Abstract
Background
Owing to its pharmacodynamic properties, especially the rapid onset and short duration of its action, the use of remifentanil in obstetric anesthesia, as well as in neonatology, might be increasingly used.
Objective
We conducted a systematic review to assess the efficacy and safety of remifentanil in preterm and term neonates. Outcomes of interest were neonatal adaptation after fetal exposure; neonatal pain, distress, and discomfort control during invasive procedures; and the occurrence of hemodynamic effects or respiratory depression induced by remifentanil infusion.
Methods
Given the different contexts of use, we have organized this work into three parts: (A) use of remifentanil for labor or cesarean section, with exposure of the fetus before birth, (B) brief use for neonatal procedural analgesia, and (C) prolonged use for sedation/analgesia of neonates. The bibliographic search was conducted based on keywords using electronic medical databases (DATABASE, Cochrane Library, PubMed, and EMBASE) from 1 January 2000 until 31 December 2022.
Results
Twenty-two articles were included (10 in part A, 5 in part B and 7 in part C). Prospective, controlled, randomized, blinded, and intention-to-treat trials were retained. Neonates were well adapted after exposure to remifentanil in the fetal period. Pain, stress, and discomfort were controlled during a brief or prolonged invasive procedure when remifentanil was used for sedation/analgesia. The physiological parameters were stable and the procedures were straightforward. Chest wall rigidity appeared to be a common side effect, but this can be managed by slow and continuous infusion and by using the minimum effective dose.
Conclusions
Remifentanil appears to be effective and safe in the short term in preterm and full-term neonates. However, its safety is compromised by the risk of chest wall rigidity. It should be used in appropriate neonatal units and in the presence of physicians able to monitor its side effects. Long-term outcomes have not been evaluated, to our knowledge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Remifentanil appears to be effective in preterm and term neonates. However, concerning its safety, adverse effects (particularly chest wall rigidity) are a potential risk, possibly related to the dose received. |
Remifentanil should be used in appropriate care units and in the presence of physicians able to monitor its side effects. |
1 Introduction
Remifentanil, a synthetic opioid, is increasingly used in obstetrics for labor analgesia or cesarean section analgesia and anesthesia. There is transplacental passage leading to fetal exposure in utero [1,2,3,4]. Literature concerning the impact of fetal exposure to remifentanil on neonatal outcomes and literature concerning the use of remifentanil in neonatal intensive care units (NICU) are poor. Insufficient data are available regarding optimal dosing, effects, and side effects. In fact, its use is not common practice in NICU although the use of opioids is widespread, as painful and stressful procedures are performed daily. Both, preterm and full-term newborns with repeated and prolonged exposures to painful procedures end up with long-term effects such as hyperalgesia and psychomotor and cognitive disorders [5]. It has been reported that exposure to anesthetic drugs during the neonatal period can lead to altered brain development [6]. Therefore, the ideal anesthetic agent should have a rapid onset, short duration of action, strong analgesic potency, and no short- or long-term adverse effects. Remifentanil, which is a potent selective µ-opioid receptor agonist, is potentially a good candidate for neonatal analgesia and sedation. It has a rapid onset of action (1–2 min), a short half-life (3–10 min), a brief offset of action, and immediate recovery of the clinical effect after interruption of its administration. It is metabolized by non-specific blood and tissue esterases, irrespective of any renal or hepatic metabolisms [3]. It has a low volume of distribution and its plasma clearance rate is high [2]. Non-specific esterase activity is present in preterm infants, irrespective of the gestational age [7]. In addition, the usually encountered adverse effects are similar to those observed with others opioids, in particular bradycardia, hypotension, chest wall rigidity, nausea, and vomiting [8]. The aim of the present study was to review the efficacy and safety aspects in neonates of remifentanil use in the perinatal period. In order to facilitate the data analysis and to reflect clinical practices, we divided our research into three parts: (A) use of remifentanil for labor or cesarean section, with exposure of the fetus before birth, (B) brief use for neonatal procedural analgesia, and (C) prolonged use for sedation/analgesia of neonates.
2 Methods
2.1 Preliminary Search and Registration
Before drafting the protocol for the systematic review, a preliminary PubMed search was conducted to check for similar systematic reviews and to explore articles of relevance to the review. Our study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) in August 2018 (Use of remifentanil in preterm or term neonates: efficacy and safety [CRD42018099873]). We used the PRISMA checklist to write our manuscript.
