (e.g., vancomycin, aminoglycosides, amphotericin, colistin, acyclovir), only a handful are routinely subjected to therapeutic drug monitoring (TDM): vancomycin and aminoglycosides. Most of the other higher risk drugs do not regularly undergo TDM in children, which creates challenges for clinicians, despite dose-dependent toxicities and known toxicity thresholds [19]. For instance, many of these drugs have important indications for use in immunocompromised individuals: colistin (multidrug-resistant Gram-negative infections), amphotericin (invasive fungal disease), trimethoprim/sulfamethoxazole (Pneumocystis jiroviecii infections), and cidofovir (cytomegalovirus infection). Furthermore, these drugs are often used in the setting of severe or life-threatening infections, resulting in the use of high dosages to mitigate the risk of treatment failure. Without TDM, however, there is a significant risk of the administration of toxic dosages, which may contribute to the high rates of AKI seen with these agents. Therefore, implementation of TDM may provide benefits and improve the safety of some of these higher risk medications.
Second, piperacillin/tazobactam has become a drug of particular interest in the AKI research field of late. It is frequently co-administered with vancomycin and several studies have found an increased rate of AKI in recipients of vancomycin plus piperacillin/tazobactam compared with vancomycin plus other β-lactam agents [20,21,22]. The pooled AKI rate for piperacillin/tazobactam was noticeably higher than for other β-lactams in our summative review, as well, which is consistent with these reports. Yet, recent studies have called into question the validity of this association as the mechanism of the added nephrotoxicity risk has yet to be defined and some experts theorize that much of the AKI seen during concomitant vancomycin and piperacillin/tazobactam may be a product of a false elevation of creatinine rather than true kidney injury [23, 24], similar to what can be seen with trimethoprim, which can inhibit tubular secretion of creatinine. All studies evaluating AKI in recipients of piperacillin/tazobactam in our review included a change in serum creatinine as part of the definition. Regardless of whether this finding reflects true or false AKI, changes in serum creatinine impact clinical care. As vancomycin is a known nephrotoxin, clinicians cannot ignore creatinine, unless an alternative renal function biomarker is used. It would be potentially dangerous to ignore a doubling of creatinine in a patient receiving vancomycin simply because they are also taking piperacillin/tazobactam. Until methods to clinically distinguish true from false elevations in creatinine are implemented (i.e., use of alternative urinary or plasma biomarkers), vancomycin plus piperacillin/tazobactam remains a precarious combination of drugs.
One of the goals of our review was to summarize the incidence of AKI among pediatric recipients of various antimicrobials. A significant challenge to this work is that the majority of studies did not report concomitant medication exposure with sufficient detail to tease out the unique contribution of an antimicrobial to AKI development. Receipt of other nephrotoxins, including secondary antimicrobials, is common among hospitalized children being treated for infection [20]. Future studies examining pediatric antimicrobial-associated AKI should report concomitant medications to better assess the intrinsic and additive effects of combination therapies on the development of AKI. Although some studies examined specific combination therapies (e.g., vancomycin plus cefepime), many studies lumped secondary antimicrobials together, making it impossible to isolate all antimicrobial use across studies. For instance, within the 12 included studies that evaluated AKI during piperacillin/tazobactam treatment, all 2500+ children received concomitant vancomycin, as well as other treatments. There were no included studies that evaluated AKI among recipients of piperacillin/tazobactam monotherapy. Thus, we cannot conclude that 16% of all piperacillin/tazobactam recipients develop AKI. The study by Lu et al. [25] was the only piperacillin/tazobactam study included that focused on AKI during piperacillin/tazobactam treatment (as opposed to vancomycin plus piperacillin/tazobactam). Although the AKI rate in this study was 7.7%, 89% of recipients received concomitant nephrotoxins including vancomycin, acyclovir, aminoglycosides, amphotericin, meropenem, or other non-antimicrobial agents. Ultimately, as very few studies reported AKI incidence separately for all combinations of antimicrobials, we opted not to try to distinguish AKI during monotherapy from AKI during combination treatments and summarized AKI for each drug reported. This highlights the difficulty in gauging the true nephrotoxicity risk associated with any given drug.
Finally, we encountered substantial variability in AKI rates across different studies of the same antimicrobial. This was not unexpected and was, in part, due to the use of different AKI definitions among studies. However, even among studies that used similar AKI definitions (KDIGO, AKIN, or pRIFLE criteria), the AKI rates reported varied widely across studies of the same drug. This is likely because of the substantial heterogeneity of patient populations studied (i.e., neonates, general ward patients, critically ill), as well as ascertainment bias. As detailed above, AKI is a heterogeneous process influenced by many factors. Nephrotoxic medication exposure is just one contributory component to the development of AKI in hospitalized children. While we generated pooled estimates for AKI across numerous studies, the range of AKI rates reported across studies is also a valuable piece of information. In many instances, clinicians can gauge the risk of AKI based on the number of factors present in an individual patient (e.g., severity of illness, underlying comorbidities, concurrent medication exposure) and the range of reported rates may allow better estimation of the likelihood of AKI development than a single point estimate. However, ascertainment bias also plays into the range of AKI rates. Most studies of antimicrobial AKI were retrospective such that detection of AKI was limited based on when creatinine was measured clinically. A lack of a standardized approach to creatinine monitoring across patients, institutions, and drugs can significantly influence the capture rate of AKI across studies. Multinational registries that utilize both standardized and defined entry and outcome criteria can also be used to help estimate AKI rates across antimicrobials.
