Abstract
Eosinophilic esophagitis (EoE) is a chronic immune-mediated inflammatory disorder characterized by symptomatic esophageal dysfunction and an eosinophil-predominant inflammation of the esophagus. EoE arises from interaction between genetic and environmental factors. In pediatric patients, clinical manifestations vary depending on age, from a gastroesophageal reflux disease (GERD)-like condition to severe dysphagic symptoms. Upper endoscopy is considered the gold standard for diagnosis and monitoring of EoE; however, significant efforts are underway to identify noninvasive diagnostic tools and biomarkers to avoid repetitive invasive procedures. Therapeutic first-line options currently available for EoE are elimination diets, proton pump inhibitors (PPIs), and steroids. The aim of treatment is to improve clinical symptoms while obtaining mucosal healing and avoiding long-term complications. Dietary treatment options comprise different empiric diets or an exclusively amino acid formula. Despite the efficacy of diets, compliance is often challenging. PPIs and topical steroids represent the main pharmacological options for EoE, and both can induce and maintain remission. Topical steroids have been reported as more effective, but data on long-term safety remain insufficient for both these and PPIs. Endoscopic dilations are currently reserved for severe untreated fibrostenotic disease unresponsive to medical therapies. Several biologic agents are available but not yet approved for EoE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Eosinophilic esophagitis (EoE) is a chronic immune-mediated inflammatory disorder characterized by symptoms of esophageal dysfunction and an eosinophil-predominant inflammation of the esophagus. |
The therapeutic options currently available for EoE are elimination diets, proton pump inhibitors, and topical steroids. |
More evidence is needed about the role of biologic agents and maintenance therapy for EoE. |
1 Introduction
Eosinophilic esophagitis (EoE) is a chronic immune-mediated inflammatory disorder characterized clinically by symptoms of esophageal dysfunction and histologically by an eosinophil-predominant inflammation of the esophagus [1]. EoE represents the main cause of dysphagia among children and young adults in Europe and North America and is the most prevalent cause of chronic esophagitis after gastroesophageal reflux disease (GERD) [2]. It was recognized as a unique clinicopathologic syndrome only in 1993 [3]. The awareness of this disease has increased over time, as has its incidence and prevalence. A recent meta-analysis reported an incidence of 6.6/100,000 per year and a prevalence of 34/100,000 in children [4]. In adults, incidence and prevalence were estimated at 7.7/100,000 and 42.2/100,000, respectively [4]. The remarkable increase in recent years might result from an improved diagnostic definition. On the other hand, the diagnosis of EoE is also rising at rates that outpace improvements in diagnostic accuracy. Epidemiological data indicate a change in environmental, rather than genetic and immune, factors [7]. The importance of these factors is suggested by the lower incidence in Eastern countries than in Europe and North America, where a western lifestyle is more common [7]. EoE is more frequent in rural areas with low population densities. A higher exposure to plant-based allergens or differences in exposure to particulate matter (different size and origin in rural than in urban areas, where finer particulate matter is typically associated with industrial activity) might be an explanation [5]. EoE varies according to climate zone and season, with more frequent diagnoses during summer [6]. It is more common in males, with a ratio of 3:1. Although 65% of cases occur during childhood, there is a second peak of incidence between 30 and 44 years of age [7]. This review aims to summarize the epidemiology/risk factors, clinical features, diagnostic management, and pharmacological approach in pediatric EoE, with a comprehensive review of dietary treatments. Lastly, we analyze and report the unsolved questions on the management of this relatively new disease.
2 Pathogenesis
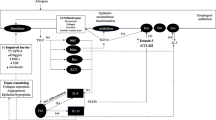
As mentioned, EoE derives from interaction between genetic and environmental factors (Fig. 1) [8]. Increased EoE frequency among first-degree family members supports a role for genetic patterns in the development of the disease [9, 10]. Alexander et al. [9] reported a 64-fold and 43-fold increased risk of developing EoE in fathers and brothers of a patient with EoE, respectively, whereas monozygotic and dizygotic twins exhibited an EoE frequency of 41 and 22%. Familial patterning of EoE does not follow a Mendelian inheritance, suggesting a complex multifactorial component. Most of the EoE genetic components identified affect epithelial barrier functions and T-helper cell type 2 (Th2)-mediated immune responses [11, 12].
2.1 Epithelial Dysfunction
Several genes involved in epithelial cell differentiation are located in a “hot spot” of dysregulation at the epidermal differentiation complex (1q21). These are included in a set of 574 differentially expressed genes that represent the EoE transcriptome [13]. The role of this complex in epithelial dysregulation was suggested by specific clinical syndromes caused by single mutations. Desmoglein 1 (DSG1) is downregulated by interleukin (IL)-13. The absence of DSG1 is sufficient to induce epithelial cell barrier dysfunction in EoE, even in the absence of dysregulation of tight junction genes; the importance of DSG1 has been demonstrated in SAM (severe atopic dermatitis, multiple allergies, and metabolic wasting) syndrome, caused by loss-of-function mutations of DSG1 or desmoplankin. Calpain 14 (CALPN14) is an esophagus-specific proteolytic enzyme induced by IL-13 [14]. In EoE, increases in CAPN14 lead to a loss of DSG1 expression and impaired epithelial barrier function. However, absence of CAPN14 also impairs the ability of the epithelium to repair following IL-13-mediated damage [15]. Hence, it has been hypothesized that CAPN14 is an intermediary of IL-13-mediated esophageal epithelial homeostasis.
A fundamental epithelial protein in EoE pathogenesis is filaggrin (FLG), which has been reported to be downregulated in this condition [12]. Lastly, the serine peptidase inhibitors kazal type 5 (SPINK5) and SPINK7 also participate in EoE pathogenesis, likely because of being related to uncontrolled activity of serine proteases. All these genetic variants may have an additive effect in vivo, although individual proteins have independent action on the epithelial function. As a result, it is possible that an increased tissue permeability and antigen uptake may lead to an inappropriate Th2 immune response.
2.2 Th2-Mediated Immune Response
This immune response pattern involves several cytokines, such as IL-4, IL-5, and IL-13. In particular, IL-13 has been shown to induce esophageal eosinophilia in an experimental model and regulates several genes implicated in EoE. Eotaxin 3 (CCL26) causes chemotaxis of eosinophils mediated by its receptor (CCR3) while being upregulated by IL-13. Another molecule, periostin (POSTN), induced by IL-13 and transforming growth factor β, increases the eosinophil adhesion to fibronectin [16]. The interaction between epithelial barrier dysfunction and the Th2-mediated immune response is highlighted by a tight relationship between DSG1 and POSTN. When DSG1 is absent, POSTN increases, leading to epithelial production of thymic stromal lymphopoietin (TSLP). TSLP is implicated in the regulation of the dendritic cell-mediated Th2 response, eosinophil survival, and mast cell and basophil activation [17,18,19]. STAT6 (12q13) has also been shown to independently associate with EoE. It is activated by IL-4 and IL-13 and encodes for a transcription factor activating many EoE genes [11, 12].
