Abstract
Background
Some patients with complex healthcare needs become high users of healthcare services. Case management allows these patients and their interprofessional team to work together to evaluate their needs, priorities and available resources. High-user patients must make an informed decision when choosing whether to engage in case management and currently there is no tool to support them.
Objective
The objective of this study was to develop and conduct a pilot alpha testing of a patient decision aid that supports high-user patients with complex needs and the teams who guide those patients in shared decision making when engaging in case management.
Methods
We chose a user-centered design to co-develop a patient decision aid with stakeholders informed by the Ottawa Research Institute and International Patient Decision Aid Standards frameworks. Perceptions and preferences for the patient decision aid’s content and format were assessed with patients and clinicians and were iteratively collected through interviews and focus groups. We developed a prototype and assessed its acceptability by using a think-aloud method and a questionnaire with three patient-partners, six clinicians and seven high-user patients with complex needs.
Results
The three rounds of evaluation to assess the decision aid’s acceptability highlighted comments related to simplicity, readability and visual aspect. A section presenting clinical vignettes including story telling was identified as the most helpful.
Conclusions
We created and evaluated a patient decision aid. Considering the positive comments, we believe that this aid has the potential to help high-user patients with complex care needs make better choices concerning case management.
Plain Language Summary
Some patients are living with physical and mental health problems. They also may have handicaps and unsuitable backgrounds. This may lead them to use health services more often. Case management is a service offered by a team of health professionals. They help patients to decide what is important to them based on their values and preferences. Currently, no tools exist for that service. We built and assessed a tool to support patients in their decisions. With this tool, they think about engaging in case management or continuing with usual care. They can also postpone their decision to a later time. This tool will present data based on scientific studies about case management. It will help patients to clarify their values and preferences to make the best decision for them. This tool was built with a team of researchers, healthcare professionals, managers and patient-partners. It was built according to several guidelines. We met participants and they answered questions that helped us to build our tool. We also ensured the tool was acceptable to them. The most frequent comments were to make it simpler and to use simple vocabulary. The look was also important for the participants. The latter found that the section where patients could write their own story was useful. Patients also found that reading stories about other patients like them was helpful. Our tool will help patients with complex care needs make better choices concerning their health based on their values and scientific data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Complex interventions such as case management need to be better described so that they can be improved by researchers and better translated to patients. |
Current guidelines for the creation of a patient decision aid are not optimal for complex interventions that depend on multiple elements. |
The co-creation of a patient decision aid must involve several stakeholders such as patient-partners, decision-makers and clinicians. |
1 Introduction
“Patients with complex care needs” is a term used to describe a subpopulation of patients with multimorbidity, psychiatric comorbidities and/or psychosocial factors with or without functional limitations [1, 2]. Their level of independence and functionality may bring a part of this population to use healthcare services more frequently (high users) and involve more complexity than the general population [1, 3,4,5,6]. A recent systematic review on high users showed that they are generally older and experience multiple chronic conditions [7]. They often have circulatory diseases and mental and behavioural disorders [7]. For the remainder of the article, we use the term “patients” and it will refer to patients with complex care needs and who are high users of healthcare services. In the Province of Quebec (Canada), the majority of those patients are elderly women who present with coronary heart diseases or diabetes mellitus [8]. Some of them are persistent high users and others are occasional users [8]. More than 80% of these patients have chronic conditions such as asthma, chronic obstructive pulmonary disease, diabetes, hypertension and atherosclerosis [9, 10]. In Canada, which has a publicly funded health system [11], those patients are responsible for 50% of the expenditures [12]. Clinicians must better address patients’ needs to improve patient-related outcomes by using patient-centred care that is adapted for patients with complex conditions [13].
Case management (CM) may help support those specific patients and their clinicians [14]. Case management programmes involve both an interprofessional team (nurses, physician, social workers) and the patient to work together to evaluate needs, priorities and available resources [15,16,17,18]. Case managers plan, facilitate and coordinate patient-centred healthcare to provide patients with the right service at the right time [18, 19]. Moreover, they also provide education, self-management support and offer a personalised service allowing direct communication. Case management can reduce emergency department visits, improve patients’ quality of life and increase clinicians’ satisfaction [6, 20]. However, it requires a high level of engagement from both patients and clinicians to produce positive outcomes [19].