There are several important limitations to this work. First, we intentionally summarized AKI rates across studies that used different AKI definitions. This was done to explore variability in the rates reported. However, even when summarizing data among studies with similar AKI definitions, heterogeneity in the study designs and biases within studies likely contributed to variability in the AKI rates reported. Thus, the pooled AKI rates should be interpreted with caution. Second, as mentioned above, concomitant medication exposure was common but seldomly reported with sufficient detail to reliably tease out unique antimicrobial exposures. We summarized data for drugs that were explicitly reported, but it is likely that many of the children studied had additional antimicrobial treatments that could not be accounted for. Third, we did not attempt to determine the relative risk of AKI during treatment with any antimicrobial agent. The goal of our review was primarily descriptive, so that clinicians could understand the range of AKI rates reported among children treated with different drugs. Readers should not use the pooled estimates nor the ranges reported in our figures to compare the risk of toxicity from one agent to another. The studies included in our review were highly variable and our analytic approach was insufficient to inform direct comparisons. Further, pooling of different populations (e.g., neonatal, critically ill) may obscure differences in toxicity rates among various patient groups and disease states. As data amass, future studies should focus on these subpopulations. Last, like any structured review, the results of this study are limited by the underlying data available. Several of the antimicrobials were evaluated in <100 children or only in a small number of studies. This likely reflects our study’s strict selection criteria and limited research on antimicrobial-associated AKI in children. However, the certainty around the AKI point estimates is limited, which is why we opted not to generate confidence intervals surrounding the pooled estimates.
5 Conclusions
Among published studies, antimicrobial-associated AKI occurred commonly in children exposed to antimicrobials. Over 10% of the more than 27,000 antimicrobial courses included in our review led to AKI. To our knowledge, this is the first review to systematically evaluate rates of antimicrobial-associated AKI in pediatric patients. However, the substantial heterogeneity in AKI definitions, patient populations, and study designs contributed to significant variability in AKI rates across studies and drugs. Further, while we evaluated all eligible published studies, AKI rates were less commonly reported for drugs deemed to be “safer.”
Data availability
Data is not available in a repository but authors can provide upon request.
References
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. https://doi.org/10.1038/s41572-021-00284-z.
Perazella MA, Rosner MH. Drug-induced acute kidney injury. Clin J Am Soc Neprol. 2022;17(8):1220–33. https://doi.org/10.2215/CJN.11290821.
Downes KJ, Hayes M, Fitzgerald JC, et al. Mechanisms of antimicrobial-induced nephrotoxicity in children. J Antimicrob Chemother. 2020;75(1):1–13. https://doi.org/10.1093/jac/dkz325.
Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children’s hospitals. Pediatrics. 2010;126(6):1067–73. https://doi.org/10.1542/peds.2010-1275.
Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):23–31. https://doi.org/10.1542/peds.2011-2879.
Pierson-Marchandise M, Gras V, Moragny J, et al. The drugs that mostly frequently induce acute kidney injury: a case-noncase study of a pharmacovigilance database. Br J Clin Pharmacol. 2017;83(6):1341–9. https://doi.org/10.1111/bcp.13216.
Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–61. https://doi.org/10.2215/CJN.01900214.
Parikh RV, Tan TC, Salyer AS, et al. Community-based epidemiology of hospitalized acute kidney injury. Pediatrics. 2020;146(3): e20192821. https://doi.org/10.1542/peds.2019-2821.
Kaddourah A, Basu RK, Bagshaw SM, Goldstein S, AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20. https://doi.org/10.1056/NEJMoa1611391.
Uber AM, Sutherland SM. Acute kidney injury in hospitalized children: consequences and outcomes. Pediatr Nephrol. 2020;35(2):213–20. https://doi.org/10.1007/s00467-018-4128-7.
Lewis SJ, Mueller BA. Antibiotic dosing in patients with acute kidney injury: “enough but not too much.” J Intensive Care Med. 2016;31(3):164–76. https://doi.org/10.1177/0885066614555490.
Kim JY, Yee J, Yoon HY, Han JM, Gwak HS. Risk factors for vancomycin-associated acute kidney injury: a systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88(9):3977–89. https://doi.org/10.1111/bcp.15429.
Kwiatkowska E, Domański L, Dziedziejko V, Kajdy A, Stefańska K, Kwiatkowski S. The mechanism of drug nephrotoxicity and the methods for preventing kidney damage. Int J Mol Sci. 2021;22(11):6109. https://doi.org/10.3390/ijms22116109.
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–35. https://doi.org/10.1038/sj.ki.5002231.
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. https://doi.org/10.1186/cc5713.
Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. https://doi.org/10.1038/kisup.2012.1.
Goswami E, Ogden RK, Bennett WE, et al. Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Health Syst Pharm. 2019;76(22):1869–74. https://doi.org/10.1093/ajhp/zxz203.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Downes KJ, Goldman JL. Too much of a good thing: defining antimicrobial therapeutic targets to minimize toxicity. Clin Pharmacol Ther. 2021;109(4):905–17. https://doi.org/10.1002/cpt.2190.
Downes KJ, Cowden C, Laskin BL, et al. Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr. 2017;171(12): e173219. https://doi.org/10.1001/jamapediatrics.2017.3219.
Hundeshagen G, Herndon DN, Capek KD, et al. Co-administration of vancomycin and piperacillin-tazobactam is associated with increased renal dysfunction in adult and pediatric burn patients. Crit Care. 2017;21(1):318. https://doi.org/10.1186/s13054-017-1899-3.
Kalligeros M, Karageorgos SA, Shehadeh F, Zacharioudakis IM, Mylonakis E. The association of acute kidney injury with the concomitant use of vancomycin and piperacillin/tazobactam in children: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2019;63(12):e01572-19, AAC.01572-19. https://doi.org/10.1128/AAC.01572-19.
Miano TA, Hennessy S, Yang W, et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144–55. https://doi.org/10.1007/s00134-022-06811-0.