2.3 Environmental Factors
Another important factor in EoE development is the environment. In particular, allergic sensitization seems to heavily influence disease pathogenesis, as indicated by the success of dietary elimination and several animal models [19,20,21]. The esophageal microbiome may play a role, but dysbiosis could also result from EoE rather than being a cause [22, 23]. Helicobacter pylori and herpes simplex virus (HSV) have been shown to be inversely and directly associated with EoE, respectively. H. pylori has been hypothesized to polarize the immune system toward a Th1 response [24]. Some reports described an association between EoE and HSV, but the cause–effect relationship remains unclear [25].
Lastly, geographical localization could be another EoE risk factor. According to the hygiene and microbial dysbiosis hypotheses, a higher EoE incidence should be expected within urban settings and in developed countries. Air pollutants and allergens could affect esophageal epithelial development and barrier function. On the other hand, living in a rural environment may place individuals at higher risk of developing EoE because of increased exposure to aeroallergens [21]. A recent review argued against this hypothesis but did not rule out the possibility of pollen–food allergen cross reactivity [26]. Overall, more rigorous studies are needed to establish the true impact of geographic factors in EoE.
3 Clinical Features
EoE clinical manifestations vary with age. Infants and younger children generally present GERD-like symptoms, such as regurgitation, vomiting, abdominal pain, food refusal, and failure to thrive. In children and young adults, dysphagia is the main symptom. Difficulty in swallowing solid food, food impaction (defined as difficulty in advancing bolus through the esophageal lumen and sense of foreign body), and non-swallowing-associated heartburn and chest pain are also common in this age range [27].
Although not associated with an increased risk of mortality or malignancies, EoE has a chronic and progressive course with a negative impact on the patient’s quality of life [28]. The progressive EoE course is characterized by repeated mucosal inflammation, damage and remodeling of esophageal wall, collagen deposition, and lamina propria fibrosis, leading to the development of strictures and narrow-caliber esophagus [29, 30]. The evolution of symptoms from childhood to adult age reflects the progressive inflammation and damage. Adolescents and adults tend to change their eating behaviors and hide dysphagia by avoiding specific foods (particularly meat and bread) and increasing water intake during the meal. Thus, symptom assessment is not a reliable instrument in monitoring the disease. Dysphagia can also be an intermittent symptom, caused more by inflammation than by fibrosis. Indeed, EoE has been recently recognized as a transmural disease involving muscular layers and neuronal plexus [31]. Nonetheless, several studies have reported progression to fibrostenosis in most untreated EoE [32, 33].
Several scores have been proposed to assess EoE activity and predict remission and relapses. The Pediatric Eosinophilic Esophagitis Symptoms Score is a parent- or self-reported measure of EoE activity currently used for pediatric patients because it has good correlation with esophageal inflammation [35]. For adult patients, the Eosinophilic Esophagitis Activity Index patient-reported outcomes instrument is available: this quantifies the difficulties encountered by patients in swallowing different food and behavioral modification (during the meal), with modest predictive capacity to assess endoscopic or histologic remission [34]. Further and wider studies are needed to confirm the ability of the scores to predict actual EoE activity.
4 Diagnosis
Diagnosis of EoE is based on a combination of symptoms and histologic features. Multiple esophageal mucosal biopsies (at least six) are necessary to evaluate the peak eosinophil count, which represents the current main diagnostic criterion [1]. The diagnostic threshold is > 15 eosinophils/high power field (eos/HPF; standard size of around 0.3 mm2). This cut-off provides uniformity in EoE diagnosis, thus distinguishing it from other esophageal inflammatory diseases (mainly GERD with < 5 eos/HPF). This threshold has been shown have a sensitivity of 100% and a specificity of 96% [36].
The endoscopic lesions suggestive of EoE vary. The most common are edema, decreased vascularity of mucosal surfaces, longitudinal furrows, white plaques (spots or exudate), and concentric rings (also indicated as trachealization) up to the reduction of the esophageal caliber. Esophageal mucosa may also appear normal in 10–25% of patients with EoE [133, 134]. A meta-analysis of EoE endoscopic findings in more than 100 publications, comprising a total of 4678 patients with EoE and 2742 controls (both adults and children), revealed modest sensitivity and negative and positive predictive values for endoscopic features in predicting histologic inflammation [37]. An EoE endoscopic score, the EREFS (Exudate, Rings, Edema, Furrows, Strictures) was proposed as a standardized tool to classify the severity of mucosal lesions, with good interobserver agreement [38]. Histologic examination is then mandatory to correctly diagnose EoE. The six biopsies should be from different locations, mostly focusing in areas with mucosal abnormalities [1]. Beyond the eosinophil count, other nonspecific histologic alterations (i.e., eosinophil abscesses, basal-layer hyperplasia, dilated intercellular spaces, papillary elongation) have been associated with EoE. These abnormalities were recently scored for severity (grade) and extent (stage) in a specific scoring system (EoHSS) [39]. Lamina propria fibrosis was not included in this score because it is not always available on mucosal biopsy, but it should be evaluated to determine the effect of therapy on fibrotic changes [40].
Currently, no noninvasive biomarkers are available for EoE diagnosis and monitoring. Neither serum eosinophil count [41] nor level of eosinophil-derived proteins (including cationic eosinophil protein, eosinophil-derived neurotoxin, basic major protein [42, 43]) have been useful for this purpose. Minimally invasive devices, such as Cytosponge (an ingestible gelatin capsule comprising compressed mesh attached to a string), String test, and blind esophageal brushing have shown promising results in detecting esophageal eosinophilia. However, none of them are currently recommended in EoE clinical practice [44, 45].
The use of esophageal pH-multichannel luminal impedance is debated, and strong evidence is lacking. This tool may be used in patients with symptoms suggestive of GERD during the initial EoE assessment [46].
Lastly, barium swallow series have a role in esophageal stricture assessment when endoscopy is not technically possible, but not in EoE diagnosis. Upper gastrointestinal (GI) endoscopy and barium swallow series have similar sensitivity in identifying the remodeling consequences of EoE, but inflammatory features are better assessed on endoscopy [47, 48].