To decrease patients’ decisional conflict, the use of shared decision making is known to have a positive impact on both the patient and healthcare providers [21]. The purpose of shared decision making is to help patients understand the evidence-based healthcare involved in their care before making any decision and to help practitioners explore and consider patient values related to the decision. It helps patients clarify their values and identify the influence of external societal pressures, allowing them to regain control over their health and to be comfortable with their decisions. From this process, patients can have clear and realistic expectations about their care, and they become more aware of the conflicting aspects of the decision [22,23,24,25]. Shared decision making is also known to improve patients’ affective, behavioural and health outcomes [26]. In such a model, patients and clinicians relate to, and influence, each other as they collaborate in making the right decision corresponding to patients’ values and needs.
Although some tools have been developed for shared decision making for specific populations, currently, there is no patient decision aid (PtDA) promoting an interprofessional approach supporting these patients in their decision-making process to engage in CM. This study aims to develop and evaluate a PtDA to help patients in engaging in CM, which presents the following options: (1) to engage in CM; (2) to not engage in CM; or (3) to postpone their decision and to assess its acceptability.
2 Methods
We obtained approval to conduct this study from the Ethics Committee of the Centre Intégré Universitaire de Santé et de Services Sociaux du Saguenay-Lac-Saint-Jean.
2.1 Theoretical Frameworks and Conceptual Models
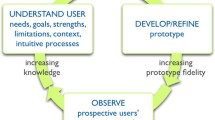
We used the Ottawa Decision Support Framework [24], a highly relevant, evidence-based theoretical model including input from several domains that facilitates the development of interventions for healthcare providers involving shared decision making [27]. It allows professionals to improve the quality of decision processes through the evaluation of what could influence decision making. The interprofessional shared decision-making conceptual model [28] also guided the creation of our primary care PtDA allowing all stakeholders [29] to share their knowledge. This model allows the adaptation of the aid in response to the actual needs of current health and social services networks and therefore uses an integrative and coherent approach. As suggested by Coulter et al. [30], we also based our work on the user-centred design [31] conceptual model, which is a proven framework for the development of products and services. The user-centred design model is an iterative method allowing optimisation of the user experience and maximisation of usability and understandability [32]. Finally, we also used the criteria of the International Patient Decision Aid Standard [33] to produce a good-quality and effective PtDA. Figure 1 illustrates the methodology used and the four design steps needed prior to the prototype drafting.

adapted from Coulter et al. [30]
Schematic of the systematic development process for our patient decision aid,
2.2 Development of the Decision Aid
2.2.1 Designs 1 and 2: Scoping and Patients’ and Clinicians’ Views on Decisional Needs
Our team performed the scoping of more than 70 patients and clinicians’ views on decisional needs between 2016 and 2018. This study took a pragmatic approach [34, 35] and the complete results are published elsewhere [29]. Briefly, results revealed that patients frequently face difficult dilemmas regarding their choices, or even priorities, in terms of health management [29]. We also found that, according to patients and clinicians, the decision about engaging in CM (or not) was crucial to reach patient health-related outcomes. Patients and clinicians revealed that a decision aid could better support shared decision-making processes to engage (or not) in CM. More specifically, patients revealed that this decision aid could inform them about the harms and benefits of each option. Clinicians described that a decision aid could help them be more comfortable when they presented options and scientific evidence. Clinicians perceived that a decision aid could support patients in reiterating their choice to remain engaged in a CM program. Indeed, clinicians observed that the patients’ engagement decreases over time. Including several stakeholders from multiple backgrounds allowed us to obtain a wider spectrum of comments representing different perspectives on the decision aid.
2.2.2 Design 3: Content, Design and Distribution Plan
This part of the user-centred design was embedded in the study aiming to assess decisional needs. While assessing the clinicians and patients’ views on decisional needs (design steps 1 and 2), we also asked them about their preferences regarding content, visual aspect and format of the PtDA (Electronic Supplementary Material [ESM]). Focus groups and individual interviews were recorded and transcribed. Analysis was performed in an iterative manner. We performed a qualitative hybrid thematic analysis (deductive/inductive) assisted by NVivo 11 Software to identify relevant content and format for the PtDA. We also identified facilitators and barriers of the use of the PtDA to build an efficient distribution plan in further steps. This type of analysis allows the combination of themes derived from philosophical frameworks (deductive) and those emerging from participants’ discussions (inductive). The coding scheme was supported by the user experience honeycomb that allows exploration of several facets of experience such as usability, accessibility, credibility and usefulness.