Pais GM, Liu J, Avedissian SN, et al. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother. 2020;75(5):1228–36. https://doi.org/10.1093/jac/dkz563.
Lu H, Thurnherr E, Meaney CJ, Fusco NM. Incidence and risk factors for acute kidney injury in hospitalized children receiving piperacillintTazobactam. J Pediatr Pharmacol Ther. 2021;26(6):597–602. https://doi.org/10.5863/1551-6776-26.6.597.
Ericson JE, Gostelow M, Autmizguine J, et al. Safety of high-dose acyclovir in infants with suspected and confirmed neonatal herpes simplex virus infections. Pediatr Infect Dis J. 2017;36(4):369–73. https://doi.org/10.1097/INF.0000000000001451.
Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. 2014;27(14):1485–90. https://doi.org/10.3109/14767058.2013.860522.
Rao S, Abzug MJ, Carosone-Link P, et al. Intravenous acyclovir and renal dysfunction in children: a matched case control study. J Pediatr. 2015;166(6):1462-8.e4. https://doi.org/10.1016/j.jpeds.2015.01.023.
Kendrick JG, Ensom MHH, Steer A, White CT, Kwan E, Carr RR. Standard-dose versus high-dose acyclovir in children treated empirically for encephalitis: a retrospective cohort study of its use and safety. Pediatr Drugs. 2014;16(3):229–34. https://doi.org/10.1007/s40272-014-0066-4.
Downes KJ, Boge CLK, Baro E, et al. Acute kidney injury during treatment with intravenous acyclovir for suspected or confirmed neonatal herpes simplex virus infection. J Pediatr. 2020;219:126-32.e2. https://doi.org/10.1016/j.jpeds.2019.12.056.
Sandery BJ, Erlich JH, Kennedy SE. Acute kidney injury following intravenous acyclovir in children. Arch Dis Child. 2020;105(12):1215–9. https://doi.org/10.1136/archdischild-2019-317990.
Yalçınkaya R, Öz FN, Kaman A, et al. Factors associated with acyclovir nephrotoxicity in children: data from 472 pediatric patients from the last 10 years. Eur J Pediatr. 2021;180(8):2521–7. https://doi.org/10.1007/s00431-021-04093-0.
Sonia SF, Hassan MS, Ara F, Hanif M. Fractional excretion of magnesium, a marker of aminoglycoside induced nephrotoxicity in neonates. Saudi J Kidney Dis Transpl. 2016;27(5):902–7. https://doi.org/10.4103/1319-2442.190781.
Guadalupe Vásquez-Mendoza MA, Vargas-Origel A, Del Carmen R-J, Aguilar-Orozco G, Romero-Gutiérrez G. Efficacy and renal toxicity of one daily dose of amikacin versus conventional dosage regime. Amer J Perinatol. 2007;24(2):141–6. https://doi.org/10.1055/s-2006-958159.
Endo A, Hanawa K, Nemoto A, et al. Evaluation of nephrotoxicity and ototoxicity following amikacin administration once daily or every 48 hours in neonates. Medicine. 2022;101(43): e31425. https://doi.org/10.1097/MD.0000000000031425.
Medjebeur Hanna R, Levy M, Bille E, et al. Assessment of the effects of a high amikacin dose on plasma peak concentration in critically ill children. Pediatr Drugs. 2021;23(4):395–401. https://doi.org/10.1007/s40272-021-00456-0.
Sandler ES, Mustafa MM, Tkaczewski I, et al. Use of amphotericin B colloidal dispersion in children. J Pediatr Hematol Oncol. 2000;22(3):242–6. https://doi.org/10.1097/00043426-200005000-00009.
Alexander BD, Wingard JR. Study of renal safety in amphotericin B lipid complex-treated patients. Clin Infect Dis. 2005;40(Suppl. 6):S414–21. https://doi.org/10.1086/429335.
Cetin H, Yalaz M, Akisu M, Hilmioglu S, Metin D, Kultursay N. The efficacy of two different lipid-based amphotericin B in neonatal Candida septicemia. Pediatr Int. 2005;47(6):676–80. https://doi.org/10.1111/j.1442-200x.2005.02135.x.
Ambreen G, Rehman A, Hussain K, et al. Neonatal fluid and electrolytes profile effect on amphotericin B associated nephrotoxicity in neonatal tertiary care unit of Karachi-Pakistan. Expert Opin Drug Saf. 2020;19(9):1209–17. https://doi.org/10.1080/14740338.2020.1781813.
Le J, Adler-Shohet FC, Nguyen C, Lieberman JM. Nephrotoxicity associated with amphotericin B deoxycholate in neonates. Pediatr Infect Dis J. 2009;28(12):1061–3. https://doi.org/10.1097/INF.0b013e3181af6201.
Turcu R, Patterson MJ, Omar S. Influence of sodium intake on amphotericin B-induced nephrotoxicity among extremely premature infants. Pediatr Nephrol. 2009;24(3):497–505. https://doi.org/10.1007/s00467-008-1050-4.
Jeon GW, Koo SH, Lee JH, et al. A comparison of Am Bisome to amphotericin B for treatment of systemic candidiasis in very low birth weight infants. Yonsei Med J. 2007;48(4):619–26. https://doi.org/10.3349/ymj.2007.48.4.619.
Holler B, Omar SA, Farid MD, Patterson MJ. Effects of fluid and electrolyte management on amphotericin B-induced nephrotoxicity among extremely low birth weight infants. Pediatrics. 2004;113(6):e608–16. https://doi.org/10.1542/peds.113.6.e608.
Krepis P, Argyri I, Krepi A, Syrmou A, Spyridis N, Tsolia M. Short-course regimens of liposomal amphotericin B for the treatment of Mediterranean visceral leishmaniasis in children: an 11-year retrospective study at a tertiary care center. Pediatr Infect Dis J. 2017;36(9):849–54. https://doi.org/10.1097/INF.0000000000001602.