5 Treatment
Therapy of EoE should aim for symptoms improvement and esophageal inflammation resolution. Esophageal mucosa healing avoids long-term fibrotic sequelae and quality-of-life deterioration [49]. EoE therapy includes dietary modifications, PPI, and steroids. All are considered as first-line therapy in children with EoE, with no approach preferable to another (Fig. 2) [1], although poor patient or parental compliance might discourage a dietary intervention [79]. Endoscopic dilation is reserved for long-term untreated disease with fibrotic stenosis. Table 1 summarizes the indications for and the pros and cons of each option, as well as drug dosages.
Therapeutic algorithm for treatment of eosinophilic esophagitis in clinical practice. Reproduced with permission from Lucendo et al. [1]
5.1 Dietary Therapy
The rationale for diet modifications in EoE comes from the efficacy of dietary allergens as a trigger in the disease pathogenesis. Three major classes of elimination diets are in use: elemental, targeted by allergy testing, and empiric diet [50].
In 1995, Kelly et al. [51] demonstrated the efficacy of an exclusively amino acid-based formula in a small pediatric EoE series. All patients had normalized esophageal histology, and eight of ten also exhibited complete clinical remission. Since then, numerous studies, mostly retrospective, have corroborated these initial promising data. In a recent meta-analysis, the effectiveness of an elemental diet has been shown to be around 90% in adults and children [52]. However, the use of this diet is challenging for several factors: poor palatability, use of nasogastric tubes, lack of adherence, social and psychological impact, important quality of life impairment, and high costs. Thus, an elemental diet should be reserved for persistently active EoE resistant to other conventional drugs and/or empiric diets [1].
Targeted dietary therapy guided by allergy tests (including skin prick tests, atopy patch test, and serum-specific immunoglobulin E [IgE]) has demonstrated efficacy of about 45–53% in different studies in childhood [53, 54]. The main criticism of one of these studies was that food triggers were not identified by histologic remission but rather by symptom relapse reported by parents after individual food reintroduction [53]. A meta-analysis including 1128 children and 128 adults with EoE confirmed these data, reporting an effectiveness of allergy test-targeted diets in 45.5% of cases, although with a wide heterogeneity (I2 = 75%) and low reproducibility [52]. Its effectiveness was significantly lower in adults than in children (32.2 vs. 47.9%) [52]. Similar results were reported in some adult cases series [55, 56]. In a comprehensive study from Australia, Philpott et al. [57] assessed the accuracy of combining five different food allergy tests (skin prick and patch tests, serum allergen-specific IgE, basophil activation test, and serum food-specific IgG) to detect causative foods in adults with EoE. None of these tests could accurately predict food triggers [57].
An empiric diet is currently the best studied and most established dietary treatment in pediatric EoE; the elimination of the most frequent foods involved in immediate hypersensitivity is the rationale for this diet. The 6-Food Elimination Diet (6FED; avoiding milk, wheat, egg, soy, nuts, and fish/seafood) was reported for the first time in 2006, when Kagalwalla et al. [58] described a clinical and histological remission rate of 74% in 60 children with EoE. Similar results were obtained in other pediatric studies [64, 135]. By comparison, lower success rates were reported in an adult study (remission rate of 52% after 6 weeks of 6FED [59]). After the induction period, the aim is to identify the triggering foods, with a gradual reintroduction. The most common causative foods identified were cow’s milk, followed by wheat/gluten, egg, and, to a lesser extent, soy/legumes (with the exception of studies conducted in Spain, where legumes are consumed regularly) [60, 61]. By contrast, studies have consistently shown that nuts and fish/seafood rarely trigger EoE. After 8 weeks of using 6FED, the reintroduction process should include a histologic remission evaluation by endoscopy after each food challenge [1]. Although timeframes are not clear and standardized, a period of 8–12 weeks between the challenges, and 4–6 weeks from the latter to endoscopy, should be reasonable [50]. This process entails a rigorous sequence of upper endoscopy to assess histological remission after a food class reintroduction, with a negative psychological impact and high healthcare costs. On this basis, a step-up approach was developed to optimize dietary restrictions and endoscopic procedures, beginning with a two- or four-food elimination diet [63]. A first multicenter study in 78 patients from the USA showed a remission rate of 64% with a 4FED [62]. The frequency of triggering foods was similar to that in previous studies, and 45% of patients registered cow’s milk as a unique trigger. These data suggest that 2FED (exclusion of cow’s milk and wheat/gluten) could be sufficient to identify foods that cause EoE in a substantial proportion of affected children. This step-up approach was first assessed in a multicenter study conducted in 14 centers involving 130 consecutive adult and pediatric patients: all patients underwent 2FED (milk and gluten), and nonresponders were offered to escalate to 4FED and eventually to 6FED [63]. 2FED achieved EoE remission in 43% of patients, and 4FED and 6FED led to remission rates similar to those previously reported. Compared with starting with a 6FED, this step-up strategy allowed reduction of endoscopic procedures and shortening of the diagnostic process time [63]. Taking these results into consideration, a 6FED might be reserved for motivated patients unresponsive to a less restrictive empiric elimination diet.
Overall, although these dietary treatments have shown good efficacy in obtaining remission and identifying triggering foods, very weak evidence has been reported regarding long-term effectiveness to maintain drug-free disease remission. A first pediatric study described a relapse following rechallenge with triggering food after 4 years of an elimination diet [64]. Other more recent studies in adults reported a stable remission after 1 year of diet [59, 65], but data about longer follow-up with a diet maintenance therapy are needed.