2.2 Eligibility Criteria
We selected studies published in English or French. The PICOS framework was set as Table 1. Only prospective, controlled, randomized, blinded trials in intention-to-treat, with high level of evidence were included.
2.3 Search Strategy
The electronic search of the published literature was performed by two pediatricians using DATABASE, the Cochrane Library (Cochrane Central Register of Controlled Trials—CENTRAL), PubMed (Medline), and EMBASE for randomized controlled trials (RCTs). The databases were searched for papers published from January 2000 to October 2022. Part A of the study was in regard to effects of remifentanil, used for delivery analgesia and sedation and before umbilical cord clamping, in full-term neonates during the first 24 hours of life. We used the following search strategy by keywords: remifentanil AND cesarean section AND fetus/fetal effects/neonatal effects, remifentanil AND labor AND fetus/fetal effects/neonatal effects. Part B of the study was in regard to efficacy and safety aspects of remifentanil used in the postnatal period for short-term procedures (e.g., premedication before elective endotracheal intubation or analgesia for insertion of a venous catheter). We used the following search strategy by keywords: remifentanil AND neonate AND intubation, remifentanil AND neonate AND analgesia, remifentanil AND neonate AND sedation. Part C of the study was in regard to efficacy and safety aspects of remifentanil used in the postnatal period for long-term procedures (e.g., sedation for mechanical ventilation or anesthesia for surgery). We used the following search strategy by keywords: remifentanil AND neonate AND mechanical ventilation, remifentanil AND neonate AND anesthesia, remifentanil AND neonate AND surgery.
2.4 Study Selection
Two reviewers independently screened the titles and abstracts and selected articles for full-text review, and potentially eligible articles were retrieved for perusal in full text. Any discrepancies between the assessments were resolved through discussion. Articles were excluded from this review when they did not meet one or more of the eligibility criteria.
2.5 Data Extraction
We extracted data from selected RCTs using the French National Health Authority standardized reading grid for therapeutic articles (items: clearly defined objectives, comparative trial, number of patients needed, adjusted population, adjusted statistical analysis, relevant variable and intention-to-treat analysis) [9]. From each RCT, we extracted (1) the first author and the year of publication, (2) the study design, (3) the study population, (4) the protocol of intervention, (5) the statistical analysis, (6) the outcomes, and (7) the summary estimate of the intervention effects.
2.6 Risk of Bias Assessment
The two reviewers explored the quality of selected RCTs using Cochrane’s risk-of-bias tool for randomized controlled trials (RoB2). This tool required evaluation of seven domains: random sequence generation, allocation concealment, blinding of the medical staff and patients, baseline characteristics of similar patients, assessment of incomplete outcome data, and exempt of selective reporting. We rated each domain as containing a low, uncertain, or high risk of bias. We assessed the overall risk for each study and the risk of bias in each domain for all included RCTs.
3 Results
3.1 Study Selection and Characteristics
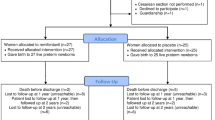
Our literature search strategy identified 323 potentially relevant studies. After screening the titles and abstracts, 55 manuscripts remained for full-text critical review. Ultimately, we selected 22 studies for inclusion based on our predetermined criteria of inclusion (Fig. 1). All studies were randomized, blinded, controlled trials and were published between 2000 and 2019. Ten studies concerned use of remifentanil in the fetal period (part A) [10,11,12,13,14,15,16,17,18,19]. Three studies concerned use of remifentanil for premedication before intubation (part B) [20,21,22]. Two studies concerned use of remifentanil for analgesia for an invasive procedure (part B) [23, 24]. Two studies concerned use of remifentanil for sedation of mechanical ventilation (part C) [25, 26]. Five studies concerned use of remifentanil for anesthesia of surgery (part C) [27,28,29,30,31]. In total, 1014 preterm and full-term neonates were included (Table 2).
3.2 Risk of Bias in the Studies
The results of this analysis are summarized in Table 3. Most RCTs had a low risk of bias. Except for two studies [20, 31], the studies had blinded medical staff and patients, and the baseline patient characteristics were similar. For seven studies, the outcomes were not clearly stated and defined, resulting in a significant risk of bias [10, 11, 15, 16, 18, 27, 28]. One study was at high risk of selective reporting [20], and another had incompletely assessed the outcomes data [10].