Manzoni P, Galletto P, Rizzollo S, et al. Liposomal amphotericin B does not induce nephrotoxicity or renal function impairment in premature neonates. Early Hum Dev. 2012;88(Suppl. 2):S86-91. https://doi.org/10.1016/S0378-3782(12)70024-5.
Kolve H, Ahlke E, Fegeler W, Ritter J, Jürgens H, Groll AH. Safety, tolerance and outcome of treatment with liposomal amphotericin B in paediatric patients with cancer or undergoing haematopoietic stem cell transplantation. J Antimicrob Chemother. 2009;64(2):383–7. https://doi.org/10.1093/jac/dkp196.
McLintock LA, Cook G, Holyoake TL, Jones BL, Kinsey SE, Jackson GH. High loading dose Am Bisome is efficacious and well tolerated in the management of invasive fungal infection in hematology patients. Haematologica. 2007;92(4):572–3. https://doi.org/10.3324/haematol.10531.
Devrim F, Çağlar İ, Acar SO, et al. Evaluation of renal effects of liposomal amphotericin B in children with malignancies with KDIGO and RIFLE criteria. Nephrol Ther. 2021;17(7):507–11. https://doi.org/10.1016/j.nephro.2021.06.007.
Ibrahim SL, Zhang L, Brady TM, Hsu AJ, Cosgrove SE, Tamma PD. Low-dose gentamicin for uncomplicated Enterococcus faecalis bacteremia may be nephrotoxic in children. Clin Infect Dis. 2015;61(7):1119–24. https://doi.org/10.1093/cid/civ461.
Cesaro S, Giacchino M, Locatelli F, et al. Safety and efficacy of a caspofungin-based combination therapy for treatment of proven or probable aspergillosis in pediatric hematological patients. BMC Infect Dis. 2007;7(1):28. https://doi.org/10.1186/1471-2334-7-28.
Maertens JA, Madero L, Reilly AF, et al. A randomized, double-blind, multicenter study of caspofungin versus liposomal amphotericin B for empiric antifungal therapy in pediatric patients with persistent fever and neutropenia. Pediatr Infect Dis J. 2010;29(5):415–20. https://doi.org/10.1097/INF.0b013e3181da2171.
Imburgia TA, Engdahl SR, Pettit RS. Evaluation of the safety of cefepime prolonged infusions in pediatric patients with cystic fibrosis. Pediatr Pulmonol. 2022;57(4):919–25. https://doi.org/10.1002/ppul.25817.
Ganapathi L, Arnold A, Jones S, et al. Use of cidofovir in pediatric patients with adenovirus infection. F1000Res. 2016;5:758. https://doi.org/10.12688/f1000research.8374.2.
Vora SB, Brothers AW, Englund JA. Renal toxicity in pediatric patients receiving cidofovir for the treatment of adenovirus infection. J Pediatr Infect Dis Soc. 2017;6(4):399–402. https://doi.org/10.1093/jpids/pix011.
Caruso Brown AE, Cohen MN, Tong S, et al. Pharmacokinetics and safety of intravenous cidofovir for life-threatening viral infections in pediatric hematopoietic stem cell transplant recipients. Antimicrob Agents Chemother. 2015;59(7):3718–25. https://doi.org/10.1128/AAC.04348-14.
Pérez V, Saénz D, Madriz J, et al. A double-blind study of the efficacy and safety of multiple daily doses of amikacin versus one daily dose for children with perforated appendicitis in Costa Rica. Int J Infect Dis. 2011;15(8):e569–75. https://doi.org/10.1016/j.ijid.2011.04.012.
Ilhan O, Bor M, Ozdemir SA, Akbay S, Ozer EA. Efficacy and safety of intravenous colistin in very low birth weight preterm infants. Pediatr Drugs. 2018;20(5):475–81. https://doi.org/10.1007/s40272-018-0301-5.
Sahbudak Bal Z, Kamit Can F, Yazici P, et al. The evaluation of safety and efficacy of colistin use in pediatric intensive care unit: results from two reference hospitals and review of literature. J Infect Chemother. 2018;24(5):370–5. https://doi.org/10.1016/j.jiac.2017.12.017.
İpek MS, Aktar F, Okur N, Celik M, Ozbek E. Colistin use in critically ill neonates: a case-control study. Pediatr Neonatol. 2017;58(6):490–6. https://doi.org/10.1016/j.pedneo.2016.10.002.
Çağan E, Kıray Baş E, Asker HS. Use of colistin in a neonatal intensive care unit: a cohort study of 65 patients. Med Sci Monit. 2017;23:548–54. https://doi.org/10.12659/MSM.898213.
Kumar PP, Giri SR, Shaikh FAR, Panigrahy N, Chirla D. Safety and efficacy of intravenous colistin in children. Indian Pediatr. 2015;52(2):129–30. https://doi.org/10.1007/s13312-015-0586-1.
Karli A, Paksu MS, Karadag A, et al. Colistin use in pediatric intensive care unit for severe nosocomial infections: experience of an university hospital. Ann Clin Microbiol Antimicrob. 2013;12:32. https://doi.org/10.1186/1476-0711-12-32.
Ozsurekci Y, Aykac K, Cengiz AB, et al. Is colistin effective in the treatment of infections caused by multidrug-resistant (MDR) or extremely drug-resistant (XDR) Gram-negative microorganisms in children? Diagn Microbiol Infect Dis. 2016;85(2):233–8. https://doi.org/10.1016/j.diagmicrobio.2016.02.017.