5.2 Proton Pump Inhibitors
The role of PPIs in the management of EoE has changed over time. They were originally used in patients with esophageal eosinophilia to distinguish GERD from EoE. Later on, patients who responded to PPI were diagnosed as having a specific condition named “PPI-responsive esophageal eosinophilia” (PPI-REE) [66], considered an entity distinct from EoE and GERD. However, in 2017, the AGREE conference, involving experts from 14 countries, recognized significant overlap between PPI-REE and EoE in terms of Th2-mediated inflammation and abnormal gene expression [67]. Therefore, challenge with PPI was excluded from the diagnostic criteria, and PPI-REE was recognized within the same spectrum as EoE. Consequently, PPI therapy is now recognized as a valid first-line therapeutic option in patients with EoE, with recommended doses of omeprazole 1–2 mg/kg twice daily (BID) or equivalent in children and 20–40 mg BID in adults [1]. The efficacy of PPI in inducing remission seems to be related not only to the reduction of esophageal damage caused by acid exposure [86] but also to the anti-inflammatory effect. By blocking the Th2 response, PPIs lead to a reduction in inflammatory cytokines, thus repairing the epithelium [87, 88]. Evidence of PPI efficacy derives from several prospective randomized controlled trials (RCTs) [68,69,70,71], with clinic and histologic remission rates (defined as < 15 eos/HPF at biopsy) ranging from 33 to 57%, depending on the study design and patient population. Recently, a systematic review and meta-analysis of 33 studies, including 431 adults and 188 children, reported an overall efficacy of PPI in inducing histologic remission in about 50.5% of cases, with clinical improvement in 60.8% [72]. No differences were observed between children and adults. Moreover, the authors observed a trend toward significance for a higher efficacy of PPI when administered BID compared with once daily (55.9 vs. 49.7%, respectively). Higher remission rates have been reported in those with documented GERD, ranging from 70 to 80% [73]; nevertheless, esophageal pH monitoring failed to predict responsiveness to PPI treatment in other studies [74, 80, 81]. A negative correlation between the number of eos/HPF and response to PPI has been reported [74, 80]: patients with a higher peak eosinophil count were less likely to respond to PPIs. However, no defined threshold for PPI responsiveness has been identified. Clinicopathologic recurrence typically occurs 3–6 months after stopping treatment [75, 76]. Gutierrez-Junquera et al. [74] first demonstrated a sustained clinicopathologic remission at 1-year follow-up in children with PPI-REE receiving maintenance doses of PPI (esomeprazole 1 mg/kg/day) [74]. A sustained remission was demonstrated in about 80% of the patients when reducing the induction dose by between 25 and 50% [77]. Optimum maintenance doses have not been defined, so some authors have suggested that decreasing the dose to the lowest that keeps the disease in remission could be acceptable to minimize potential treatment complications [1, 78, 79, 82, 83]. PPIs are recognized as generally safe drugs, but possible recognized side effects of long-term acid suppression are dysbiosis, micronutrient malabsorption, osteoporosis, and possible higher risks for GI and respiratory infections [97, 98].
5.3 Topical Steroids
Swallowed/topical steroids are a recommended first-line therapy option in children with EoE [1]. Their action seems to be related to the nonspecific inhibition of the Th2 immune response, with secondary reduction of esophageal inflammation, remodeling, and fibrosis [84, 85]. Initially, Liacouras et al. [89] demonstrated the clinical effectiveness of corticosteroids in an observational study conducted in 20 children by using systemic (oral) methylprednisolone 1.5 mg/kg BID for 4 weeks. However, an RCT comparing oral prednisolone and swallowed (topical) fluticasone propionate over 12 weeks demonstrated an equal effectiveness in inducing clinicopathologic remission, with a lower rate of side effects in the topical steroid arm [90]. In this study, 40% of children treated with oral prednisolone and none of those in the topical steroid arm exhibited systemic effects (weight gain, hyperphagia, and/or cushingoid features), although 15% of patients receiving the topical steroid reported esophageal candidiasis. Therefore, systemic steroids are not recommended as first-line therapy in EoE [1]. Topical steroids are effective in children, but their efficacy or safety profile for chronic use is poorly understood [107]. Although the European Medicines Agency (EMA) approved a swallowed effervescent budesonide tablet in 2017 for use in adult patients with EoE [103], they are yet to approve a corticosteroid formulation for use in children, so current options are limited to swallowing aerosolized or nebulizer corticosteroids, with fluticasone and budesonide the best studied. Several RCTs have demonstrated the efficacy of topical steroids in inducing histologic remission in patients with EoE [91,92,93,94,95,96]. Topical steroids were superior to placebo in inducing improvements in dysphagia scores, despite not having statistically significant differences [93]. However, a meta-analysis of five RCTs conducted in children and/or adults comparing fluticasone and budesonide demonstrated that the latter was significantly superior to placebo in terms of symptomatic relief [odds ratio (OR) 7.20; 95% confidence interval (CI) 2.15–24.05] but not superior to fluticasone propionate (OR 1.27; 95% CI 0.44–3.65) [92]. No head-to-head trials have compared budesonide and fluticasone in children; available data are limited to indirect comparisons of data from different trials. Dellon et al. [100] evaluated the efficacy of oral viscous budesonide (OVB) and swallowed fluticasone in a randomized trial with placebo involving 129 adult and young adult patients with a new diagnosis of EoE: both the pharmacological arms showed an analogous clinical-endoscopic and histological improvement. Fluticasone propionate is administered directly into the patient’s mouth using a metered dose inhaler without a spacer and then swallowed; patients should then not drink for at least 30 min. Recommended doses are as follows: fluticasone 880–1760 mcg/day in children and 1760 mcg/day in adults; swallowed/topical budesonide 1–2 mg/day in children and 2–4 mg/day in adults [1].
Maintenance treatment is crucial in children, as disease generally relapses after cessation of therapy, and active inflammation may lead to progressive fibrosis over time [108]. Little evidence is available regarding a maintenance strategy. Oliva et al. [104] recently reported a sustained remission after induction therapy in 20 children receiving maintenance treatment with a 50% dose reduction of OVB. Similar results have been reported for half doses of swallowed fluticasone [105]. Guidelines support the use of long-term therapy with topical corticosteroids in maintaining remission in steroid-responsive patients, decreasing the dose by 50% [1]. However, available data are poor and conflicting regarding the long-term safety of these drugs [109,110,111].
Predictors of response to steroids are not understood. Wolf et al. [106] evaluated several potential predictive factors of response to topical steroids in a retrospective study conducted in 221 patients: multivariate regression analysis indicated the need for esophageal dilation at baseline endoscopy, and decreased levels of mast cells and eotaxin-3 were demonstrated to predict nonresponse; conversely, the presence of abdominal pain was a predictor of response to topical steroids. Oliva et al. [137] recently identified a different messenger RNA expression of a serine protease inhibitor (SERPINB12) in a pediatric cohort with a different tendency to relapse after discontinuation of topical steroids.
Unlike systemic steroids, topical steroids are generally considered safe. The most common associated complication is esophageal candidiasis, which occurs in 5–30% of cases [99]. Recently, herpes esophagitis has been reported as a possible complication [101, 102]. No evidence is currently available regarding the potential role of topical steroids in reduction of bone mineral density or growth retardation.
5.4 Biologic Agents
The role of immune cells and cytokines in EoE inflammatory processes suggests that agents directly modulating immune response might have substantial efficacy by minimizing the risk of adverse effects. Table 2 summarizes the most relevant clinical trials, both published and ongoing, using biologic agents in children and adolescents with EoE.