2.2.3 Design 4: Review and Synthesise Evidence
Informed by the results of a systematic review on the characteristics of CM in primary care for frequent users of healthcare by Hudon and colleagues [19], we aimed to include data on the frequency of hospitalisation, length of hospitalisation, emergency visits and the cost of hospitalisation. Briefly, this systematic review, guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines, identified 22 eligible publications. Because of the low number and high heterogeneity of the studies, the pooling of the studies and meta-analysis was feasible for the cost of hospitalisation only (no difference was observed, data not shown). More details about the methods used for this systematic review can be found in the published paper [19].
Taking this into account and to better translate evidence to patients, our team chose to support our decision aid with the literature synthesis of articles (without a meta-analysis) included in Hudon and colleagues’ systematic review [19], which considers the influence of contexts and interactional elements on patient outcomes (harms and benefits).
2.2.4 Prototype
Deductive analysis complemented by inductive analysis allowed the identification of new themes emerging from interviews. Data were triangulated among sources and discussed according to the conceptual frameworks used to support the development process in a shared decision context. With the comments of stakeholders and data generated from the literature synthesis, we created a prototype of a PtDA.
2.3 Alpha Testing
Coulter and colleagues [30] recommend conducting alpha testing with both patients and clinicians. We therefore included three patient-partners and six case managers in the design step based on their availability and interest. We also recruited seven patients, through regional case managers, who evaluated the aid and allowed us to reach data saturation. Individual interviews using think-aloud methods [37] were conducted using the user experience honeycomb [38]. As we used a user-centred design, which is iterative, the number of evaluation rounds needed is not predefined and is rather defined by the needs expressed by the stakeholders. In our case, three rounds were required to reach acceptability.
After the interview, participants were invited to complete an adaptation of the Decision Self Efficacy Scale Questionnaire developed by O’Connor [39] and Lalonde (ESM) to measure the acceptability of our PtDA, both quantitatively and qualitatively. Briefly, this survey contains nine questions to assess the content and presentation of the PtDA, two questions graded from 1 to 10 to measure the general appreciation of content and visual aspect and finally, three open-ended questions to identify aspects that were appreciated, disliked and may need improvement. Quantitative data were analysed with Excel software and qualitative data with content analysis. After each round, the research team adapted the PtDA according to participants’ feedback and a final version of the prototype was available for alpha testing.
3 Results
3.1 Determination of Content and Design
We found that a meta-analysis was not the appropriate method to document the effectiveness and outcomes of a complex intervention such as CM, even less so when patients are also presenting complex conditions. In the context of the literature synthesis for the construction of the PtDA, the meta-analysis was possible only for the cost of hospitalisation and there was no difference between control and intervention groups (data not shown). According to patient-partners and clinicians, this outcome was not relevant for shared decision making in a Canadian context of care because patients do not have to pay for their hospitalisation as it is publicly funded. We therefore did not include this result in our PtDA. The previous work performed by Hudon et al. [19] allowed us to identify and include in our PtDA the following categories of patient-related outcomes: healthcare condition; quality of life, use of healthcare services, relationship between patients and healthcare professionals, and accessibility to information and healthcare services.
All stakeholders agreed on the relevance of a PtDA to help patients assess their preferences and make a decision on their engagement in CM. All participants wanted an aid that is accessible, simple and easy to use to avoid burdening their tasks. We did not reach a consensus on the format because some participants, regardless of their occupation, preferred a paper format and others a digital format. For clinicians and decision makers who preferred the digital format, many of them mentioned that it would be preferable to connect the PtDA to current electronic medical software. Clinicians would like to have a section where the patient could write down his or her needs. Decision makers mentioned that patients would appreciate videos on the PtDA.
Clinicians expressed some concerns about the confidentiality aspect as a limit to PtDA use. Some of them reported the fact that using a tool in a paper format could allow anyone to have access to personal data recorded on the tool. Thus, it would make anyone able to read a patient’s confidential data or medical records as it is easier for a paper format tool to be inadvertently left on the corner of a table, for example, for anyone to see. That would obviously not be the case if using an electronic version on a computer. Additionally, in their view, their current workload (reports and forms to fill out), could reduce the tool’s usefulness. To optimise the usability of the PtDA, they told us that it must be simple, easily available (visibility), adaptable (patients, relatives, caregivers) and accessible among clinicians.