Alan S, Yildiz D, Erdeve O, et al. Efficacy and safety of intravenous colistin in preterm infants with nosocomial sepsis caused by Acinetobacter baumannii. Am J Perinatol. 2014;31(12):1079–86. https://doi.org/10.1055/s-0034-1371361.
Tamma PD, Newland JG, Pannaraj PS, et al. The use of intravenous colistin among children in the United States: results from a multicenter, case series. Pediatr Infect Dis J. 2013;32(1):17–22. https://doi.org/10.1097/INF.0b013e3182703790.
Paksu MS, Paksu S, Karadag A, et al. Old agent, new experience: colistin use in the paediatric intensive care unit: a multicentre study. Int J Antimicrob Agents. 2012;40(2):140–4. https://doi.org/10.1016/j.ijantimicag.2012.04.010.
Fatehi S, Eshaghi H, Sharifzadeh M, et al. A randomized clinical trial evaluating the efficacy of colistin loading dose in critically ill children. J Res Pharm Pract. 2019;8(4):196. https://doi.org/10.4103/jrpp.JRPP_19_68.
Ambreen G, Salat MS, Hussain K, et al. Efficacy of colistin in multidrug-resistant neonatal sepsis: experience from a tertiary care center in Karachi. Pakistan Arch Dis Child. 2020;105(9):830–6. https://doi.org/10.1136/archdischild-2019-318067.
Wacharachaisurapol N, Phasomsap C, Sukkummee W, et al. Greater optimisation of pharmacokinetic/pharmacodynamic parameters through a loading dose of intravenous colistin in paediatric patients. Int J Antimicrob Agents. 2020;55(6): 105940. https://doi.org/10.1016/j.ijantimicag.2020.105940.
Aksoy GK, Özkan SEÖ, Tezel G, Dayar GT, Köşker M, Doğan ÇS. Assesment of colistin related side effects in premature neonates. Turk J Pediatr. 2020;62(5):795. https://doi.org/10.24953/turkjped.2020.05.011.
Wacharachaisurapol N, Kawichai S, Chanakul A, Puthanakit T. No increased acute kidney injury rate through giving an intravenous colistin loading dose in pediatric patients. Int J Infect Dis. 2021;106:91–7. https://doi.org/10.1016/j.ijid.2021.03.059.
Meysam S, Khosravi Z, Rashti R, et al. Colistin induced acute kidney injury in critically ill children: a prospective study utilizing RIFLE criteria. DARU J Pharm Sci. 2022;30(1):11–5. https://doi.org/10.1007/s40199-021-00421-9.
Lee J, Kim HS, Shin SH, et al. Efficacy and safety of fluconazole prophylaxis in extremely low birth weight infants: multicenter pre-post cohort study. BMC Pediatr. 2016;16:67. https://doi.org/10.1186/s12887-016-0605-y.
Vora SB, Brothers AW, Waghmare A, Englund JA. Antiviral combination therapy for cytomegalovirus infection in high-risk infants. Antivir Ther. 2018;23(6):505–11. https://doi.org/10.3851/IMP3238.
Carapetis JR, Jaquiery AL, Buttery JP, et al. Randomized, controlled trial comparing once daily and three times daily gentamicin in children with urinary tract infections. Pediatr Infect Dis J. 2001;20(3):240–6. https://doi.org/10.1097/00006454-200103000-00004.
Constance JE, Reith D, Ward RM, et al. Risk of nonsteroidal anti-inflammatory drug-associated renal dysfunction among neonates diagnosed with patent ductus arteriosus and treated with gentamicin. J Perinatol. 2017;37(10):1093–102. https://doi.org/10.1038/jp.2017.80.
Lau L, Al-Ismaili Z, Harel-Sterling M, et al. Serum cystatin C for acute kidney injury evaluation in children treated with aminoglycosides. Pediatr Nephrol. 2017;32(1):163–71. https://doi.org/10.1007/s00467-016-3450-1.
Constance JE, Balch AH, Stockmann C, et al. A propensity-matched cohort study of vancomycin-associated nephrotoxicity in neonates. Arch Dis Child Fetal Neonatal Ed. 2016;101(3):F236–43. https://doi.org/10.1136/archdischild-2015-308459.
Jansen D, Peters E, Heemskerk S, et al. Tubular injury biomarkers to detect gentamicin-induced acute kidney injury in the neonatal intensive care unit. Am J Perinatol. 2016;33(2):180–7. https://doi.org/10.1055/s-0035-1563714.
McWilliam SJ, Antoine DJ, Sabbisetti V, et al. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS ONE. 2012;7(8): e43809. https://doi.org/10.1371/journal.pone.0043809.
Martínková J, Pokorná P, Záhora J, et al. Tolerability and outcomes of kinetically guided therapy with gentamicin in critically ill neonates during the first week of life: an open-label, prospective study. Clin Ther. 2010;32(14):2400–14. https://doi.org/10.1016/j.clinthera.2011.01.013.
Serane TV, Zengeya S, Penford G, Cooke J, Khanna G, McGregor-Colman E. Once daily dose gentamicin in neonates: is our dosing correct? Acta Paediatr. 2009;98(7):1100–5. https://doi.org/10.1111/j.1651-2227.2009.01297.x.
Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;26(1):144–50. https://doi.org/10.1093/ndt/gfq375.
Khan AM, Ahmed T, Alam NH, Chowdhury AK, Fuchs GJ. Extended-interval gentamicin administration in malnourished children. J Trop Pediatr. 2006;52(3):179–84. https://doi.org/10.1093/tropej/fmi085.
Chong CY, Tan ASL, Ng W, Tan-Kendrick A, Balakrishnan A, Chao SM. Treatment of urinary tract infection with gentamicin once or three times daily. Acta Paediatr. 2003;92(3):291–6.