5.4.1 Interleukin (IL)-5 and Immunoglobulin E
The cytokine IL-5, as mentioned, has a recognized central role in the chronic Th2 inflammation that occurs in EoE. Monoclonal antibodies against IL-5 (mepolizumab, reslizumab) were evaluated in five studies in adults and children with EoE, the results of which indicated a significant reduction of esophageal eosinophilia and a mild reduction of clinical symptoms but without a complete histological remission, and with rapid relapse after discontinuation. The most frequent adverse events were headache, cough, nasal congestion, and upper respiratory tract infections [112,113,114,115,116]. In particular, a multicenter, double-blind randomized trial investigated the effect of mepolizumab in pediatric patients with EoE and reported a decrease of peak eosinophil counts from 122.5 to 40.2 eos/HPF. However, the primary outcome (< 5 eos/HPF at week 12) was achieved in only 8% of patients, and significant improvement in symptoms was not detected across dosing arms [114]. Another placebo-controlled trial with mepolizumab is currently ongoing in patients aged ≥ 16 years (clinicaltrials.gov NCT03656380). In a large phase III trial, 226 children with EoE received reslizumab in three different dosing arms every 4 weeks: < 25% of subjects achieved the threshold of < 15 eos/HPF, and no changes were observed in symptoms compared with placebo [115]. Preliminary data demonstrated the clinical and histologic efficacy of benralizumab (an antibody binding to the IL-5 receptor α on eosinophils) in patients with hypereosinophilic syndrome [136], and it is currently being investigated in adolescent and adult patients with eosinophilic gastritis (clinicaltrials.gov NCT03473977).
Omalizumab (a serum IgE-blocking agent) demonstrated no efficacy in reducing symptoms or tissue eosinophilia compared with placebo in a trial in 30 adults with EoE [117].
5.4.2 IL-13 and IL-4
As previous described, a large number of EoE-associated genes are induced by IL-13, so these molecules could be a promising target for biological therapy. A study in adult patients with monoclonal antibodies against IL-13 (QAX576, dectrekumab) showed a decrease of 60% in eosinophil load and a decrease in EoE-related gene expression but did not significantly improve clinical symptoms [118]. QAX576 appears to have been discontinued in all indications. RPC4046 (cendakimab), another anti–IL-13 monoclonal antibody, reduced esophageal eosinophilia and improved endoscopic features and dysphagia in adult patients, particularly in patients with steroid-refractory EoE [119]. The lack of full symptomatic response in this trial, despite histologic remission, may be due, in adults, to fibrotic remodeling of the esophageal wall and not necessarily to the pharmacologic failure of the drug.
A promising effective biologic is dupilumab, a monoclonal antibody that acts on the IL-4 receptor, thereby inhibiting IL-4/IL-13 signaling and negatively regulating the Th2 response. In a very recent phase II study, dupilumab reduced dysphagia, histologic features of disease (including eosinophilic infiltration and a marker of type 2 inflammation), and abnormal endoscopic features compared with placebo in 23 adult patients with EoE [120]. A large phase III trial of dupilumab in adolescents and adults with EoE is ongoing and scheduled to complete in 2023 (clinicaltrials.gov NCT03633617). In conclusion, of the already evaluated biologics, dual targeting of IL-13 and IL-4 appears to represent the most promising approach in EoE.
5.4.3 Novel Targets
Other possible targets for biologic agents are receptors and cytokines involved in epithelial barrier disruption and eosinophil migration. Although clinical trials are not available yet, some studies on IL-33-blocking agents, macrophage migration inhibition factor, and TSLP receptors in murine models of EoE seem to show promising results [121, 122, 138].
5.5 Other Medical Therapies
The mast cell stabilizer cromolyn sodium is not effective in EoE. In a randomized double-blind trial in 16 children, the efficacy of viscous oral cromolyn (100 or 200 mg/day for 8 weeks) was compared with placebo. Cromolyn failed to improve clinical symptoms and did not decrease the peak eosinophil count [127].
Montelukast, a leukotriene receptor antagonist, was shown in an open-label trial to induce symptomatic relief in adult patients with EoE without affecting esophageal eosinophilia [123]. Unfortunately, these data have not been confirmed in controlled trials of this medication [124, 125]. The use of montelukast in pediatric patients with EoE has not been assessed. The use of leukotriene inhibitors for treatment of EoE is therefore not recommended [1].
In analogy with inflammatory bowel disease, immunomodulators have been proposed as sparing agents for topical steroid therapy. Indeed, in a small series of adult patients with refractory EoE, purine analogs were effective in maintaining remission [126]. However, pediatric trials with these drugs are not available, and this possible therapeutic strategy has not been further evaluated with controlled clinical trials, so thiopurines are not currently recommended for EoE.
5.6 Endoscopic Dilation
The most serious complication of EoE, both in pediatric and adult patients, is the development of tight esophageal strictures as a consequence of remodeling and consequent fibrosis of mucosa, especially in the lamina propria. Although the causes leading to the “stenosing” phenotype in some patients remain unclear, the duration of untreated disease is currently the most important risk factor for strictures [1]. Esophageal dilation is usually indicated in severe fibrostenotic EoE strictures unresponsive to medical/dietary treatment; these represent the minority of strictures in children. Indeed, the first medical treatment indicated in mild recent inflammatory EoE strictures should be topical steroids before esophageal endoscopic dilation [128]. Esophageal dilation may be considered first-line treatment for very severe strictures and/or significant dysphagia and frequent symptoms necessitating prompt improvement. In this eventuality, a concomitant pharmacological therapy should also be promptly initiated. Endoscopic dilation using hydrostatic balloon dilation or Savary-Gilliard-Bougie (both are safe and effective [129, 130]) should be considered in children with lumen narrowing not responsive to steroid and/or PPI therapy, with serious symptoms that interfere with adequate diet or—in severe stenosis with a marked fibrotic character, where a significant benefit from drug therapy is not expected [131, 132].
6 Conclusions
Many questions regarding the management of EoE in children remain unanswered (Table 3). Several studies are evaluating the molecular mechanisms underlying EoE and possible therapeutic targets [138]. The ultimate aim is to introduce a personalized approach. Meanwhile, current efforts should focus on optimization of PPIs and topical steroids, either alone or in combination with a tailored elimination diet. Dilation is a rescue therapy for the narrowed, fibrostenotic, symptomatic esophagus, but this should be rare in children with adequate maintenance therapies. The complexity of the treatment regimens and the frequent follow-up evaluations require a multimodal, multidisciplinary management approach for optimization of patient care.