3.3 Results of the Main Outcomes
The following sections present narrative summaries for each outcome measure. Table 4 presents details of the 22 included studies, and the outcomes measured by the studies are presented in Table 5.
3.3.1 Fetal Exposure to Remifentanil and Neonatal Outcomes
3.3.1.1 Umbilical arterial and venous blood gases
Irrespective of the study protocol used in the studies, none of the neonates experienced neonatal hypoxemia.
3.3.1.2 Apgar score and resuscitative measures in the delivery room
There is no evidence of effect that remifentanil is associated with an increased risk for newborns with Apgar scores < 7 at 5 min. Moreover, neonatal respiratory depression, if present, usually resolved in a few minutes without a need for prolonged resuscitation measures. Ngan Kee et al. reported similar Apgar scores and neonatal outcomes for the remifentanil and the control group, although two (out of 20) neonates from the remifentanil group needed naloxone [11]. Draisci et al. observed lower Apgar scores or required endotracheal intubation in 14% of neonates in the remifentanil group. This was a significant proportion of newborns, but they were born by emergency cesarean section under general anesthesia, which could have a negative effect on their neonatal adaptation. The resolution of respiratory depression was, however, quick, without a need for naloxone, and the Apgar scores at 5 minutes were 8 or more in most cases [13]. Yu et al. reported that the frequency of 1-min Apgar scores < 7 was significantly higher in the remifentanil group versus dexmedetomidine. Nevertheless, at 5 minutes, there was no difference in the Apgar scores between the groups [17].
3.3.2 Brief and Prolonged Uses of Remifentanil in the Postnatal Period
The results of brief and prolonged uses of remifentanil are combined here to facilitate and synthetize the presentation.
3.3.2.1 Efficacy of remifentanil (control of pain and stress, number of intubation attempts, quality of sedation, time to successful intubation)
Primarily, there is evidence of effect that remifentanil provides adequate sedation and analgesia during endotracheal intubation or brief invasive procedures. Silva et al. compared remifentanil versus a combination of morphine and midazolam for endotracheal intubation of preterm neonates. There were no significant differences between the groups in terms of pain, stress, or variations of physiological parameters (heartbeats) [20]. Lago et al. compared remifentanil versus 5% dextrose for percutaneous intravenous central catheter placement [24]. During skin preparation and needle puncture, remifentanil provides adequate analgesia. Secondly, remifentanil appeared to control the surgical stress response and provided adequate analgesia during surgical procedures. Chambers et al. and Weale et al. observed that remifentanil reduced blood endocrine markers of stress during surgical procedures in both neonates and children [28, 29]. In a prospective study, Ben Khalifa et al. showed that there was a greater intraoperative cardiovascular response in full-term neonates receiving isoflurane compared with remifentanil [30]. Thirdly, remifentanil appeared to provide efficacy for analgesia and sedation during mechanical ventilation. Silva et al. compared continuous infusion of remifentanil versus continuous infusion of morphine during mechanical ventilation in preterm neonates. The assessments of pain and stress were similar between the groups [25]. In the same way, Welzing et al. compared remifentanil versus fentanyl during mechanical ventilation in full-term neonates, and they found no difference in the pain and stress assessments [26]. Fourth, remifentanil appears to provide good conditions for endotracheal intubation. Silva et al. showed that the endotracheal intubation conditions with remifentanil were better than the conditions with morphine [20]. Choong et al. indicated that doctors rated the intubation conditions for preterm neonates more favorably with fentanyl. Nevertheless, most of the intubation conditions were good with remifentanil [21].
3.3.2.2 Short-term safety of remifentanil (time to return of spontaneous respiration, time to extubation, and time to recovery)
Remifentanil presents a major pharmacokinetic advantage. Its short half-life allows a rapid return to spontaneous ventilation. Indeed, after termination of morphine or remifentanil infusion for mechanical ventilation in preterm infants, Silva et al. showed that the time to recovery and the time to extubation were significantly longer in patients receiving morphine [25]. In the same way, concerning mechanical ventilation in full-term neonates, Welzing et al. observed that the median time to tracheal extubation was significantly shorter in the remifentanil group compared with those on fentanyl [26].