Sridharan K, Al DA. Clinical audit of gentamicin use by Bayesian pharmacokinetic approach in critically ill children. J Infect Chemother. 2020;26(6):540–8. https://doi.org/10.1016/j.jiac.2020.01.007.
Sridharan K, Al Jufairi M, Al Segai O, et al. Biomarkers in neonates receiving potential nephrotoxic drugs. Eur Rev Med Pharmacol Sci. 2021;25(22):7078–88. https://doi.org/10.26355/eurrev_202111_27260.
Uijtendaal EV, Rademaker CM, Schobben AF, et al. Once-daily versus multiple-daily gentamicin in infants and children. Ther Drug Monit. 2001;23(5):506–13. https://doi.org/10.1097/00007691-200110000-00002.
Polat M, Kara SS. Once-daily intramuscular amikacin for outpatient treatment of lower urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli in children. Infect Drug Resist. 2017;10:393–9. https://doi.org/10.2147/IDR.S148703.
Bayram N, Düzgöl M, Kara A, Özdemir FM, Devrim İ. Linezolid-related adverse effects in clinical practice in children. Arch Argent Pediatr. 2017;115(5):470–5. https://doi.org/10.5546/aap.2017.eng.470.
Shabaan AE, Nour I, Elsayed Eldegla H, Nasef N, Shouman B, Abdel-Hady H. Conventional versus prolonged infusion of meropenem in neonates with Gram-negative late-onset sepsis: a randomized controlled trial. Pediatr Infect Dis J. 2017;36(4):358–63. https://doi.org/10.1097/INF.0000000000001445.
Yoshikawa K, Nakazawa Y, Katsuyama Y, et al. Safety, tolerability, and feasibility of antifungal prophylaxis with micafungin at 2 mg/kg daily in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Infection. 2014;42(4):639–47. https://doi.org/10.1007/s15010-014-0601-9.
Arrieta AC, Maddison P, Groll AH. Safety of micafungin in pediatric clinical trials. Pediatr Infect Dis J. 2011;30(6):e97-102. https://doi.org/10.1097/INF.0b013e3182127eaf.
Wang PL, Liu P, Zhang QW, et al. Population pharmacokinetics and clinical outcomes of polymyxin B in paediatric patients with multidrug-resistant Gram-negative bacterial infections. J Antimicrob Chemother. 2022;77(11):3000–8. https://doi.org/10.1093/jac/dkac265.
Barco-Cabrera C, Reina YA, Dávalos DM, et al. Use of polymyxins for carbapenem-resistant infections in children and adolescents. JAC Antimicrob Resist. 2022;4(3):dlac073. https://doi.org/10.1093/jacamr/dlac073.
Jia X, Yin Z, Zhang W, Guo C, Du S, Zhang X. Effectiveness and nephrotoxicity of intravenous polymyxin B in carbapenem-resistant Gram-negative bacterial infections among Chinese children. Front Pharmacol. 2022;13: 902054. https://doi.org/10.3389/fphar.2022.902054.
Yamada T, Kubota T, Nakamura M, et al. Evaluation of teicoplanin concentrations and safety analysis in neonates. Int J Antimicrob Agents. 2014;44(5):458–62. https://doi.org/10.1016/j.ijantimicag.2014.07.005.
Yamada T, Kubota T, Yonezawa M, et al. Evaluation of teicoplanin trough values after the recommended loading dose in children with associated safety analysis. Pediatr Infect Dis J. 2017;36(4):398–400. https://doi.org/10.1097/INF.0000000000001456.
Sidi V, Roilides E, Bibashi E, Gompakis N, Tsakiri A, Koliouskas D. Comparison of efficacy and safety of teicoplanin and vancomycin in children with antineoplastic therapy-associated febrile neutropenia and gram-positive bacteremia. J Chemother. 2000;12(4):326–31. https://doi.org/10.1179/joc.2000.12.4.326.
Ju M, Zheng M, Yuan J, Lin D, Qian Y. Prevalence and risk factors of trimethoprim/sulfamethoxazole-related acute kidney injury in pediatric patients: an observational study from a public database. Transl Pediatr. 2022;11(8):1285–91. https://doi.org/10.21037/tp-21-600.
Sierra CM, Tran Y, Oana L, Bahjri K. Renal impairment associated with trimethoprim-sulfamethoxazole use in the pediatric population. J Pediatr Pharmacol Ther. 2022;27(7):663–8. https://doi.org/10.5863/1551-6776-27.7.663.
Arends A, Pettit R. Safety of extended interval tobramycin in cystic fibrosis patients less an 6 years old. J Pediatr Pharmacol Ther. 2018;23(2):152–8. https://doi.org/10.5863/1551-6776-23.2.152.
Imani S, Fitzgerald DA, Robinson PD, Selvadurai H, Sandaradura I, Lai T. Personalized tobramycin dosing in children with cystic fibrosis: a comparative clinical evaluation of log-linear and Bayesian methods. J Antimicrob Chemother. 2022;77(12):3358–66. https://doi.org/10.1093/jac/dkac324.
Hayes M, Boge CLK, Sharova A, et al. Antiviral toxicities in pediatric solid organ transplant recipients. Am J Transpl. 2022;22(12):3012–20. https://doi.org/10.1111/ajt.17171.
LeCleir LK, Pettit RS. Piperacillin-tazobactam versus cefepime incidence of acute kidney injury in combination with vancomycin and tobramycin in pediatric cystic fibrosis patients. Pediatr Pulmonol. 2017;52(8):1000–5. https://doi.org/10.1002/ppul.23718.
Cook KM, Gillon J, Grisso AG, et al. Incidence of nephrotoxicity among pediatric patients receiving vancomycin with either piperacillin-tazobactam or cefepime: a cohort study. J Pediatr Infect Dis Soc. 2019;8(3):221–7. https://doi.org/10.1093/jpids/piy030.
