Abstract
Ewing sarcoma is a translocation-associated sarcoma mainly impacting adolescents and young adults. The classic translocation (EWSR1::FLI1) leads to a fusion oncoprotein that functions as an aberrant transcription factor. As such, the oncogenic driver of this disease has been difficult to target pharmacologically and, therefore, the systemic therapies used to treat patients with Ewing sarcoma have typically been non-selective cytotoxic chemotherapy agents. The current review highlights recent clinical trials from the last decade that provide the evidence base for contemporary drug therapy for patients with Ewing sarcoma, while also highlighting novel therapies under active clinical investigation in this disease. We review recent trials that have led to the establishment of interval-compressed chemotherapy as an international standard for patients with newly diagnosed localized disease. We further highlight recent trials that have shown a lack of demonstrable benefit from high-dose chemotherapy or IGF-1R inhibition for patients with newly diagnosed metastatic disease. Finally, we provide an overview of chemotherapy regimens and targeted therapies used in the management of patients with recurrent Ewing sarcoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with localized Ewing sarcoma treated with conventional chemotherapy have favorable cure rates, but a large burden of late effects. |
Patients with metastatic or recurrent Ewing sarcoma have poor outcomes despite a range of interventions evaluated. |
Novel approaches are needed to improve quality of cure for patients with localized disease and survival rates for patients with metastatic or recurrent Ewing sarcoma. |
1 Introduction
Ewing sarcoma is an aggressive malignancy, mainly diagnosed in adolescents and young adults. Ewing sarcoma is driven by characteristic fusion oncoproteins generated by translocation events, typically involving EWSR1 or FUS together with a member of the ETS transcription factor family (most commonly FLI1, leading to the pathognomonic EWSR1::FLI1 translocation). Despite recognition of the presence of these translocations for more than 30 years and increasing understanding of their oncogenic role in these tumors, therapy for patients with Ewing sarcoma still largely consists of conventional modalities, including cytotoxic chemotherapy and local control of sites of disease involvement.
The current treatment paradigm for a patient with newly diagnosed Ewing sarcoma is shown in the Fig. 1. Patients typically receive neoadjuvant chemotherapy followed by local control of the primary tumor and then adjuvant chemotherapy. For patients with metastatic disease, local control to sites of metastatic disease often takes place after completion of adjuvant chemotherapy. In contrast to a well-established standard approach used for patients with newly diagnosed disease, the management of a patient with recurrent Ewing sarcoma is much more individualized. A management plan depends on several individual factors, including time from diagnosis to first relapse, sites of relapse, therapies received during frontline therapy and toxicities experienced during frontline therapy.
In our review article of available literature from the last decade, we will summarize the data that have informed current drug therapies used for patients with newly diagnosed disease. We will review data supporting commonly used regimens for patients with recurrent Ewing sarcoma, as well as emerging data about novel approaches for these patients. While local control approaches (including surgery and/or radiotherapy) to sites of disease play a critical role in managing patients with newly diagnosed and recurrent Ewing sarcoma, this review will focus on systemic therapies for these indications.
2 Drug Therapy for Newly Diagnosed Ewing Sarcoma
Several decades of clinical trials have established intense, multi-agent chemotherapy as a backbone of Ewing sarcoma treatment. In this section, we review trials reported over the last 10 years that have helped to define current front-line therapies in localized as well as metastatic Ewing sarcoma. For a comprehensive review of more historic clinical trials, please refer to an excellent review by Zöllner et al. [1].
2.1 Systemic Therapy in Newly Diagnosed Localized Disease
After initial evidence demonstrating activity of multiagent chemotherapy, several trials over the last decade have evaluated strategies to increase chemotherapy dose intensity in Ewing sarcoma. Chemotherapy dose intensity can be increased by adding new chemotherapeutic agents, increasing the administered dose of medication, shortening the interval between doses, or by prolonging the total duration of chemotherapy.
In 2012, the Children’s Oncology Group (COG) published results from AEWS0031, a prospective randomized trial evaluating whether shortening the interval between cycles of chemotherapy improved survival outcomes in 564 patients with newly diagnosed localized Ewing sarcoma [2]. Patients received 14 cycles of standard dose vincristine/doxorubicin/cyclophosphamide (VDC) cycles alternating every 3 weeks (standard arm) versus every 2 weeks (experimental arm) with ifosfamide/etoposide (IE) cycles (VDC/IE regimen). Patients received myeloid growth factor support to enable interval compression. The 5-year event-free survival (EFS) was significantly higher in the experimental group (73 vs 65%) and both regimens had similar toxicity. These results contrasted with those reported in North American cooperative group trial INT-0154 [3]. In this trial, 478 patients with previously untreated, localized Ewing sarcoma received 17 cycles of standard dose VDC/IE every 3 weeks over 48 weeks or 11 cycles of a dose-intensified VDC/IE regimen every 3 weeks over 30 weeks. There was no significant difference in 5-year survival between both groups (72 vs 70%) and the dose-intensified regimen resulted in greater toxicity. These studies showed that dose intensification by interval compression, rather than dose escalation, improves survival outcomes in Ewing sarcoma. Interval compressed VDC/IE remains the backbone of most Ewing sarcoma chemotherapy regimens in North America.