3.3.2.3 Incidence of side effects and threats to safety (respiratory depression, hemodynamic effects, chest wall rigidity, and tolerance)
Concerning short-term use of remifentanil, infusion of remifentanil appears to increase the risk of chest wall rigidity. Choong et al. compared remifentanil with a combination of fentanyl and succinylcholine. Chest wall rigidity occurred in two neonates in the remifentanil group compared with none in the fentanyl and succinylcholine group [21]. The latter being a curare, its effect can reduce the risk of chest wall rigidity. In the study by Badiee et al., 25% of neonates receiving remifentanil exhibited chest wall rigidity, 45% exhibited laryngospasm, and 25% needed naloxone [22]. Shin et al. found that, in mechanically ventilated preterm infants, a remifentanil infusion of 0.25 µg/kg/min was superior to 0.1 µg/kg/min for providing superior analgesia during insertion of a venous catheter, although there were more apnea and bradycardia events [23]. Concerning prolonged use of remifentanil, none of the studies reported adverse effects attributable to remifentanil. Some complications occurred post-extubation or post-surgery, but these could not be directly related to the drugs used during procedures [25,26,27, 29].
3.3.2.4 Remifentanil dosing
Concerning premedication before intubation or sedation for INSURE (intubation-surfactant-extubation)/LISA (Less Invasive Surfactant Administration) procedures, boluses of 1–3 µg/kg over 1 minute were used [20,21,22, 26]. Concerning analgesia and sedation for short procedures, continuous infusion of 0.03–0.75 µg/kg/min was used [23, 24]. Concerning analgesia and sedation for mechanical ventilation, continuous infusion of 0.075–0.5 µg/kg/min was used [25, 26]. Concerning surgical anesthesia, continuous infusion of 0.25–0.4 µg/kg/min was used [27,28,29,30,31].
3.3.2.5 Long-term safety of remifentanil
No long-term safety data or large trials were found.
4 Discussion/Conclusion
This review describes the safety and efficacy of remifentanil in neonates, including after fetal exposure shortly before birth.
4.1 Chest Wall Rigidity
The use of remifentanil in the fetal period has been recommended and is considered one of the most suitable opioids for obstetric anesthesia and analgesia. Remifentanil used for general anesthesia during cesarean section or for analgesia during labor before umbilical cord clamping crosses the placental barrier, thus resulting in fetal drug exposure. At birth, neonates exposed in utero exhibited good adaptation to extra-uterine life, without signs of perinatal asphyxia. They exhibited opioid side effects, such as chest wall rigidity requiring mask ventilation or tactile stimulation, without a need for intubation. Concerning naloxone, its use is not recommended in the delivery room in neonates presenting with respiratory depression secondary to administration of opioids to the mother. Ventilator support must be the first line of treatment [32].
According to the studies reported in our review, there was frequently a risk of chest wall rigidity when remifentanil was used in the postnatal period for short procedures. This appears to be the main factor limiting the use of remifentanil in neonates. Fentanyl-induced rigid chest syndrome is quite well described, even in neonatology [33,34,35]. Muscle rigidity appears to be more frequent with high doses for anesthetic induction. In fact, the total received dose of remifentanil could be one of the factors that determine the occurrence of chest wall rigidity. These side effects are reversed by naloxone and/or a neuromuscular blocking agent [36]. Remifentanil or alternative analgesic has been maintained until the effect of the neuromuscular blocking agent has worn off. In addition, the risk of remifentanil-induced chest wall rigidity appears to be related to rapid administration. The development of chest wall rigidity can be minimized by use of a slow, continuous infusion of remifentanil instead of boluses, and by aiming for a minimum effective dose. In a prospective study concerning sedation for the INSURE procedure, de Kort et al. described chest well rigidity in six patients (43%), and they discontinued the study prematurely due to side effects and the lack of efficacy [37]. However, as discussed in a letter to the editor by Chollat et al., an accumulative dose of >3 µg/kg may partly promote chest wall rigidity [38]. Otherwise, the diagnosis of chest wall rigidity is clinical, but the studies cited in our systematic review do not use an objective definition. To our knowledge, there is no objective scale to assess the presence or degree of chest wall rigidity, which may partly explain the variability of its occurrence from one study to another, as its evaluation was subjective and operator-dependent. This may lead to over-diagnosis of thoracic rigidity, especially in preterm neonates, who may have low lung compliance.