References
Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5:335–58.
Gonzales-Cervera J, Lucendo AJ. Eosinophilic esophagitis: an evidence-based approach to therapy. J Investig Allergol Clin Immunol. 2016;26:8–18.
Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16.
Navarro P, Arias Á, Arias-González L, Laserna-Mendieta EJ, Ruiz-Ponce M, Lucendo AJ. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2019;49:1116–25.
Jensen ET, Hoffman K, Shaheen NJ, Genta RM, Dellon ES. Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol. 2014;109:668–75.
Jensen ET, Shah ND, Hoffman K, Sonnenberg A, Genta RM, Dellon ES. Seasonal variation in detection of oesophageal eosinophilia and eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;42:461–9.
Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 2018;154:319–32.
Lyles J, Rothenberg M. Role of genetics, environment, and their interactions in the pathogenesis of eosinophilic esophagitis. Curr Opin Immunol. 2019;60:46–53.
Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, Mukkada VA, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134:1084–92.
Allen-Brady K, Firszt R, Fang JC, Wong J, Smith KR, Peterson KA. Population-based familial aggregation of eosinophilic esophagitis suggests a genetic contribution. J Allergy Clin Immunol. 2017;140:1138–43.
O’Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology. 2018;154:333–45.
Kottyan LE, Rothenberg ME. Genetics of eosinophilic esophagitis. Mucosal Immunol. 2017;10:580–8.
Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson P, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–47.
Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593.
Litosh VA, Rochman M, Rymer JK, Porollo A, Kottyan LC, Rothenberg ME. Calpain-14 and its association with eosinophilic esophagitis. J Allergy Clin Immunol. 2017;139:1762–71.
Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–96.
Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Bio. 2010;43:305–15.
Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–8.
Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–133.
Rajavelu P, Rayapudi M, Moffitt M, Mishra A. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G645–G654654.
Rayapudi M, Mavi P, Zhu X, Pandey AK, Abonia JP, Rothenberg ME, et al. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2010;88:337–46.
Benitez AJ, Hoffman C, Muir AB, Dods KK, Spergel JM, Bushman FD, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome. 2015;1(3):23.
Harris JK, Fang R, Wagner BD, Choe HN, Kelly CJ, Schroeder S, et al. Esophageal microbiome in eosinophilic esophagitis. PLoS ONE. 2015;10:e0128346.
Von Armin U, Wex T, Link A, Messerschmidt M, Venerito M, Miehlke S, et al. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic esophagitis. Aliment Pharmacol Ther. 2016;43:825–30.
Jensen ET, Dellon ES. Environmental factors and eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142:32–40.
Guajardo JR, Zegarra-Bustamante MA, Brooks EG. Does aeroallergen sensitization cause or contribute to eosinophilic esophagitis? Clin Rev Allergy Immunol. 2018;55:65–9.
Lucendo AJ, Sanchez-Cazalilla M. Adult versus pediatric eosinophilic esophagitis: important differences and similarities for the clinician to understand. Expert Rev Clin Immunol. 2012;8:733–45.
Lucendo AJ, Arias-González L, Molina-Infante J, Arias Á. Systematic review: health-related quality of life in children and adults with eosinophilic oesophagitis—measure instruments and determinant factors. Aliment Pharmacol Ther. 2017;46:401–9.
Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–85.
Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and oesophageal diameter. J Clin Gastroenterol. 2016;50:134–40.
Runge TM, Dellon ES. Do we know what causes eosinophilic esophagitis? A mechanistic update. Curr Gastroenterol Rep. 2015;17:33.
Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(577–585):e4.
Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon H-U, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time dependent manner. Gastroenterology. 2013;145:1230–6.
Schoepfer AM, Straumann A, Panczak R, Coslovsky M, Kuehni CE, Maurer E, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255–66.
Martin LJ, Franciosi JP, Collins MH, Abonia JP, Lee JJ, Hommel KA, et al. Pediatric eosinophilic esophagitis symptom scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol. 2015;135(6):1519–28. https://doi.org/10.1016/j.jaci.2015.03.004.
Dellon ES, Speck O, Woodward K, Covey S, Rusin S, Shaheen NJ, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol. 2015;28:383–90.
Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988–96.
Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–95.
Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43:257–68.
Kagalwalla AF, Akhtar N, Woodruff SA, Rea BA, Masterson JC, Mukkada V, et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol. 2012;129:1387–96.
Min SB, Nylund CM, Baker TP, Ally M, Reinhardt B, Chen YJ, et al. Longitudinal evaluation of noninvasive biomarkers for eosinophilic esophagitis. J Clin Gastroenterol. 2017;51(2):127–35.
Rao GS, Mitchell L, Ohnuki JF, Fitzgerald J, Gleich GJ, Corkins M, et al. Can eosinophil derived neurotoxin (EDN) act as a surrogate marker of disease activity in children with allergic eosinophilic esophagitis (AEE)? Gastrointest Endosc. 2004;59:P103.
Dellon ES, Rusin S, Gebhart JH, Covey S, Higgins LL, Beitia R, et al. Utility of a noninvasive serum biomarker panel for diagnosis and monitoring of eosinophilic esophagitis: a prospective study. Am J Gastroenterol. 2015;110:821–7.
Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395–405.
Katzka DA, Geno DM, Ravi A, Smyrk TC, Lao-Sirieix P, Miremadi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:77–83.
Rosen R, Amirault J, Johnston N, Haver K, Khatwa U, Rubinstein E, et al. The utility of endoscopy and multichannel intraluminal impedance testing in children with cough and wheezing. Pediatr Pulmonol. 2014;49(11):1090–6.
Menard-Katcher C, Swerdlow MP, Mehta P, Furuta GT, Fenton LZ. Contribution of esophagram to the evaluation of complicated pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2015;61(5):541–6.
Nelson MJ, Miller FH, Moy N, Zalewski A, Gonsalves N, Gregory DL, et al. Comparison of endoscopy and radiographic imaging for detection of esophageal inflammation and remodeling in adults with eosinophilic esophagitis. Gastrointest Endosc. 2018;87:962–8.
Straumann A, Katzka DA. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterology. 2018;154:346–59.
Molina-Infante J, Lucendo AJ. Dietary therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142:41–7.
Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–12.
Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639–48.
Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–8.
Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–7.
Molina-Infante J, Martin-Noguerol E, Alvarado-Arenas M, Porcel-Carreno SL, Jimenez-Timon S, Hernandez-Arbeiza FJ. Selective elimination diet based on skin testing has suboptimal efficacy for adult eosinophilic esophagitis. J Allergy Clin Immunol. 2012;130:1200–2.