Additional studies have sought to evaluate whether other methods of dose intensification confer additional survival benefit. In Israel, a pilot study retrospectively evaluated an innovative dose-intensive protocol called SCMCIE94 in patients with treatment-naïve Ewing sarcoma [4]. SMCIE94 (Protocol 3) involves higher doses of chemotherapy (vincristine/actinomycin/cyclophosphamide/doxorubicin [VACD], or VACD-IE) with increased dose intensity than two earlier protocols from the same institution with VACD (Protocol 1) or lower doses of VACD-IE (Protocol 2). Compared with these earlier protocols, SCMCIE94 was associated with an increase in 10-year EFS of 16% overall and 33% in patients with Ewing sarcoma localized to the extremities.
Dose intensification with high-dose chemotherapy with stem cell rescue may be beneficial in a subgroup of patients with localized Ewing sarcoma at high risk of relapse. The R2Loc portion of the EuroEwing99 trial compared consolidation treatment with busulfan and melphalan (BuMel) followed by autologous stem cell rescue to standard consolidation treatment (7 courses of vincristine/actinomycin/ifosfamide, or VAI) in 240 patients with newly diagnosed, localized, high-risk Ewing sarcoma [5]. High-risk disease was defined as patients who had large tumor volume (≥ 200 mL) at diagnosis or a poor histological response to induction chemotherapy with a less intensive regimen referred to as VIDE (vincristine/ifosfamide/doxorubicin/etoposide). The primary endpoint was EFS, defined as progression, relapse, second malignancy, or death by any cause. Patients were stratified by multiple factors including prior local therapy before balanced randomization to mitigate the confounding effects previous surgery and/or radiation therapy could have on study results. The BuMel arm had improved 8-year EFS (60.7 vs 47.1%) and overall survival (OS) (64.5 vs 55.6%) compared with the standard treatment arm, but more severe toxicity. These results supported implementing BuMel consolidation as a standard approach for this subgroup of patients with high-risk localized disease who were treated with the VIDE induction. This study also corroborated results from the French EW93 study which suggested potential benefit to a risk-adapted strategy where patients received more intensive consolidation treatment if they were stratified as having a high-risk of relapse [6]. The Brazilian Ewing1 trial confirmed the feasibility of risk-adapted treatment with comparable results in a lower income setting [7].
Other groups have evaluated the incorporation of additional chemotherapy agents in the context of standard backbone regimens. For example, a recent study retrospectively analyzed three consecutive CWS trials (CWS-91, CWS-96, CWS-2002P) in patients with localized extraskeletal Ewing sarcoma. CWS-91 evaluated whether the addition of etoposide improves response to VAIA (vincristine/actinomycin/ifosfamide/doxorubicin) induction chemotherapy [8]; CWS-96 compared a more intensive regimen of CEVAIE (carboplatin, epirubicin, vincristine, actinomycin-D, ifosfamide, etoposide) to standard VAIA [9]; and CWS-2002P evaluated the benefit of optional maintenance therapy with cyclophosphamide and vinblastine [10]. The retrospective analysis of these trials observed no significant differences in 5-year EFS or OS by adding additional agents to the VAIA backbone regimen, highlighting the importance of anthracyclines and alkylators in this disease [11].
More recently, the COG reported AEWS1031, a phase III, randomized trial that evaluated whether adding vincristine/topoptecan/cyclophosphamide (VTC) to standard interval compressed VDC/IE chemotherapy improved outcomes in patients with newly diagnosed localized Ewing sarcoma [12]. Both the experimental and standard arms had similar survival outcomes, with estimated 5-year EFS and OS of 78 and 87%, respectively. While VTC did not improve outcomes in this context, these results were nevertheless the best reported among cooperative group phase III trials.
Intense multi-agent chemotherapy is often associated with significant toxicity. Several trials have evaluated whether certain chemotherapy agents can be substituted to reduce toxicity without sacrificing efficacy. For example, given that ifosfamide is well associated with nephrotoxicity [13], the Euro-EWING99-R1 trial evaluated whether cyclophosphamide could replace ifosfamide in consolidation treatment of localized Ewing sarcoma [14]. Patients received either cyclophosphamide or ifosfamide in combination with vincristine and dactinomycin (VAC or VAI) after standard VIDE induction chemotherapy. Compared with the EICESS-92 study that observed similar efficacy of cyclophosphamide and ifosfamide in standard-risk Ewing sarcoma, the experimental and standard arms in the Euro-EWING99-R1 trial had similar 3-year EFS of 75.4 and 78.2%, suggesting that it may be possible to substitute cyclophosphamide for ifosfamide in this context [15].