For the most part, with continuous infusion and low doses, remifentanil provided safe and effective analgesia and sedation during invasive procedures. The use of excessively high doses raises the question of the intended purpose of the use of this opioid. Its use alone may lead to increased doses to achieve adequate analgesia or even sedation. Its association with a hypnotic agent seems preferable for long and/or very painful procedures.
Some questions remain about the optimal administration of remifentanil. There are no guidelines available on the dosages of remifentanil to be used in preterm and full-term neonates. Analgesia is usually obtained by titration (boluses) then maintained by continuous infusion if necessary. Dosages used are variable depending on indication and ventilatory support.
4.2 Efficacy of Remifentanil
The studies with high levels of evidence presented here showed that remifentanil has an effective analgesic effect in neonates. Remifentanil provides adequate sedation and/or analgesia during endotracheal intubation or brief invasive procedures. Among the brief invasive procedures, remifentanil could allow analgesia during laser treatment of retinopathy of prematurity, especially in hospitals without readily available pediatric anesthetists. In a pilot study, Demirel et al. described no major adverse effects except in two neonates (3%) with bradycardia and hypotension during infusion of remifentanil for laser treatment of retinopathy [39]. Remifentanil controls the surgical stress response and provides adequate analgesia during surgical procedures. Moreover, it provides effective analgesia and sedation during mechanical ventilation.
4.3 Neuroprotection
Other drugs have been proposed for sedation and analgesia in neonates, but their use is still controversial. Propofol has been associated with bradycardia, desaturation, and prolonged hypotension in newborns, and it is not an analgesic agent [40,41,42]. Further research is needed to establish the safety profiles for the use of ketamine in neonates due to concerns regarding possible neurotoxicity in animal studies [43]. Regarding remifentanil, studies performed in mice have shown that it has an antiapoptotic impact and that it exerts beneficial effects against excitotoxicity on the developing mouse brain that are associated with a reduction in the brain lesion size as well as prevention of a number of behavioral deficits in young mice [44, 45]. It should be noted that the neurotoxicity of some anesthetic agents has mainly been demonstrated in animals, and most of the time in the absence of painful procedures. The potential neurotoxicity of the anesthetic could be therefore counterbalanced by the beneficial effect of pain reduction. In humans, a recent randomized trial (GAS Trial) did not reveal neurodevelopmental disorders at 5 years of age after exposure to < 1 h of general anesthesia before 3 months of life [46].
Morphine exposure in very preterm neonates is independently associated with impaired cerebellar growth in the neonatal period and poorer neurodevelopmental outcomes in early childhood [47].
Fentanyl exposure demonstrated that cerebellar growth decreases as cumulative fentanyl exposure increases on term equivalent magnetic resonance imaging. Higher cumulative fentanyl dose in preterm infants correlated with a higher incidence of cerebellar injury and lower cerebellar diameter at term-equivalent age [48].
Midazolam has been associated with a high risk of transient hypotension and decreased mean cerebral blood flow velocity [49]. The trial NOPAIN demonstrated the risk of severe intraventricular hemorrhage, periventricular leukomalacia, or death in preterm neonates who received midazolam infusion during mechanical ventilation [50]. Clinical cohort studies demonstrated that midazolam exposure was associated with macro- and microstructural alterations in hippocampal development and poorer outcomes consistent with hippocampal dysmaturation [51].
Dexmedetomidine presented a novel option for the management of pain and sedation in preterm neonates without higher incidence of adverse neurologic effects [52]. Dexmedetomidine was neuroprotective in both in vitro and in vivo models of hypoxic-ischemic injury and this action is mediated via the α2A-adrenoceptor subtype [53, 54].
Isoflurane induced widespread cerebral neuroapoptosis in neonatal rat pups with subsequent long-term neurocognitive impairment of the animals. As the injury occurred in the neonatal period and animal training and testing followed this injury, this indicates impairment in learning and memory consistent with a significant hippocampal lesion [55].
4.4 Limitations
The number of studies included in the systematic review is relatively low, in connection with a rigorous selection of articles. Moreover, the use and indication of remifentanil differ between studies. Therefore, it is difficult to assess remifentanil in a specific indication.