3.2 Prototype
With the feedback from stakeholders, the research team created a prototype of the decision aid in French. The prototype contained the following six sections: (1) definition of CM and roles of case managers; (2) benefits and harms of CM for patients and for healthcare organisations compared to usual care and some statistics about pre- and post-intervention outcomes based on scientific evidence; (3) clinical vignettes on real cases that can help patients understand how CM can help them in managing their health; (4) a series of questions to help patients identify their personal values and the importance they place on the advantages and disadvantages of CM; (5) a series of questions assessing patient healthcare situations and personal objectives; and (6) the SURE test to evaluate patients’ decisional conflicts and their comfort with their decisions [40].
3.3 Alpha Testing (Acceptability)
To investigate PtDA acceptability according to patient-partners, patients and clinicians, we performed a small-scale in-depth exploration. Three back-and-forth rounds were required to improve the PtDA and reach acceptability (Figs. 1–4 of the ESM). Globally, all stakeholders found the aid very relevant and patient centred. Recurring comments related to the quantity and the complexity of the information presented recommended decreasing the amount of information to keep the PtDA as simple as possible and to use simpler vocabulary (lay language). Everyone appreciated the section presenting clinical vignettes and proposed to improve these by adding barriers and facilitators of the decision-making process. Stakeholders also helped the research team developing an aid that presents options in a balanced manner and that is not skewed towards on one of the options.
Specifically, clinicians suggested including factors influencing the success of CM to inform patients that impacts of CM vary. They also recommended showing benefits and disadvantages of the decision options (engage in CM, continue with usual care or postpone the decision), and not only the advantages and disadvantages of CM. Clinicians also said that the aid was helpful to understand the way patients think and it was useful to measure the gap between clinician and patient perspectives. They also mentioned that they could use the PtDA to promote health services. In this sense, they recommended providing the aid in a kit from which they could select sections they needed according to clinical settings and patients.
Patient-partners provided relevant recommendations such as making the facts and examples more concrete, removing vocabulary that patients might perceive as derogatory and addressing the message directly to them (message expressed in the second person). They also proposed to add a small section describing who were the potential users of the PtDA.
They appreciated the clinical vignettes and reported that it was eloquent and that they could identify with the fictive high-user patients. They suggest adding a blank clinical vignette in which patients could write about their own stories, values and health conditiosn. They also stated that it was rewarding for them to know that CM exists and that they could benefit from it. Half of them expressed the need for some information about community organisations and available services.
According to these results and suggestions provided by alpha testing, we modified and improved the prototype and produced a ten-page final version (ESM). This version was simplified and refined. It contains enough clear information to better guide patients in their decision-making process (Fig. 2).
4 Discussion
We developed and evaluated a PtDA, based on the Ottawa Decision Support Framework, to help patients with complex care needs who are frequent users of healthcare services in engaging in CM. This PtDA included three options: engaging in CM, maintaining usual care or postponing their decision. First, we found that systematic reviews and a meta-analysis were not appropriate for complex interventions with patients living with complex conditions. Overall, we found that all stakeholders agreed on the relevance of a PtDA. However, they did not reach a consensus on the format: paper vs digital. In addition, between the initial version of the PtDA and the version produced by three iterative cycles, the most significant changes were the number of pages, the vocabulary used and a substantial reduction in written content. These results led us to make the following observations.
First, we found that a meta-analysis was not the best method to report the effectiveness of complex interventions with patients living with complex conditions. Indeed, a meta-analysis tends to find modest effects of behavioural change interventions [41], even less in regard to complex interventions and context [42]. In addition to the heterogeneity of interventions included in the same review, results depend on several elements such as patient and clinician behaviours and the level of involvement in the process. For these reasons, some authors conduct a realist synthesis [36] to better understand contexts and mechanisms of complex interventions conducting to positive patient reported experience measures rather than their measurable impacts. Integrating both qualitative and quantitative data [43] allows more explicit details on the importance of context and patient engagement to reach positive health-related outcomes.