Until recently, there was no international standard treatment for localized Ewing sarcoma. The recent results of the Euro-Ewing-2012 trial have helped to clarify best available therapy for these patients. This trial was an open-label, randomized trial that compared the North American standard of care (interval compressed VDC/IE) with the European standard of care (VIDE induction with VAI or VAC consolidation) in 640 patients with localized or metastatic Ewing sarcoma [16]. The VDC/IE regimen showed significantly greater 3-year EFS (67 vs 61%), less toxicity and was on average 12 weeks shorter than the European regimen. These results support establishing interval compressed VDC/IE as an international standard of first-line care in patients with Ewing sarcoma.
The approach to drug therapy for Ewing sarcoma has been centered almost exclusively on conventional cytotoxic chemotherapy and very few trials have evaluated novel systemic agents in patients with newly diagnosed localized Ewing sarcoma. The EWING2008R1 study evaluated the effect of zoledronic acid maintenance therapy in standard-risk EWS and observed no clear benefit, indicating that other novel agents are needed for these patients [17].
2.2 Chemotherapy in Newly Diagnosed Metastatic Disease
Patients with newly diagnosed metastatic disease have historically had significantly worse outcomes compared with patients with localized disease. This population has therefore been the focus of additional novel interventions. Much of this work has studied incorporation of additional chemotherapy agents or chemotherapy intensification through the use of high-dose chemotherapy with autologous stem cell rescue.
The EuroEwing consortium performed a window-of-opportunity trial of single-agent irinotecan [18]. Twenty-three patients with newly diagnosed extra-pulmonary metastatic Ewing sarcoma received two courses of irinotecan prior to standard VIDE induction. Five patients (24%) responded to single-agent irinotecan, contrasting results from a COG study that observed no responders among a group of 16 patients with relapsed/refractory Ewing sarcoma treated with single-agent irinotecan [19].
The ISG/AIEOP EW-2 trial evaluated front-line window therapy with two courses of temozolomide and irinotecan (TEMIRI) in 34 patients with primary disseminated multifocal Ewing sarcoma [20]. The response rate was 59%, but the 3-year EFS and OS rates were 21 and 36%, similar to other studies in patients with primary disseminated multifocal EWS [21, 22]. Given these encouraging results in the front-line setting, its known activity in relapsed disease and low hematological toxicity, TEMIRI may warrant further study in metastatic EWS. Indeed, an ongoing trial at Memorial Sloan Kettering Cancer Center is investigating the effects of adding TEMIRI to standard chemotherapy in patients with newly diagnosed EWS (ClinicalTrials.gov identifier: NCT01864109).
GEIS-21 was a prospective, multicenter, non-randomized trial by the Spanish Sarcoma Group that evaluated the efficacy of window phase gemcitabine and docetaxel (G/D) in patients with high-risk Ewing sarcoma [23]. Patients received two cycles of G/D prior to mP6 induction chemotherapy. Patients with an objective response (complete response [CR], partial response [PR], stable disease [SD]) to the G/D regimen received an additional 12 monthly cycles of G/D as maintenance therapy after mP6. Twelve of 17 (70%) patients had an objective response to G/D, demonstrating the activity of this doublet in newly diagnosed patients.
At Memorial Sloan Kettering Cancer Center, a modified version of the P6 regimen with dose intensification of ifosfamide was retrospectively evaluated in 30 patients with newly diagnosed metastatic Ewing sarcoma [24]. Dose intensification did not significantly improve survival outcomes, with 3-year EFS and OS being 27 and 39%, respectively. These results add to growing literature that patients with metastatic disease likely require approaches other than further dose intensification.
A EuroEwing consortium study evaluated whether tandem high-dose chemotherapy with thiotepa and melphalan-busulfan after VIDE induction was feasible in patients with primary disseminated multifocal metastatic EWS [25]. The regimen was deemed feasible, but outcomes were unsatisfactory with 3-year EFS and OS of 11 and 22%, respectively. These outcomes did not improve on those reported in prior studies using high-dose chemotherapy with stem cell rescue in poor prognosis Ewing sarcoma [26,27,28].
The R2Pulm portion of the EuroEwing99 trial compared busulfan-melphalan (BuMel) high-dose chemotherapy with autologous stem-cell rescue to standard chemotherapy with whole-lung irradiation in 287 patients with newly diagnosed Ewing sarcoma with only pulmonary or pleural metastases [29]. Despite positive findings in high-risk localized patients treated with BuMel (see Sect. 2.1), there was no significant benefit to BuMel in this metastatic population.