Also, the studies included in the systematic review are small, and thus there is limited power to ascertain the incidence of adverse events, particularly those that are uncommon but potentially severe. Furthermore, enrollment in small RCTs is plagued by population selection bias.
Finally, although remifentanil seems to have potential neuroprotection effects, nevertheless, the lack of studies on long-term outcomes is an additional limitation.
4.5 Perspectives
Remifentanil is potentially a good candidate for premedication before neonatal intubation. Nevertheless, given the risk of chest wall rigidity, its use is controversial. The routine addition of a neuromuscular blocking agent and/or a hypnotic agent may be a promising way to use remifentanil before intubation. If a neuromuscular blocker is administered, the infusion of remifentanil must be adjusted to cover the duration of muscle blockade.. Questions remain regarding the optimal mode of administration of remifentanil. No guidelines are available for the dosages of remifentanil to be used in preterm and full-term neonates. Insufficient data are available regarding optimal dosing, effects, and side effects. Research on the clinical applicability of remifentanil in preterm and term neonates should continue, in particular pharmacokinetic and pharmacodynamic studies. To our knowledge, there are no data on the economic impact of remifentanil. Further economic research may be considered.
References
Egan TD, Kern SE, Muir KT, White J. Remifentanil by bolus injection: a safety, pharmacokinetic, pharmacodynamic, and age effect investigation in human volunteers. Br J Anaesth. 2004;92(3):335–43.
Glass PSA, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999;89(4 Suppl):7.
Glass PSA. Remifentanil: a new opioid. J Clin Anesth. 1995;7(7):558–63.
Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88(6):1467–74.
Grunau RE, Holsti L, Peters JWB. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11(4):268–75.
Lim Y, Godambe S. Prevention and management of procedural pain in the neonate: an update, American Academy of Pediatrics, 2016. Arch Dis Child Educ Pract Ed. 2017;102(5):254–6.
Welzing L, Ebenfeld S, Dlugay V, Wiesen MHJ, Roth B, Mueller C. Remifentanil degradation in umbilical cord blood of preterm infants. Anesthesiology. 2011;114(3):570–7.
Nakatani T. Opioid therapy and management of side effects associated with opioids. Gan To Kagaku Ryoho. 2017;44(4):294–7.
Agence Nationale d’Accréditation et d’Evaluation en Santé (ANAES). Acta Endosc. 1998;28(2):151–5.
Volikas I, Male D. A comparison of pethidine and remifentanil patient-controlled analgesia in labour. Int J Obstet Anesth. 2001;10(2):86–90.
Ngan Kee WD, Khaw KS, Ma KC, Wong ASY, Lee BB, Ng FF. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: a randomized, double-blind, controlled trial. Anesthesiology. 2006;104(1):14–20.
Bouattour L, Ben Amar H, Bouali Y, Kolsi K, Gargouri A, Khemakhem K, et al. Maternal and neonatal effects of remifentanil for general anaesthesia for Caesarean delivery. Ann Fr Anesth Reanim. 2007;26(4):299–304.
Draisci G, Valente A, Suppa E, Frassanito L, Pinto R, Meo F, et al. Remifentanil for cesarean section under general anesthesia: effects on maternal stress hormone secretion and neonatal well-being: a randomized trial. Int J Obstet Anesth. 2008;17(2):130–6.
Ng TKT, Cheng BCP, Chan WS, Lam KK, Chan MTV. A double-blind randomised comparison of intravenous patient-controlled remifentanil with intramuscular pethidine for labour analgesia. Anaesthesia. 2011;66(9):796–801.
Behdad S, Ayatollahi V, Harrazi H, Nazemian N, Heiranizadeh N, Baghianimoghadam B. Remifentanil at induction of general anesthesia for cesarean section: Double blind, randomized clinical trial. Colomb Med (Cali). 2013;44(2):87–91.
Varposhti MR, Ahmadi N, Masoodifar M, Shahshahan Z, Tabatabaie MH. Comparison of remifentanil: Entonox with Entonox alone in labor analgesia. Adv Biomed Res. 2013;2:87.
Yu Z, Zhang P, Wang H, Zhang L, Wei W, Fang W, et al. Effects of dexmedetomidine versus remifentanil on mothers and neonates during cesarean section under general anesthesia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020;164(4):417–24.