Moreover, in the context of a CM program for high users of healthcare services, a meta-analysis has some limitations for patient-related outcomes because they did not inform about the intervention’s process or the patient engagement level in his/her own self-management process [36, 44]. In other words, the measured outcomes sometimes do not reflect what the patient is really experiencing. A higher complexity of intervention brings higher heterogeneity and it became difficult to present evidence-based outcomes to the patients. Another factor that makes the data synthesis difficult is the inability to pool the studies together. This may be explained by the fact that multiple different time points can be used and that no clear descriptions of the intervention are presented in most of the published articles [45]. None of the frameworks or guidelines available really mention how to report intervention characteristics [41], which are most of the time multi-component and depend on the behaviours of the people involved. Currently, the development of PtDAs is informed by theoretical models and intuitive methods rather than systematic methods [46, 47], which lead to poor reproducibility. Nevertheless, current frameworks provide important key steps to fulfil in the development process of a PtDA specific for complex interventions. As the current available guidelines and frameworks are not sufficient to guide the co-creation of PtDA used in complex interventions, additional work is still needed to document this process.
Second, we found that all stakeholders agreed on the relevance of a PtDA but did not reach a consensus on the format: paper vs digital. Patients would prefer to have a paper format because it is more accessible and simpler, which is consistent with the literature [48, 49]. This can also be explained by the fact that most of our patients had a lower socioeconomic status and that this may increase the preference for paper PtDA format [49]. Clinicians preferred digital PtDA, which can be explained by the fact that they can add their own notes in the file, save it for later consultations and track the changes in the patient’s decision-making process. Those observations are consistent with the literature [49]. As the format does not influence the knowledge acquisition and reduction in decisional conflict [50], the next important step is to target the audience’s preference regarding the PtDA format to maximise its utilisation.
Finally, between the initial version of the PtDA and the version produced by three iterative cycles, the most significant changes were the number of pages, the vocabulary used and the substantial reduction in written content. As shown in our evaluation process, the inclusion of several stakeholders in the development of PtDA, as suggested by guidelines [30], is essential to capture all different perspectives. This is consistent with previous studies showing that clinicians and patients have different points of view regarding health issues and content of PtDA [51, 52]. Their perspectives, when brought together, allowed the creation of a patient-centred tool that can be used by patients and clinicians. However, as reported by Ankolekar and colleagues [51], involving a large number of stakeholders in a co-creation process can increase developmental time and cost. It took 3 years for a part-time coordinator to recruit participants, conduct interviews, and process and analyse the data generated by more than 70 participants. Consequently, researchers must plan enough human resources for the development of a PtDA. In our study, the major concern expressed by the patient-partners and the clinicians in each evaluation round of the alpha testing was the complexity of vocabulary used and the amount of information in the PtDA. As recommended by the Ottawa Hospital Research Institute, language used in the tool must be readable at a grade 8 level [27] and this is what we have tried to achieve with the feedback from our stakeholders. For the next steps prior to implementation, case managers will be validating (beta testing) our aid in primary care settings to evaluate its effects on the knowledge of the patients, their decision comfort and decision durability, for which we expect improvements.
4.1 Strengths and Limitations
The user-centred design is a strength of our study compared with other studies reviewed, as only about half of those involved patients in the development of their decision aids [30]. It is essential in the co-creation of a PtDA to incorporate patient perspectives and expertise and to use a user-centred design. For example, the inclusion of clinical vignettes was made following a suggestion from a patient-partner and this section was one of the most appreciated by all stakeholders. Another supporting example is that the need to include information on community organisations and available services emerged from several patients’ feedback. Co-creation with an interprofessional team is also a strength of our study as this promoted efficiency and positive outcomes for the patients [19]. We included five types of clinicians and some decision makers. This allowed us to merge the expertise of several individuals and create an aid that can be used by a wide variety of professionals.
Our co-creation process involved various participants, leading to a large spectrum of points of view. This made the integration of all those opinions challenging. The research team had to come to a decision on some elements, such as the PtDA’s format, as the stakeholders reached no consensus. Even though small-scale in-depth exploration is recommended for alpha testing by the Ottawa Decision Support Framework [27], the inclusion of only 16 stakeholders in the PtDA’s evaluation may represent a limitation. We also had to deal with the limited availability of the clinicians and some difficulty while working with patients, as they constitute a population with specific needs. However, we did reach data saturation.