The Ewing 2008R3 trial evaluated whether the addition of treosulfan and melphalan high-dose chemotherapy (Treomel-HDT) followed by autologous stem cell rescue improved outcomes in patients with disseminated Ewing sarcoma [30]. The entire cohort for each treatment arm had similar 3-year EFS (20.9 vs 19.2%), but a post-hoc analysis showed that patients younger than 14 years had improved 3-year EFS on the Treomel-HDT arm (39.3 vs 9%). Taken together, these three trials of high-dose chemotherapy argue strongly against further evaluation of this approach in patients with newly diagnosed metastatic disease.
2.3 Novel Agents for Metastatic Ewing Sarcoma
The experience trying to improve outcomes for metastatic Ewing sarcoma with conventional cytotoxic chemotherapy has been disappointing, with little improvement despite a range of rational interventions. Ultimately, incorporation of novel therapies may be needed to improve survival in these patients with poor prognosis.
Few trials over the last decade have evaluated the use of novel therapies in the front-line setting for these patients. COG conducted a pilot study evaluating the feasibility of a low-dose regimen of anti-angiogenic chemotherapy with vinblastine and celecoxib in combination with standard VDC/IE in patients with newly diagnosed metastatic Ewing sarcoma [31]. The 2-year EFS was 35% in the cohort of 35 patients, but 71% in the seven patients who only had pulmonary metastases, the latter comparing favorably with other studies with patients with pulmonary metastases only [32]. An ongoing ISG/AEIOP EW2 study is evaluating the feasibility and efficacy of maintenance therapy with oral cyclophosphamide plus celecoxib in patients with metastatic EWS (NCT02727387).
Based upon evidence implicating the IGF-1R axis in Ewing sarcoma, the COG conducted a phase III randomized trial (AEWS1221) comparing VDC/IE with VDC/IE plus the anti-IGF-1R monoclonal antibody ganitumab in patients with newly diagnosed metastatic Ewing sarcoma [33]. No differences in outcomes were observed between arms and future evaluation of IGF-1R inhibition in this context is unlikely.
3 Drug Therapy for Relapsed Ewing Sarcoma
With the results of the Euro-Ewing-2012 trial, VDC/IE has become a common international backbone for patients with newly diagnosed Ewing sarcoma. In contrast, management of patients with relapsed disease remains more individualized, with a number of factors influencing selection of therapy. While all patients with relapse have a low probability of long-term survival with current available therapies, time to first relapse appears to be a key determinant of outcome after relapse. Relapse within 0–2 years of diagnosis is associated with a 4-fold lower 5-year EFS in comparison with relapse more than 2 years after diagnosis [34, 35]. For these patients, a usual goal of care is to control the disease with reasonable quality of life. In this context, patients with relapsed disease have a number of conventional chemotherapy approaches as well as targeted therapy approaches available for consideration (Table 1). These patients are also candidates for clinical trials.
3.1 Conventional Chemotherapy for Relapsed Disease
Historically, two of the most used chemotherapy relapse regimens have relied on camptothecins. Irinotecan is a camptothecin prodrug that is metabolized by carboxylesterase enzymes to a topoisomerase I inhibitor, SN-38, which is about 1000 times more potent than the prodrug. This offers the advantages of cytotoxicity at relatively non myelosuppressive doses and manageable nonhematological toxicity, most commonly diarrhea [36]. The risk of diarrhea can be mitigated with concurrent use of oral cephalosporins [37]. While some studies demonstrated antitumor activity in Ewing sarcoma when irinotecan was used as a single agent (see Sect. 2.2), the most common use in the relapse setting is in combination with temozolomide (IT regimen). The addition of vincristine to irinotecan significantly decreased disease progression in relapsed sarcoma [38], so vincristine is also commonly added (VIT regimen). For example, a small study of 22 patients treated with VIT demonstrated complete response in five patients and partial response in seven patients, for a response rate of 68.1% [39]. In addition, there are many retrospective reports demonstrating the activity of IT or VIT in patients with relapsed Ewing sarcoma [40, 41].
Topotecan is another camptothecin topoisomerase I inhibitor used in combination with cyclophosphamide (TC regimen). A Pediatric Oncology Group phase II trial studied the TC regimen administered daily for 5 days every 21 days in pediatric patients with recurrent solid tumors, including Ewing sarcoma [42]. Complete or partial responses were noted in 6 of 17 patients, for a response rate of 35%. Hematologic and gastrointestinal toxicities were reported in 53 and 34% of patients, respectively.
Other groups have reported activity of other chemotherapy regimens in this population. For example, a phase II trial of the combination of gemcitabine/docetaxel (GD regimen) reported responses in 2 of 14 patients with relapsed Ewing sarcoma [43]. Likewise, high-dose ifosfamide given as monotherapy has also been reported to have activity in this context, with one study reporting a 34% response rate [44].