Li C, Li Y, Wang K, Kong X. Comparative evaluation of remifentanil and dexmedetomidine in general anesthesia for cesarean delivery. Med Sci Monit. 2015;21:3806–13.
Güneş S, Türktan M, Güleç ÜK, Hatipoğlu Z, Ünlügenç H, Işık G. The comparison of patient-controlled remifentanil administered by two different protocols (bolus and bolus+infusion) and intramuscular meperidine for labor analgesia. Turk J Anaesthesiol Reanim. 2014;42(5):264–9.
e Silva YP, Gomez RS, Marcatto J de O, Maximo TA, Barbosa RF, Simões e Silva AC. Morphine versus remifentanil for intubating preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2007;92(4):F293–294.
Choong K, AlFaleh K, Doucette J, Gray S, Rich B, Verhey L, et al. Remifentanil for endotracheal intubation in neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):F80-84.
Badiee Z, Vakiliamini M, Mohammadizadeh M. Remifentanil for endotracheal intubation in premature infants: a randomized controlled trial. J Res Pharm Pract. 2013;2(2):75–82.
Shin SH, Kim H-S, Lee J, Choi KY, Lee JH, Kim E-K, et al. A comparative study of two remifentanil doses for procedural pain in ventilated preterm infants: a randomized, controlled study*. Pediatr Crit Care Med. 2014;15(5):451–5.
Lago P, Tiozzo C, Boccuzzo G, Allegro A, Zacchello F. Remifentanil for percutaneous intravenous central catheter placement in preterm infant: a randomized controlled trial. Paediatr Anaesth. 2008;18(8):736–44.
e Silva YP, Gomez RS, Marcatto J de O, Maximo TA, Barbosa RF, e Silva ACS. Early awakening and extubation with remifentanil in ventilated premature neonates. Paediatr Anaesth. 2008;18(2):176–83.
Welzing L, Oberthuer A, Junghaenel S, Harnischmacher U, Stützer H, Roth B. Remifentanil/midazolam versus fentanyl/midazolam for analgesia and sedation of mechanically ventilated neonates and young infants: a randomized controlled trial. Intensive Care Med. 2012;38(6):1017–24.
Davis PJ, Galinkin J, McGowan FX, Lynn AM, Yaster M, Rabb MF, et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. I. Emergence and recovery profiles. Anesth Analg. 2001;93(6):1380–6 (table of contents).
Chambers N, Lopez T, Thomas J, James MFM. Remifentanil and the tunnelling phase of paediatric ventriculoperitoneal shunt insertion. A double-blind, randomised, prospective study. Anaesthesia. 2002;57(2):133–9.
Weale NK, Rogers CA, Cooper R, Nolan J, Wolf AR. Effect of remifentanil infusion rate on stress response to the pre-bypass phase of paediatric cardiac surgery. Br J Anaesth. 2004;92(2):187–94.
Ben Khalifa S, Blidi S, Trifa M, Skhiri A, Drira M, Regaya T, et al. Time to extubation in infants undergoing pyloromyotomy—isoflurane inhalation vs remifentanil infusion. Middle East J Anaesthesiol. 2009;20(2):277–80.
Silibuldu C, Ozcengiz D, Gunes Y. Comparison of two new different anaesthetic techniques for neonatal surgical emergencies. J Anaesthesiol Clin Pharmacol. 2010;26(3):307–10.
Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 Suppl 2):S516-538.
Tammen AJ, Brescia D, Jonas D, Hodges JL, Keith P. Fentanyl-induced rigid chest syndrome in critically ill patients. J Intensive Care Med. 2022;8850666221115635.
Wells S, Williamson M, Hooker D. Fentanyl-induced chest wall rigidity in a neonate: a case report. Heart Lung. 1994;23(3):196–8.
Gaertner VD, Restin T, Bassler D, Fauchère J-C, Rüegger CM. Case report: intrapulmonary tidal volumes in a preterm infant with chest wall rigidity. Front Pediatr. 2022;10: 979763.
Pacifici GM. Clinical pharmacology of fentanyl in preterm infants. A review. Pediatr Neonatol. 2015;56(3):143–8.
de Kort EHM, Hanff LM, Roofthooft D, Reiss IKM, Simons SHP. Insufficient sedation and severe side effects after fast administration of remifentanil during INSURE in preterm newborns. Neonatology. 2017;111(2):172–6.