5 Conclusions
We developed and assessed alpha testing of a PtDA to support patients with complex care needs and who are high users of healthcare services. This patient-oriented tool should contribute to improve shared decision making with patients and allow them to make their decision while considering all advantages and disadvantages of their options in terms of engaging in CM or continuing with usual care. At the end of the process, patients will make their decision according to their personal objectives and values. We now need to evaluate the aid in the field with patients and clinicians with beta testing and develop an implementation strategy. Further research is needed to support the process of creating decision aids in the context of complex interventions that require the integration of contextual data to inform us of the effectiveness of those interventions and its impact on patient-related outcomes.
References
Chan BT, Ovens HJ. Frequent users of emergency departments. Do they also use family physicians' services? Can Fam Physician. 2002;48:1654–60.
Lee KH, Davenport L. Can case management interventions reduce the number of emergency department visits by frequent users? Health Care Manag. 2006;25(2):155–9. https://doi.org/10.1097/00126450-200604000-00008.
Hayes SL, Salzberg CA, McCarthy D, Radley DC, Abrams MK, Shah T, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1–14.
Byrne M, Murphy AW, Plunkett PK, McGee HM, Murray A, Bury G. Frequent attenders to an emergency department: a study of primary health care use, medical profile, and psychosocial characteristics. Ann Emerg Med. 2003;41(3):309–18. https://doi.org/10.1067/mem.2003.68.
Hansagi H, Olsson M, Sjoberg S, Tomson Y, Goransson S. Frequent use of the hospital emergency department is indicative of high use of other health care services. Ann Emerg Med. 2001;37(6):561–7. https://doi.org/10.1067/mem.2001.111762.
Kumar GS, Klein R. Effectiveness of case management strategies in reducing emergency department visits in frequent user patient populations: a systematic review. J Emerg Med. 2013;44(3):717–29. https://doi.org/10.1016/j.jemermed.2012.08.035.
Wammes JJG, van der Wees PJ, Tanke MAC, Westert GP, Jeurissen PPT. Systematic review of high-cost patients’ characteristics and healthcare utilisation. BMJ Open. 2018;8(9):e023113. https://doi.org/10.1136/bmjopen-2018-023113.
Chiu YM, Vanasse A, Courteau J, Chouinard MC, Dubois MF, Dubuc N, et al. Persistent frequent emergency department users with chronic conditions: a population-based cohort study. PLoS ONE. 2020;15(2):e0229022. https://doi.org/10.1371/journal.pone.0229022.
Billings J, Raven MC. Dispelling an urban legend: frequent emergency department users have substantial burden of disease. Health Affairs (Project Hope). 2013;32(12):2099–108. https://doi.org/10.1377/hlthaff.2012.1276.
Aggarwal, M. et Hutchison, B. (2012). Vers une stratégie des soins primaires pour le Canada. Fondation canadienne pour l'amélioration des services de santé. http://www.cfhi-fcass.ca/Libraries/Reports/Primary-Care-Strategy-FR.sflb.ashx
Government of Canada. Canada's health care system. 2020. https://www.canada.ca/en/health-canada/services/health-care-system/reports-publications/health-care-system/canada.html. Accessed 14 Jul 2020.
Wodchis WP, Austin PC, Henry DA. A 3-year study of high-cost users of health care. Can Med Assoc J. 2016;188(3):182–8. https://doi.org/10.1503/cmaj.150064.
Poitras ME, Maltais ME, Bestard-Denommé L, Stewart M, Fortin M. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv Res. 2018;18(1):446. https://doi.org/10.1186/s12913-018-3213-8.
Roberts SR, Crigler J, Ramirez C, Sisco D, Early GL. Working with socially and medically complex patients: when care transitions are circular, overlapping, and continual rather than linear and finite. J Healthc Qual. 2015;37(4):245–65. https://doi.org/10.1097/jhq.0000000000000006.
Reeves S, Zwarenstein M, Goldman J, Barr H, Freeth D, Hammick M, et al. Interprofessional education: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2008. https://doi.org/10.1002/14651858.CD002213.pub2.
Reeves S, Zwarenstein M, Goldman J, Barr H, Freeth D, Koppel I, et al. The effectiveness of interprofessional education: key findings from a new systematic review. J Interprof Care. 2010;24(3):230–41. https://doi.org/10.3109/13561820903163405.
Zwarenstein M, Goldman J, Reeves S. Interprofessional collaboration: effects of practice-based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009. https://doi.org/10.1002/14651858.CD000072.pub2.