Wolf WA, Jerath MR, Sperry SLW, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12:1272–9.
Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. Allergy tests do not predict food triggers in adult patients with eosinophilic oesophagitis. A comprehensive prospective study using five modalities. Aliment Pharmacol Ther. 2016;44:223–33.
Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–102.
Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. A prospective open clinical trial of a proton pump inhibitor, elimination diet and/or budesonide for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43:985–93.
Hoofien A, Dias JA, Malamisura M, Rea F, Chong S, Oudshoorn J, et al. Pediatric eosinophilic esophagitis: results of the European retrospective pediatric eosinophilic esophagitis registry (RetroPEER). J Pediatr Gastroenterol Nutr. 2019;68(4):552–8.
Lucendo AJ, Arias A, Gonzalez-Cervera J, Yague-Compadre JL, Guagnozzi D, Angueira T, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol. 2013;131:797–804.
Kagalwalla AF, Wechsler JB, Amsden K, Schwartz S, Makhija M, Olive A, et al. Efficacy of a 4-food elimination diet for children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2017;15:1698–707.
Molina-Infante J, Arias A, Alcedo J, Garcia-Romero R, Casabona-Frances S, Prieto-Garcia A, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2-4-6 study. J Allergy Clin Immunol. 2018;141(4):1365–72.
Kagalwalla AF, Shah A, Li BU, Sentongo TA, Ritz S, Manuel-Rubio M, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–9.
Reed CC, Fan C, Koutlas NT, Shaheen NJ, Dellon ES. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46:836–44.
Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, American College of Gastroenterology. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–92.
Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;155(4):1022–33.e10.
Vazquez-Elizondo G, Ngamruengphong S, Khrisna M, Devault KR, Talley NJ, Achem SR. The outcome of patients with oesophageal eosinophilic infiltration after an eight-week trial of a proton pump inhibitor. Aliment Pharmacol Ther. 2013;38:1312–9.
Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108:1854–60.
Moawad FJ, Veerappan GR, Dias JA, Baker TP, Maydonovitch CL, Wong RK. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol. 2013;108:366–72.
Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci. 2010;55:1313–9.
Lucendo AJ, Arias A, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;4:13–22.
Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110–7.
Gutierrez-Junquera C, Fernandez-Fernandez S, Cilleruelo ML, Rayo A, Echeverría L, Quevedo S, et al. High revalence of response to proton-pump inhibitor treatment in children with esophageal eosinophilia. J Pediatr Gastroenterol Nutr. 2016;62:704–10.
Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.
Molina-Infante J, Rodriguez-Sanchez J, Martinek J, van Rhijn BD, Krajciova J, Rivas MD, et al. Long-term loss of response in proton pump inhibitor-responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am J Gastroenterol. 2015;110:1567–75.
Gómez Torrijos E, García-Rodríguez R, Castro-Jiménez A, Rodríguez-Sanchez J, Méndez Díaz Y, Molina-Infante J. The efficacy of step-down therapy in adult patients with proton pump inhibitorresponsive oesophageal eosinophilia. Aliment Pharmacol Ther. 2016;43:534–40.
Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147:1238–54.
Cotton CC, Durban R, Dellon ES. Illuminating elimination diets: controversies regarding dietary treatment of eosinophilic esophagitis. Dig Dis Sci. 2019;64(6):1401–8.
Dranove JE, Horn DS, Davis MA, Kernek KM, Gupta SK. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr. 2009;154:96–100.
Rea F, Caldaro T, Tambucci R, Romeo EF, Caloisi C, Torroni F, et al. Eosinophilic esophagitis: is it also a surgical disease? J Pediatr Surg. 2013;48:304–8.
Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611–6.
Ye X, Liu H, Wu C, Qin Y, Qin Y, Zang J, Gao Q, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23(9):794–800.
Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65(1):109–16.
Van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, de Jonge WJ, et al. Histological response to fluticasone propionate in patients with eosinophilic esophagitis is associated with improved functional esophageal mucosal integrity. Am J Gastroenterol. 2015;110(9):1289–97.
Richter JE. Endoscopic treatment of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2018;28:97–110.
Molina Infante J, Rivas MD, Hernandez-Alonso M, Vinagre-Rodríguez G, Mateos-Rodríguez JM, Dueñas-Sadornil C, et al. Proton pump inhibitor-responsive oesophageal eosinophilia correlates with downregulation of eotaxin-3 and Th2 cytokines overexpression. Aliment Pharmacol Ther. 2014;40:955–96.
Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS ONE. 2012;7:e50037.
Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998;26(4):380–5.
Schaefer ET, Fitzgerald JF, Molleston JP, Croffie JM, Pfefferkorn MD, Corkins MR, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165–73.
Chuang MYA, Chinnaratha MA, Hancock DG, Woodman R, Wong GR, Cock C, et al. Topical steroid therapy for the treatment of eosinophilic esophagitis (EoE): a systematic review and meta-analysis. Clin Transl Gastroenterol. 2015;6:e82.
Murali AR, Gupta A, Attar BM, Ravi V, Koduru P. Topical steroids in eosinophilic esophagitis: systematic review and metaanalysis of placebo-controlled randomized clinical trials. J Gastroenterol Hepatol. 2016;31:1111–9.
Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41:797–806.
Tan ND, Xiao YL, Chen MH. Steroids therapy for eosinophilic esophagitis: systematic review and meta-analysis. J Dig Dis. 2015;16:431–42.
De Rooij WE, Dellon ES, Parker CE, Feagan BG, Jairath V, Ma C, et al. Pharmacotherapies for the treatment of eosinophilic esophagitis: state of the art review. Drugs. 2019;79(13):1419–34.
Oliva S, Rossetti D, Papoff P, Tiberti A, Rossi P, Isoldi S, et al. A New formulation of oral viscous budesonide in treating paediatric eosinophilic oesophagitis: a pilot study. J Pediatr Gastroenterol Nutr. 2017;64(2):218–24.
Eusebi LH, Rabitti S, Artesiani ML, et al. Proton pump inhibitors: risks of long-term use. J Gastroenterol Hepatol. 2017;32(7):1295–302.
De Bruyne P, Ito S. Toxicity of long-term use of proton pump inhibitors in children. Arch Dis Child. 2018;103(1):78–82.
Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus rec-ommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.
Dellon ES, Woosley JT, Arrington A, McGee SJ, Covington J, Moist SE, et al. Efficacy of budesonide vs fluticasone for initial treatment of eosinophilic esophagitis in a randomized controlled trial. Gastroenterology. 2019;157:65.