Chollat C, Tourrel F, Marret S. Does remifentanil have a place for sedation in the case of endotracheal intubation or minimally invasive surfactant therapy in neonates? NEO. 2017;112(4):372–3.
Demirel N, Bas AY, Kavurt S, Celik IH, Yucel H, Turkbay D, et al. Remifentanil analgesia during laser treatment for retinopathy of prematurity: a practical approach in neonatal intensive care unit. Am J Perinatol. 2014;31(11):983–6.
Durrmeyer X, Breinig S, Claris O, Tourneux P, Alexandre C, Saliba E, et al. Effect of atropine with propofol vs atropine with atracurium and sufentanil on oxygen desaturation in neonates requiring nonemergency intubation: a randomized clinical trial. JAMA. 2018;319(17):1790–801.
Allegaert K, Peeters MY, Verbesselt R, Tibboel D, Naulaers G, de Hoon JN, et al. Inter-individual variability in propofol pharmacokinetics in preterm and term neonates. Br J Anaesth. 2007;99(6):864–70.
Vanderhaegen J, Naulaers G, Van Huffel S, Vanhole C, Allegaert K. Cerebral and systemic hemodynamic effects of intravenous bolus administration of propofol in neonates. Neonatology. 2010;98(1):57–63.
Jp C, Je H. Pediatric sedation—evolution and revolution. Paediatr Anaesth. 2011. https://doi.org/10.1111/j.1460-9592.2011.03617.x.
Tourrel F, de Lendeu PK, Abily-Donval L, Chollat C, Marret S, Dufrasne F, et al. The antiapoptotic effect of remifentanil on the immature mouse brain: an ex vivo study. Anesth Analg. 2014;118(5):1041–51.
Chollat C, Lecointre M, Leuillier M, Remy-Jouet I, Do Rego J-C, Abily-Donval L, et al. Beneficial effects of remifentanil against excitotoxic brain damage in newborn mice. Front Neurol. 2019;10:407.
McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393(10172):664–77.
Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. 2016;172:81-87.e2.
McPherson C, Haslam M, Pineda R, Rogers C, Neil JJ, Inder TE. Brain injury and development in preterm infants exposed to fentanyl. Ann Pharmacother. 2015;49(12):1291–7.
van Straaten HLM, Rademaker CMA, de Vries LS. Comparison of the effect of midazolam or vecuronium on blood pressure and cerebral blood flow velocity in the premature newborn. DPD. 1992;19:191–5.
Anand KJS, McIntosh N, Lagercrantz H, Pelausa E, Young TE, Vasa R. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Arch Pediatr Adolesc Med. 1999;153(4):331–8.
Duerden EG, Guo T, Dodbiba L, Chakravarty MM, Chau V, Poskitt KJ, et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann Neurol. 2016;79(4):548–59.
O’Mara K, Gal P, Wimmer J, Ransom JL, Carlos RQ, Dimaguila MAVT, et al. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. J Pediatr Pharmacol Ther. 2012;17(3):252–62.
Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, et al. Dexmedetomidine produces its neuroprotective effect via the α2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502(1):87–97.
Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010;54(6):710–6.
Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110(5):1077–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.M., C.C., and M.S.A. have no conflicts of interest to declare.
Statement of ethics
The authors have no ethical conflicts to disclose.
Funding
Not applicable.
Author contributions
A.M. drafted the protocol and manuscript; acquired, analyzed, and interpreted the data; and provided final approval for publication. C.C. helped analyze and interpret the data, revised the manuscript for important intellectual content, provided final approval for publication, and agreed to be accountable for all aspects of the work. M.S.A. contributed to the conception and design of the work, revised the manuscript for important intellectual content, helped analyze and interpret the data, and provided final approval for publication.
Data statement
Not applicable.
Consent (participation and publication)
Not applicable.
Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maroni, A., Aubelle, MS. & Chollat, C. Fetal, Preterm, and Term Neonate Exposure to Remifentanil: A Systematic Review of Efficacy and Safety. Pediatr Drugs 25, 537–555 (2023). https://doi.org/10.1007/s40272-023-00583-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00583-w