Damery S, Flanagan S, Combes G. Does integrated care reduce hospital activity for patients with chronic diseases? An umbrella review of systematic reviews. BMJ Open. 2016;6(11):e011952. https://doi.org/10.1136/bmjopen-2016-011952.
Hudon C, Chouinard MC, Pluye P, El Sherif R, Bush PL, Rihoux B, et al. Characteristics of case management in primary care associated with positive outcomes for frequent users of health care: a systematic review. Ann Fam Med. 2019;17(5):448–58. https://doi.org/10.1370/afm.2419.
Hudon C, Chouinard MC, Lambert M, Dufour I, Krieg C. Effectiveness of case management interventions for frequent users of healthcare services: a scoping review. BMJ Open. 2016;6(9):e012353. https://doi.org/10.1136/bmjopen-2016-012353.
Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Sci. 2016;11:114. https://doi.org/10.1186/s13012-016-0480-9.
Labrecque M, Paunescu C, Plesu I, Stacey D, Legare F. Evaluation of the effect of a patient decision aid about vasectomy on the decision-making process: a randomized trial. Contraception. 2010;82(6):556–62. https://doi.org/10.1016/j.contraception.2010.05.003.
O'Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Counsel. 1998;33(3):267–79. https://doi.org/10.1016/s0738-3991(98)00026-3.
Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD001431.pub4.
Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ (Clin Res Ed). 1999;319(7212):766–71. https://doi.org/10.1136/bmj.319.7212.766.
Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Mak. 2015;35(1):114–31. https://doi.org/10.1177/0272989x14551638.
The Ottawa Hospital Research Institute. Ottawa Decision Support Framework (ODSF). 2020. https://decisionaid.ohri.ca/odsf.html. Accessed 18 May 2020.
Légaré F, Stacey D, Gagnon S, Dunn S, Pluye P, Frosch D, et al. Validating a conceptual model for an inter-professional approach to shared decision making: a mixed methods study. J Eval Clin Pract. 2011;17(4):554–64. https://doi.org/10.1111/j.1365-2753.2010.01515.x.
Poitras ME, Hudon C, Godbout I, Bujold M, Pluye P, Vaillancourt VT, et al. Decisional needs assessment of patients with complex care needs in primary care. J Eval Clin Pract. 2020;26(2):489–502. https://doi.org/10.1111/jep.13325.
Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13(Suppl. 2):S2. https://doi.org/10.1186/1472-6947-13-s2-s2.
Witteman HO, Dansokho SC, Colquhoun H, Coulter A, Dugas M, Fagerlin A, et al. User-centered design and the development of patient decision aids: protocol for a systematic review. Syst Rev. 2015;4(1):11. https://doi.org/10.1186/2046-4053-4-11.
Luna D, Quispe M, Gonzalez Z, Alemrares A, Risk M, Garcia Aurelio M, et al. User-centered design to develop clinical applications: literature review. Stud Health Technol Inform. 2015;216:967.
Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ (Clin Res Ed). 2006;333(7565):417. https://doi.org/10.1136/bmj.38926.629329.AE.
Creswell J. Research design: qualitative, quantitative, and mixed methods approaches. 3rd ed. Thousand Oaks, CA: Sage Publications Inc.; 2009.
Creswell J, Clark V, Creswell JW. Designing and conducting mixed methods research. 2nd ed. Los Angeles, CA: Sage Publications Inc.; 2011.
Hudon C, Chouinard MC, Aubrey-Bassler K, Muhajarine N, Burge F, Bush PL, et al. Case management in primary care for frequent users of health care services: a realist synthesis. Ann Fam Med. 2020;18(3):218–26. https://doi.org/10.1370/afm.2499.
Charters E. The use of think-aloud methods in qualitative research: an introduction to think-aloud methods. Brock Educ. 2003;12(2):68–82.
Morville P. User experience design. Ann Arbor (MI): Semantic Studios. 2020. https://semanticstudios.com/user_experience_design/. Accessed 28 Nov 2019.
O'Connor AM. Validation of a decisional conflict scale. Med Decis Mak. 1995;15(1):25–30. https://doi.org/10.1177/0272989x9501500105.
Legare F, Kearing S, Clay K, Gagnon S, D'Amours D, Rousseau M, et al. Are you SURE? Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56(8):e308–e314314.
Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implement Sci. 2009;4:40. https://doi.org/10.1186/1748-5908-4-40.