Holsen MR, Meaney CJ, Hassinger AB, Fusco NM. Increased risk of acute kidney injury in critically ill children treated with vancomycin and piperacillin/tazobactam. Pediatr Crit Care Med. 2017;18(12):e585–91. https://doi.org/10.1097/PCC.0000000000001335.
Joyce EL, Kane-Gill SL, Priyanka P, Fuhrman DY, Kellum JA. Piperacillin/tazobactam and antibiotic-associated acute kidney injury in critically ill children. J Am Soc Nephrol. 2019;30(11):2243–51. https://doi.org/10.1681/ASN.2018121223.
Alqurashi R, Batwa M, Alghamdi B, et al. Acute kidney injury in pediatric patients treated with vancomycin and piperacillin-tazobactam versus vancomycin and cefotaxime: a single-center study. Cureus. 2020;12(1): e6805. https://doi.org/10.7759/cureus.6805.
Bartlett JW, Gillon J, Hale J, Jimenez-Truque N, Banerjee R. Incidence of acute kidney injury among infants in the neonatal intensive care unit receiving vancomycin with either piperacillin/tazobactam or cefepime. J Pediatr Pharmacol Ther. 2020;25(6):521–7. https://doi.org/10.5863/1551-6776-25.6.521.
Al-Jebawi Y, Karalic K, Shekhawat P, Mhanna MJ. The concomitant use of vancomycin and piperacillin-tazobactam is associated with acute kidney injury (AKI) in extremely low birth weight infants (ELBW). J Neonatal Perinatal Med. 2022;15(2):303–9. https://doi.org/10.3233/NPM-210866.
Abouelkheir M, Alsubaie S. Pediatric acute kidney injury induced by concomitant vancomycin and piperacillin-tazobactam. Pediatr Int. 2018;60(2):136–41. https://doi.org/10.1111/ped.13463.
Zhang H, Wang Y, Gao P, et al. Pharmacokinetic characteristics and clinical outcomes of vancomycin in young children with various degrees of renal function. J Clin Pharmacol. 2016;56(6):740–8. https://doi.org/10.1002/jcph.653.
Sun Y, Huskey RL, Tang L, et al. Adverse effects of intravenous vancomycin-based prophylaxis during therapy for pediatric acute myeloid leukemia. Antimicrob Agents Chemother. 2018;62(3):e01838-e1917. https://doi.org/10.1128/AAC.01838-17.
Knoderer CA, Gritzman AL, Nichols KR, Wilson AC. Late-occurring vancomycin-associated acute kidney injury in children receiving prolonged therapy. Ann Pharmacother. 2015;49(10):1113–9. https://doi.org/10.1177/1060028015594190.
Moffett BS, Hilvers PS, Dinh K, Arikan AA, Checchia P, Bronicki R. Vancomycin-associated acute kidney injury in pediatric cardiac intensive care patients. Congenit Heart Dis. 2015;10(1):E6-10. https://doi.org/10.1111/chd.12187.
Sinclair EA, Yenokyan G, McMunn A, Fadrowski JJ, Milstone AM, Lee CKK. Factors associated with acute kidney injury in children receiving vancomycin. Ann Pharmacother. 2014;48(12):1555–62. https://doi.org/10.1177/1060028014549185.
Linder N, Lubin D, Hernandez A, Amit L, Ashkenazi S. Duration of vancomycin treatment for coagulase-negative Staphylococcus sepsis in very low birth weight infants. Br J Clin Pharmacol. 2013;76(1):58–64. https://doi.org/10.1111/bcp.12053.
Bhargava V, Malloy M, Fonseca R. The association between vancomycin trough concentrations and acute kidney injury in the neonatal intensive care unit. BMC Pediatr. 2017;17(1):50. https://doi.org/10.1186/s12887-017-0777-0.
Wei WX, Qin XL, Cheng DH, Lu H, Liu TT. Retrospective analysis of vancomycin treatment outcomes in Chinese paediatric patients with suspected Gram-positive infection. J Clin Pharm Ther. 2016;41(6):650–6. https://doi.org/10.1111/jcpt.12437.
Seixas GTF, Araujo OR, Silva DCB, Arduini RG, Petrilli AS. Vancomycin therapeutic targets and nephrotoxicity in critically ill children with cancer. J Pediatr Hematol Oncol. 2016;38(2):e56-62. https://doi.org/10.1097/MPH.0000000000000470.
Totapally BR, Machado J, Lee H, Paredes A, Raszynski A. Acute kidney injury during vancomycin therapy in critically ill children. Pharmacotherapy. 2013;33(6):598–602. https://doi.org/10.1002/phar.1259.
McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158(3):422–6. https://doi.org/10.1016/j.jpeds.2010.08.019.
Quach HT, Esbenshade AJ, Zhao Z, Banerjee R. Incidence of acute kidney injury among pediatric hematology/oncology patients receiving vancomycin in combination with piperacillin/tazobactam or cefepime. Pediatr Blood Cancer. 2019;66(7): e27750. https://doi.org/10.1002/pbc.27750.
Reilly AM, Ding MX, Rower JE, Kiser TH. The effectiveness of a vancomycin dosing guideline in the neonatal intensive care unit for achieving goal therapeutic trough concentrations. J Clin Pharmacol. 2019;59(7):997–1005. https://doi.org/10.1002/jcph.1392.
Zhang T, Cheng H, Li Y, et al. Paediatric acute kidney injury induced by vancomycin monotherapy versus combined vancomycin and meropenem. J Clin Pharm Ther. 2019;44(3):440–6. https://doi.org/10.1111/jcpt.12806.