More recently, the European rEECur trial has provided new comparative data for these commonly used relapse regimens. rEECur was a multi-arm multi-stage (MAMS) seamless, drop-a-loser trial for patients with recurrent and primary refractory Ewing sarcoma. While additional arms are being studied, the outcomes for the first four regimens (IT, TC, GD and ifosfamide) have now been reported. In this design, regimens with low Bayesian probability of being declared the winning regimen were dropped early. Once two regimens remained, they were compared head-to-head. The GD regimen was the first to be eliminated, with response rate of 11%. The second interim analysis reported IT to have an objective response rate (ORR) of 20% and this arm of the trial was dropped due to low predicted probability of being superior to the remaining TC or ifosfamide arms. TC and ifosfamide were then compared head-to-head in a direct randomized comparison with EFS as the primary endpoint. In this comparison, ifosfamide was predicted to have a higher likelihood of being superior, with 6-month EFS of 47 versus 37% for TC [45]. Importantly, at the conclusion of this evaluation of all four arms of rEEcur, outcomes from the IT arm were reported to be more favorable compared with the TC arm. Therefore, ifosfamide and IT regimens are anticipated to be prioritized for future patients. In contrast, TC and GD regimens may be less likely to be used based upon the results of rEECur.
3.2 Targeted Therapies for Relapsed Disease
Preclinical studies nominated poly (ADP-ribose) polymerase (PARP) inhibition as a potential targeted therapy for Ewing sarcoma [46]. Olaparib monotherapy was shown to be inactive in a phase II trial specifically for patients with Ewing sarcoma [47]. Likewise, combinations of PARP inhibitors plus either temozolomide or irinotecan have had disappointing results [48, 49]. In contrast, a phase I trial of the triplet combination of talazoparib with a 5-day schedule of irinotecan and temozolomide reported a response rate of 42.9% among seven patients with relapsed Ewing sarcoma [50]. Taken together, these results suggest that a threshold exposure to DNA-damaging agents may be needed to realize the potential of PARP inhibition in these patients.
More recently, tremendous focus has been on multitargeted tyrosine kinase inhibitors for these patients. Regorafenib is an orally bioavailable multikinase inhibitor that blocks the activity of several protein kinases including those involved in tumor angiogenesis (VEGFR-1, VEGFR-2, VEGFR-3 and TIE2), oncogenesis (KIT, RAF-1, BRAF and BRAFV600E) and the tumor microenvironment (PDGFR and FGFR) [51]. The French Sarcoma Group conducted the REGOBONE trial as a monotherapy study of four parallel independent cohorts of different histological subtypes of metastatic bone cancers to assess the activity and safety of regorafenib at the adult dose of 160 mg. The Ewing cohort was randomized and enrolled 23 patients to receive treatment with regorafenib. A partial response was demonstrated in 5/23 (21.7%) patients on the regorafenib arm. Progression-free survival (PFS) rate at 24 weeks was 26%, compared with 8% for patients randomized to placebo (95% confidence interval [CI] 4.6–22.9). This outcome with regorafenib compares favorably with a pooled 6-month EFS rate of 12.7% (95% CI 7.6–19) for patients with relapsed Ewing sarcoma enrolled to seven COG phase II monotherapy trials [52, 53]. SARC024 was a phase II trial of regorafenib that included a stratum for patients with relapsed Ewing sarcoma. Regorafenib was well tolerated in this pre-treated population, with more common grade 3 or higher adverse events including hypophosphatemia, hypertension and elevated alanine transaminase. Among 19 of 30 evaluable patients, the progression-free rate at 8 weeks was 63% (95% CI 46–81). A 10% RECIST 1.1 response rate was observed with an additional 18 patients demonstrating stable disease [54].
Cabozantinib is another multitargeted tyrosine kinase inhibitor of MET and VEGFR2 that has been investigated for use in relapsed Ewing sarcoma. CABONE, a multicenter, single-arm, phase II study, was conducted by the French Sarcoma Group for patients 12 years and older. Adult patients received 60 mg daily and those < 16 years of age received 40 mg/m2/dose daily for 28-day cycles. Of 39 evaluable patients, 10 demonstrated partial responses by 6 months of treatment. Grade 3 and 4 toxicities include hypophosphatemia, pneumothorax, plantar palmar syndrome and neutropenia [55].
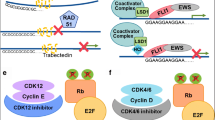
Other strategies have focused on targeting the EWSR1-FLI1 protein. Trabectedin and lurbinectedin have been shown to interfere with the EWSR1-FLI1 protein by redistributing the protein within the nucleus. Both agents have been separately combined with irinotecan and noted to have complete reversal of EWSR1-FLI1 activity in preclinical studies [56]. A recent phase II study included 28 adult patients with relapsed Ewing sarcoma treated with lurbinectedin once every 3 weeks. The ORR was 14.3% with a median PFS of 2.7 months. Grade 3/4 hematologic and febrile neutropenia toxicities were reported [57]. An ongoing phase I/II trial is evaluating trabectedin and irinotecan in this population (NCT04067115). In another phase I/II trial, 63 patients with relapsed/refractory metastatic EWS were treated with vincristine and TK216, an agent thought to interfere with the function of EWSR1-FLI1 protein [58]. The combination regimen was well tolerated with a disease control rate of 46.4%, though the need for prolonged continuous infusion may limit broad application of this agent (NCT02657005).