Lindberg GM, Van Eldik R, Saboorian MH. A case of her-pes esophagitis after fluticasone propionate for eosinophilicesophagitis. Nat Clin Pract Gastroenterol Hepatol. 2008;5:527–30.
Machicado JD, Younes M, Wolf DS. An unusual cause ofodynophagia in a patient with eosinophilic esophagitis. Gastroenterology. 2014;147:37–8.
European Medicines Agency. New medicine for rare inflammatory condition of the oesophagus: Jorveza approved under accelerated assessment. 9 November 2017. https://www.ema.europa.eu/en/news/new-medicine-rare-inflammatory-condition-oesophagus.
Oliva S, Rossetti D, Papoff P, Tiberti A, Mallardo S, Volpe D, et al. A 12-week maintenance therapy with a new prepared viscous budesonide in pediatric eosinophilic esophagitis. Dig Dis Sci. 2019;64(6):1571–8.
Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014;147:324–33.
Wolf WA, Cotton CC, Green DJ, Hughes JT, Woosley JT, Shaheen NJ, et al. Predictors of response to steroid therapy for eosinophilic esophagitis and treatment of steroid-refractory patients. Clin Gastroenterol Hepatol. 2015;13:452.
Philpott H, Dellon ES. The role of maintenance therapy in eosinophilic esophagitis: who, why, end how? J Gastroenterol. 2018;53:165–71.
Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206.
Harel S, Hursh B, Chan E, Avinashi V, Panagiotopoulos C. Adrenal suppression in children treated with oral viscous budesonide for eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2015;61:190–3.
Ahmet A, Benchimol E, Goldbloom E, Barkey JL. Adrenal suppression in children treated with swallowed fluticasone and oral viscous budesonide for eosinophilic esophagitis. Allergy Asthma Clin Immunol. 2016;12:49.
Philpott H, Dougherty MK, Reed CC, Caldwell M, Kirk D, Torpy DJ, et al. Systematic review: adrenal insufficiency secondary to swallowed topical corticosteroids in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2018;47:1071–8.
Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–9.
Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30.
Assaad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–604.
Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(456–463):463.e1–.e3.
Markowitz JE, Jobe L, Miller M, Frost C, Laney Z, Eke R. Safety and efficacy of reslizumab for children and adolescents with eosinophilic esophagitis treated for 9 years. J Pediatr Gastroenterol Nutr. 2018;66:893–7.
Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–9.
Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135:500–7.
Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer A, et al. Anti-Interleukin-13 monoclonal antibody (RPC4046) in adult patients with active Eosinophilic Esophagitis. Gastroenterology. 2019;156(3):592–603.
Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2019;158:111–22.
Venturelli N, Lexmond WS, Ohsaki A, Nurko S, Karasuyama H, Fiebiger E, et al. Allergic skin sensitization promotes eosinophilic esophagitis through the IL-33-basophil axis in mice. J Allergy Clin Immunol. 2016;138:1367–80.
De Souza HS, Tortori CA, Lintomen L, Figueiredo RT, Bernardazzi C, Leng L, et al. Macrophage migration inhibitory factor promotes eosinophil accumulation and tissue remodeling in eosinophilic esophagitis. Mucosal Immunol. 2015;8:1154–65.
Attwood SEA, Lewis CJ, Bronder CS, Morris CD, Armstrong GR, Whittam J. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut. 2003;52:181–5.
Lucendo AJ, de Rezende LC, Jimenez-Contreras S, Yagüe-Compadre JL, González-Cervera J, Mota-Huertas T, et al. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Dig Dis Sci. 2011;56:3551–8.
Alexander JA, Ravi K, Enders FT, Geno DM, Kryzer LA, Mara KC, et al. Montelukast does not maintain symptom remission after topical steroid therapy for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2017;15:214–21.
Netzer P, Gschossmann JM, Straumann A, Sendensky A, Weimann R, Schoepfer AM. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur J Gastroenterol Hepatol. 2007;19:865–9.
Lieberman JA, Zhang J, Whitworth J, Cavender C. A randomized, double-blinded, placebo-controlled study of the use of viscous oral cromolyn sodium for the treatment of eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018;120(5):527–31.
Runge TM, Eluri S, Woosley JT, Shaheen NJ, Dellon ES. Control of inflammation decreases the need for subsequent esophageal dilation in patients with eosinophilic esophagitis. Dis Esophagus. 2017;30:1–7.
Dougherty M, Runge TM, Eluri S, Dellon ES. Esophageal dilation with either bougie or balloon technique as a treatment for eosinophilic esophagitis: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86(4):581–591.e3.
Moawad FJ, Molina-Infante J, Lucendo AJ, Cantrell SE, Tmanova L, Douglas KM. Systematic review with meta-analysis: endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46(2):96–105.
Kavitt RT, Ates F, Slaughter JC, Higginbotham T, Shepherd BD, Sumner EL, et al. Randomized controlled trial comparing esophageal dilation to no dilation among adults with esophageal eosinophilia and dysphagia. Dis Esophagus. 2016;29(8):983–91.
Menard-Katcher C, Furuta GT, Kramer RE. Dilation of pediatric eosinophilic esophagitis: adverse events and short-term outcomes. J Ped Gastroenterol Nutr. 2017;64:701–6.
Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County. Minnesota Clin Gastroenterol Hepatol. 2009;7:1055–61.
Müller S, Pühl S, Vieth M, Stolte M. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy. 2007;39:339–44.
Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129:1570–8.
Kuang FL, Kumar S, Powers A, Quezado M, Wang Z, Ware JA, et al. Benralizumab (anti-IL5Ra) depletes gut tissue eosinophilia and improves symptoms in hypereosinophilic syndrome with gastrointestinal involvement. J Allergy Clin Immunol. 2018;141:196.
Oliva S, Laudadio I, Fulci V, Rossetti D, Isoldi S, Stronati L, et al. SERPINB12 as a possible marker of steroid dependency in children with eosinophilic esophagitis: a pilot study. Dig Liver Dis. 2020;52:158–63.
Oliva S, Azouz NP, Stronati L, Rothenberg ME. Recent advances in potential targets for eosinophilic esophagitis treatments. Expert Rev Clin Immunol. 2020;16(4):421–8.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Danilo Rossetti, Sara Isoldi, and Salvatore Oliva have no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Rossetti, D., Isoldi, S. & Oliva, S. Eosinophilic Esophagitis: Update on Diagnosis and Treatment in Pediatric Patients. Pediatr Drugs 22, 343–356 (2020). https://doi.org/10.1007/s40272-020-00398-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-020-00398-z