Köpke S, McCleery J. Systematic reviews of case management: too complex to manage? Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.ED000096.
Noyes J, Booth A, Moore G, Flemming K, Tunçalp Ö, Shakibazadeh E. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Global Health. 2019;4(Suppl. 1):e000893. https://doi.org/10.1136/bmjgh-2018-000893.
Hudon C, Chouinard M-C, Brousselle A, Bisson M, Danish A. Evaluating complex interventions in real context: logic analysis of a case management program for frequent users of healthcare services. Eval Prog Plan. 2020;79:101753. https://doi.org/10.1016/j.evalprogplan.2019.101753.
Reilly S, Miranda-Castillo C, Malouf R, Hoe J, Toot S, Challis D, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD008345.pub2.
Michie S. Designing and implementing behaviour change interventions to improve population health. J Health Serv Res Policy. 2008;13(Suppl. 3):64–9. https://doi.org/10.1258/jhsrp.2008.008014.
Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol. 2008;57(4):660–80. https://doi.org/10.1111/j.1464-0597.2008.00341.x.
Tomko C, Davis KM, Luta G, Krist AH, Woolf SH, Taylor KL. A comparison of web-based versus print-based decision AIDS for prostate cancer screening: participants' evaluation and utilization. J Gen Intern Med. 2015;30(1):33–42. https://doi.org/10.1007/s11606-014-2994-7.
Politi MC, Adsul P, Kuzemchak MD, Zeuner R, Frosch DL. Clinicians' perceptions of digital vs paper-based decision support interventions. J Eval Clin Pract. 2015;21(2):175–9. https://doi.org/10.1111/jep.12269.
Baptista S, Teles Sampaio E, Heleno B, Azevedo LF, Martins C. Web-based versus usual care and other formats of decision aids to support prostate cancer screening decisions: systematic review and meta-analysis. J Med Internet Res. 2018;20(6):e228. https://doi.org/10.2196/jmir.9070.
Ankolekar A, Vanneste BGL, Bloemen-van Gurp E, van Roermund JG, van Limbergen EJ, van de Beek K, et al. Development and validation of a patient decision aid for prostate. Cancer Ther BMC Med Inform Decis Mak. 2019;19(1):130. https://doi.org/10.1186/s12911-019-0862-4.
Lee CN, Dominik R, Levin CA, Barry MJ, Cosenza C, O'Connor AM, et al. Development of instruments to measure the quality of breast cancer treatment decisions. Health Expect. 2010;13(3):258–72. https://doi.org/10.1111/j.1369-7625.2010.00600.x.
Acknowledgements
The authors thank all the patients that contributed to this research, the Quebec SPOR SUPPORT Unit. France Légaré holds the Canada Research Chair in Shared Decision Making and Knowledge Translation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research project was funded by the Quebec-SPOR SUPPORT Unit, a methodological platform of the Canadian Institutes of Health Research, Strategy for Patient-Oriented Research.
Conflicts of Interest/Competing Interests
Marie-Eve Poitra, France Légaré, Vanessa T. Vaillancourt, Isabelle Godbout, Annie Poirier, Karina Prévost, Claude Spence, Maud-Christine Chouinard, Hervé Tchala Vignon Zomahoun, Lobna Khadhraoui, José Massougbodji, Mathieu Bujold, Pierre Pluye and Catherine Hudon have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Ethics approval was obtained from the Ethics Committee of the Centre Intégré Universitaire de Santé et de Services Sociaux du Saguenay-Lac-Saint-Jean.
Availability of Data and Material
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent to participate
All participants gave their informed consent to participate in the study.
Consent to publish
All authors gave their consent to publish and approved the final version of the article.
Code Availability
Not applicable.
Authors’ Contributions
MEP is the principal investigator of the study. She conceived the idea for the paper and led the writing. VTV and FL were major contributors to the drafting of the paper. CH is the principal author of the systematic review used to create the decision aid. FL, IG, MB, CP, AP, MCC, BD, PP, KP and CH were involved in the design, data collection and conduct of the study. HZTV, JM and LK were involved in the data extraction and analysis.
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12911456.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Poitras, ME., Légaré, F., Tremblay Vaillancourt, V. et al. High Users of Healthcare Services: Development and Alpha Testing of a Patient Decision Aid for Case Management. Patient 13, 757–766 (2020). https://doi.org/10.1007/s40271-020-00465-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-020-00465-0