A range of other strategies are being investigated clinically for patients with relapsed Ewing sarcoma. For example, preclinical data have implicated CDK4 as another potential vulnerability in Ewing sarcoma [59]. Separate trials of palbociclib plus IT (NCT03709680) and abemaciclib plus IT (NCT05440786) are ongoing. Likewise, preclinical data have demonstrated a potential role for LSD1 inhibition in this disease [60] and have informed an ongoing trial of seclidemstat for patients with relapsed Ewing sarcoma (NCT03600649).
4 Conclusions
Current chemotherapy regimens yield favorable survival rates for patients with newly diagnosed localized Ewing sarcoma. However, these cures come at a significant cost, with a substantial burden of chronic late effects, including reduced fertility, premature menopause, cardiotoxicity, nephrotoxicity and risk of second malignancies. These important late effects highlight the pressing need for novel, less toxic approaches for this more favorable group of patients. In contrast, patients with newly diagnosed metastatic disease have not benefited from strategies to intensify chemotherapy and innovative approaches are sorely needed to improve cure rates. Likewise, the majority of patients with recurrent Ewing sarcoma are unlikely to survive their disease with currently available therapies. A critical goal is to identify new strategies that may benefit this population and ultimately be advanced into the frontline care of patients with Ewing sarcoma. The initial chemosensitivity of this disease along with its aggressive clinical course argue strongly against monotherapy approaches and in favor of strategies that incorporate chemotherapy along with novel agents that might target resistant clones that ultimately lead to relapse.
References
Zöllner SK, et al. Ewing sarcoma-diagnosis, treatment, clinical challenges and future perspectives. J Clin Med Res. 2021;10:1685.
Womer RB, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:4148–54.
Granowetter L, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children’s Oncology Group Study. J Clin Oncol. 2009;27:2536–41.
Ash S, et al. Improved outcome in local ewing sarcoma with an Intensified Pilot Treatment Protocol SCMCIE 94. J Pediatr Hematol Oncol. 2019;41:105–11.
Whelan J, et al. High-dose chemotherapy and blood autologous stem-cell rescue compared with standard chemotherapy in localized high-risk Ewing sarcoma: results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol. 2018;36:JCO2018782516.
Gaspar N, et al. Risk adapted chemotherapy for localised Ewing’s sarcoma of bone: the French EW93 study. Eur J Cancer. 2012;48:1376–85.
Brunetto AL, et al. Carboplatin in the treatment of Ewing sarcoma: results of the first Brazilian collaborative study group for Ewing sarcoma family tumors-EWING1. Pediatr Blood Cancer. 2015;62:1747–53.
Dantonello TM, et al. Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol. 2009;27:1446–55.
Sparber-Sauer M, et al. Long-term results from the multicentric European randomized phase 3 trial CWS/RMS-96 for localized high-risk soft tissue sarcoma in children, adolescents, and young adults. Pediatr Blood Cancer. 2022;69: e29691.
Koscielniak E, et al. Long-term clinical outcome and prognostic factors of children and adolescents with localized rhabdomyosarcoma treated on the CWS-2002P Protocol. Cancers. 2022;14:899.
Koscielniak E, et al. Extraskeletal Ewing sarcoma in children, adolescents, and young adults. An analysis of three prospective studies of the Cooperative Weichteilsarkomstudiengruppe (CWS). Pediatr Blood Cancer. 2021;68: e29145.
Leavey PJ, et al. Phase III trial adding vincristine-topotecan-cyclophosphamide to the initial treatment of patients with nonmetastatic Ewing sarcoma: a Children’s Oncology Group Report. J Clin Oncol. 2021;39:4029–38.
Skinner R, Cotterill SJ, Stevens MC. Risk factors for nephrotoxicity after ifosfamide treatment in children: a UKCCSG Late Effects Group study. United Kingdom Children’s Cancer Study Group. Br J Cancer. 2000;82:1636–45.
Le Deley M-C, et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: results of the randomized noninferiority Euro-EWING99-R1 trial. J Clin Oncol. 2014;32:2440–8.
Paulussen M, et al. Results of the EICESS-92 study: two randomized trials of Ewing’s sarcoma treatment–cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol. 2008;26:4385–93.
Brennan B, et al. Comparison of two chemotherapy regimens in patients with newly diagnosed Ewing sarcoma (EE2012): an open-label, randomised, phase 3 trial. Lancet. 2022;400:1513–21.