Hing WC, Bek SJ, Lin RTP, Li SC. A retrospective drug utilization evaluation of vancomycin usage in paediatric patients. J Clin Pharm Ther. 2004;29(4):359–65. https://doi.org/10.1111/j.1365-2710.2004.00571.x.
Hurst AL, Baumgartner C, MacBrayne CE, Child J. Experience with continuous infusion vancomycin dosing in a large pediatric hospital. J Pediatr Infect Dis Soc. 2019;8(2):174–9. https://doi.org/10.1093/jpids/piy032.
Fitzgerald JC, Zane NR, Himebauch AS, et al. Vancomycin prescribing and therapeutic drug monitoring in children with and without acute kidney injury after cardiac arrest. Pediatr Drugs. 2019;21(2):107–12. https://doi.org/10.1007/s40272-019-00328-8.
Woldu H, Guglielmo BJ. Incidence and risk factors for vancomycin nephrotoxicity in acutely ill pediatric patients. J Pharm Technol. 2018;34(1):9–16. https://doi.org/10.1177/8755122517747088.
Morales Junior R, Juodinis VD, Santos ICPDF, et al. Vancomycin area under the curve-guided monitoring in pediatric patients. Rev Bras Ter Intensiva. 2022;34(1):147–53. https://doi.org/10.5935/0103-507X.20220009-en.
Hussain K, Salat MS, Rauf S, et al. Practical approaches to improve vancomycin-related patient outcomes in pediatrics- an alternative strategy when AUC/MIC is not feasible. BMC Pharmacol Toxicol. 2022;23(1):64. https://doi.org/10.1186/s40360-022-00606-1.
Mitchell B, Kormelink L, Kuhn R, Schadler A, Autry E. Retrospective review of vancomycin monitoring via trough only versus two-point estimated area under the curve in pediatric and adult patients with cystic fibrosis. Pediatr Pulmonol. 2023;58(1):239–45. https://doi.org/10.1002/ppul.26190.
Sridharan K, Al-Daylami A, Ajjawi R, Ajooz HAA. Vancomycin use in a paediatric intensive care unit of a tertiary care hospital. Pediatr Drugs. 2019;21(4):303–12. https://doi.org/10.1007/s40272-019-00343-9.
Feiten HDS, Okumura LM, Martinbiancho JK, et al. Vancomycin-associated nephrotoxicity and risk factors in critically ill children without preexisting renal injury. Pediatr Infect Dis J. 2019;38(9):934–8. https://doi.org/10.1097/INF.0000000000002391.
Olson J, Hersh AL, Sorensen J, Zobell J, Anderson C, Thorell EA. Intravenous vancomycin therapeutic drug monitoring in children: evaluation of a pharmacy-driven protocol and collaborative practice agreement. J Pediatr Infect Dis Soc. 2020;9(3):334–41. https://doi.org/10.1093/jpids/piz036.
Sosnin N, Curtis N, Cranswick N, Chiletti R, Gwee A. Vancomycin is commonly under-dosed in critically ill children and neonates. Br J Clin Pharmacol. 2019;85(11):2591–8. https://doi.org/10.1111/bcp.14084.
Zhang H, Gao P, Wang Y, et al. Baseline kidney function is associated with vancomycin-induced acute kidney injury in children: a prospective nested case-control study. Pediatr Nephrol. 2021;36(5):1299–306. https://doi.org/10.1007/s00467-020-04820-z.
Tang Z, Guan J, Li J, et al. Determination of vancomycin exposure target and individualised dosing recommendations for neonates: model-informed precision dosing. Int J Antimicrob Agents. 2021;57(3): 106300. https://doi.org/10.1016/j.ijantimicag.2021.106300.
Kwak S, Kim JY, Cho H. Vancomycin-induced nephrotoxicity in non-intensive care unit pediatric patients. Sci Rep. 2021;11(1):20681. https://doi.org/10.1038/s41598-021-00214-9.
Hambrick HR, Greco KF, Weller E, Ganapathi L, Lehmann LE, Sandora TJ. Impact of decreasing vancomycin exposure on acute kidney injury in stem cell transplant recipients. Infect Control Hosp Epidemiol. 2022;43(10):1375–81. https://doi.org/10.1017/ice.2021.454.
Dawoud TH, Khan N, Afzal U, Varghese N, Rahmani A, Abu-Sa’da O. Assessment of initial vancomycin trough levels and risk factors of vancomycin-induced nephrotoxicity in neonates. Eur J Hosp Pharm. 2022;29(1):44–9. https://doi.org/10.1136/ejhpharm-2019-002181.
Chen Q, Wan J, Shen W, et al. Optimal exposure targets for vancomycin in the treatment of neonatal coagulase-negative Staphylococcus infection: a retrospective study based on electronic medical records. Pediatr Neonatol. 2022;63(3):247–54. https://doi.org/10.1016/j.pedneo.2021.11.010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Ibram Mikhail is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number T32GM008562. Kevin J. Downes is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091365.
Conflicts of interest/competing interests
Torsten Joerger, Molly Hayes, Connor Stinson, Ibram Mikhail, and Kevin J. Downes have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
KJD conceptualized and designed the study. TJ, MH, and KJD screened articles for inclusion and exclusion criteria. TJ, MH, KJD, CS, and IM extracted rates of nephrotoxicity from selected articles and IM compiled summarizing information into Table 1 of the ESM. TJ and KJD analyzed the data and wrote the manuscript. All authors reviewed, provided critical feedback, and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joerger, T., Hayes, M., Stinson, C. et al. Incidence of Antimicrobial-Associated Acute Kidney Injury in Children: A Structured Review. Pediatr Drugs 26, 59–70 (2024). https://doi.org/10.1007/s40272-023-00607-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00607-5