Dirksen U, Koch R, Bhadri V, Brichard B, Faldum A. Efficacy of maintenance therapy with zoledronic acid in patients with localized Ewing sarcoma: report from the international Ewing 2008 trial. J Clin Oncol. 2020;38:11523–11523.
Morland B, Platt K, Whelan JS. A phase II window study of irinotecan (CPT-11) in high risk Ewing sarcoma: a Euro-E.W.I.N.G. study. Pediatr Blood Cancer. 2014;61:442–5.
Bomgaars LR, et al. Phase II trial of irinotecan in children with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2007;25:4622–7.
Asaftei SD, et al. Front-line window therapy with temozolomide and irinotecan in patients with primary disseminated multifocal Ewing sarcoma: results of the ISG/AIEOP EW-2 Study. Cancers. 2021;13:3046.
Ladenstein R, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–91.
Oberlin O, et al. Impact of high-dose busulfan plus melphalan as consolidation in metastatic Ewing tumors: a study by the Société Française des Cancers de l’Enfant. J Clin Oncol. 2006;24:3997–4002.
Mora J, et al. GEIS-21: a multicentric phase II study of intensive chemotherapy including gemcitabine and docetaxel for the treatment of Ewing sarcoma of children and adults: a report from the Spanish sarcoma group (GEIS). Br J Cancer. 2017;117:767–74.
Magnan H, et al. Ifosfamide dose-intensification for patients with metastatic Ewing sarcoma. Pediatr Blood Cancer. 2015;62:594–7.
Loschi S, et al. Tandem high-dose chemotherapy strategy as first-line treatment of primary disseminated multifocal Ewing sarcomas in children, adolescents and young adults. Bone Marrow Transplant. 2015;50:1083–8.
Lashkari A, et al. Tandem high-dose chemotherapy followed by autologous transplantation in patients with locally advanced or metastatic sarcoma. Anticancer Res. 2009;29:3281–8.
Burke MJ, Walterhouse DO, Jacobsohn DA, Duerst RE, Kletzel M. Tandem high-dose chemotherapy with autologous peripheral hematopoietic progenitor cell rescue as consolidation therapy for patients with high-risk Ewing family tumors. Pediatr Blood Cancer. 2007;49:196–8.
Rosenthal J, et al. High-dose therapy with hematopoietic stem cell rescue in patients with poor prognosis Ewing family tumors. Bone Marrow Transplant. 2008;42:311–8.
Dirksen U, et al. High-dose chemotherapy compared with standard chemotherapy and lung radiation in ewing sarcoma with pulmonary metastases: results of the European Ewing Tumour Working Initiative of National Groups, 99 trial and EWING 2008. J Clin Oncol. 2019;37:3192–202.
Koch R, et al. High-dose treosulfan and melphalan as consolidation therapy versus standard therapy for high-risk (metastatic) Ewing sarcoma. J Clin Oncol. 2022;40:2307–20.
Felgenhauer JL, et al. A pilot study of low-dose anti-angiogenic chemotherapy in combination with standard multiagent chemotherapy for patients with newly diagnosed metastatic Ewing sarcoma family of tumors: a Children’s Oncology Group (COG) Phase II study NCT00061893. Pediatr Blood Cancer. 2013;60:409–14.
Miser JS, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: evaluation of increasing the dose intensity of chemotherapy—a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;49:894–900.
DuBois S, et al. Randomized phase III trial of ganitumab with interval-compressed chemotherapy for patients with newly diagnosed metastatic Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. (in press).
Leavey PJ, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:334–8.
Stahl M, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer. 2011;57:549–53. https://doi.org/10.1002/pbc.23040.
Thompson PA, et al. Pharmacokinetics of irinotecan and its metabolites in pediatric cancer patients: a report from the children’s oncology group. Cancer Chemother Pharmacol. 2008;62:1027–37.
McGregor LM, et al. Dose escalation of intravenous irinotecan using oral cefpodoxime: a phase I study in pediatric patients with refractory solid tumors. Pediatr Blood Cancer. 2012;58:372–9.
Pappo AS, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J Clin Oncol. 2007;25:362–9.
Raciborska A, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer. 2013;60:1621–5.
Meyers PA, Ambati SR, Slotkin EK, Cruz FD, Wexler LH. The addition of cycles of irinotecan/temozolomide (i/T) to cycles of vincristine, doxorubicin, cyclophosphamide (VDC) and cycles of ifosfamide, etoposide (IE) for the treatment of Ewing sarcoma (ES). J Clin Oncol. 2018;36:10533. https://doi.org/10.1200/jco.2018.36.15_suppl.10533.
Wagner LM, et al. Phase I trial of two schedules of vincristine, oral irinotecan, and temozolomide (VOIT) for children with relapsed or refractory solid tumors: a Children’s Oncology Group phase I consortium study. Pediatr Blood Cancer. 2010;54:538–45. https://doi.org/10.1002/pbc.22407.
Saylors RL, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a pediatric oncology group phase II study. J Clin Oncol. 2001;19:3463–9. https://doi.org/10.1200/jco.2001.19.15.3463.
Fox E, et al. Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of sarcoma alliance for research through collaboration study 003. Oncologist. 2012;17:321–9. https://doi.org/10.1634/theoncologist.2010-0265.
Ferrari S, et al. Response to high-dose ifosfamide in patients with advanced/recurrent Ewing sarcoma. Pediatr Blood Cancer. 2009;(5):581–4. https://doi.org/10.1002/pbc.21917.
McCabe MG, et al. Results of the second interim assessment of rEECur, an international randomized controlled trial of chemotherapy for the treatment of recurrent and primary refractory Ewing sarcoma (RR-ES). J Clin Oncol. 2020;38:11502–11502. https://doi.org/10.1200/jco.2020.38.15_suppl.11502.
Garnett MJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–5.
Choy E, et al. Phase II study of olaparib in patients with refractory Ewing sarcoma following failure of standard chemotherapy. BMC Cancer. 2014;14:813.
Chugh R, et al. SARC025 arms 1 and 2: A phase 1 study of the poly(ADP-ribose) polymerase inhibitor niraparib with temozolomide or irinotecan in patients with advanced Ewing sarcoma. Cancer. 2021;127:1301–10. https://doi.org/10.1002/cncr.33349.
Schafer ES, et al. Phase 1/2 trial of talazoparib in combination with temozolomide in children and adolescents with refractory/recurrent solid tumors including Ewing sarcoma: a Children’s Oncology Group Phase 1 Consortium study (ADVL1411). Pediatr Blood Cancer. 2020. https://doi.org/10.1002/pbc.28073.
Federico SM, et al. A phase I trial of talazoparib and irinotecan with and without temozolomide in children and young adults with recurrent or refractory solid malignancies. Eur J Cancer. 2020;137:204–13.
Wilhelm SM, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55.
Duffaud F, et al. Results of randomized, placebo (PL)-controlled phase II study evaluating efficacy and safety of regorafenib (REG) in patients (pts) with metastatic osteosarcoma (metOS), on behalf of the French Sarcoma Group (FSG) and Unicancer. J Clin Oncol. 2018;36:11504–11504. https://doi.org/10.1200/jco.2018.36.15_suppl.11504.
Collier AB, et al. Outcome of patients with relapsed or progressive Ewing sarcoma enrolled on cooperative group phase 2 clinical trials: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2021. https://doi.org/10.1002/pbc.29333.
Attia S, et al. A phase II trial of regorafenib in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC024 trial results. Cancer Med. 2022. https://doi.org/10.1002/cam4.5044.
Italiano A, et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:446–55.
Harlow ML, et al. Lurbinectedin inactivates the Ewing sarcoma oncoprotein EWS-FLI1 by redistributing it within the nucleus. Cancer Res. 2016;76:6657–68. https://doi.org/10.1158/0008-5472.can-16-0568.
Subbiah V, et al. Antitumor activity of lurbinectedin, a selective inhibitor of oncogene transcription, in patients with relapsed Ewing sarcoma: results of a basket phase II study. Clin Cancer Res. 2022;28:2762–70. https://doi.org/10.1158/1078-0432.ccr-22-0696.
Ludwig JA, et al. TK216 for relapsed/refractory Ewing sarcoma: interim phase 1/2 results. J Clin Orthod. 2021;39:11500–11500.
Kennedy AL, et al. Functional, chemical genomic, and super-enhancer screening identify sensitivity to cyclin D1/CDK4 pathway inhibition in Ewing sarcoma. Oncotarget. 2015;6:30178–93. https://doi.org/10.18632/oncotarget.4903.
Sankar S, et al. Reversible LSD1 inhibition interferes with global EWS/ETS transcriptional activity and impedes Ewing sarcoma tumor growth. Clin Cancer Res. 2014;20:4584–97. https://doi.org/10.1158/1078-0432.ccr-14-0072.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BAS and AG declare that they have no conflicts of interest that might be relevant to the contents of this manuscript. SGD reports consulting fees for advisory board participation from Amgen, Bayer and Jazz and travel expenses from Loxo Oncology, Roche and Salarius.
Funding
Supported by Alex’s Lemonade Stand Foundation (SGD).
Ethics approval
Not applicable to this literature review.
Consent
Not applicable to this literature review.
Data availability statement
Not applicable to this literature review.
Code availability
Not applicable to this literature review.
Author contributions
All authors: literature review; drafting manuscript; editing manuscript; final approval of manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Setty, B.A., Gikandi, A. & DuBois, S.G. Ewing Sarcoma Drug Therapy: Current Standard of Care and Emerging Agents. Pediatr Drugs 25, 389–397 (2023). https://doi.org/10.1007/s40272-023-00568-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00568-9