Abstract
Background
Regulatory agencies as well as private organizations pursue programs that advocate patient centricity and emphasize the importance of dialog with patients. Various methods are applied to elicit the preferences of patients regarding the aspects of treatment they lend more importance to. Decisions on treatment choices are critical to patients with lung cancer because of their poor prognosis and the serious trade-off between safety and efficacy in traditional cytotoxic chemotherapy.
Methods
We conducted a systematic literature review of quantitative patient preference studies of patients with lung cancer. Our exhaustive search of MEDLINE, CINAHL, EMBASE, PLOS, and SpringerLink identified 15 relevant studies published from January 2000 to April 2020 that enabled us to assess the relative importance of treatment attributes according to lung cancer patients’ perspective.
Results
The literature review revealed that patients with lung cancer tend to place a higher weight on efficacy and quality of life (QoL) attributes than on other attributes. Overall survival was found to be the most important among the efficacy attributes. The consequences of adverse events seemed less important than the possible efficacy from therapies. The clinical utility of treatment, such as the route of administration, was generally not considered important. It remains inconclusive whether sociodemographic factors and/or medical history affect the relative importance of a patient’s preference.
Conclusion
Our systematic review clarified that patients generally prefer a better efficacy profile to a better safety profile, which underscores the importance of improved benefits in anti-lung cancer drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In traditional cytotoxic chemotherapy for lung cancer, the adverse event toxicities and their impact on antitumor efficacy are a serious trade-off that always lies at the center of decisions on treatment choices. |
The quantitative investigation of lung cancer patient preferences leads to better decisions in practical healthcare settings and provides vital clues for drug development strategies, both of which are essential for maximizing the value of treatment for patients. |
Patients with lung cancer tend to consider that a better benefit profile for anticancer drugs is more important than a better safety profile. The sociodemographic factors affecting the relative importance are inconclusive. |
1 Introduction
With the growing number of available drug therapies, patients have more opportunities to make treatment choices. It is becoming increasingly complicated and important to consider the preference and needs of patients when physicians and patients decide the course of therapy. A typical profile of the benefits and risks associated with the treatment for an average patient can be obtained in clinical trials, but the question of whether the treatment has value for a specific patient is measured through the value judgment of the patient. Regulatory agencies in the United States and European countries, as well as some private organizations, pursue initiatives to advocate patient centricity and emphasize the importance of dialog with patients [1,2,3]. In some recent clinical trials, patient-reported assessments of quality of life (QoL) were collected as clinical endpoints [4]. In addition, patients’ opinions on the choice and preferable attributes of therapies expressed in various questionnaires are becoming more important for drug development. The opinions and preferences of patients have been used for several purposes, including benefit-risk assessment [5,6,7,8,9,10]. The importance of patient preference in therapeutic fields with unmet medical needs has been discussed [11, 12] because patients in such therapeutic fields, including lung cancer, inevitably have to face decisions in the paucity of effective treatment options [13]. Patient preference research is an approach that examines the association between the attributes of therapy and patient choice, and several methods have been proposed to achieve this objective. Research on the usefulness and validity of such methods is also in progress in many disease areas [14, 15].
Among cancer types, lung cancer affects the largest number of patients worldwide. Approximately 1.8 million new cases and 1.6 million deaths were reported in 2012, accounting for 20% of cancer-related deaths [16]. The 5-year survival rate for people with lung cancer is reported to be 15% [17], and this poor prognosis makes the choice of therapy especially important. Recent guidelines for lung cancer treatment mention not only cytotoxic anticancer drugs (e.g., cisplatin, carboplatin, and docetaxel), but also molecularly targeted drugs (e.g., bevacizumab, gefitinib, and crizotinib) and immune checkpoint inhibitors (e.g., pembrolizumab and atezolizumab) [18, 19]. In traditional cytotoxic chemotherapy, as described in the current guidelines, adverse event toxicities and their impact on antitumor efficacy and prolonged survival are a serious trade-off that always lies at the center of decisions on treatment choices [20, 21]. It has been demonstrated that treatment selection based on the patient’s preferences has a significant impact on adherence [22, 23]. A previous study also suggested the existence of conflict between physicians and patients regarding the choice of treatment [24]. These recent challenges surrounding cancer treatment increase the need for studies on the preferences for drug attributes. Investigations of patient preferences could lead to better decisions in practical healthcare settings and provide vital clues for drug development strategies, both of which are essential in maximizing the value of treatment for patients.
Several systematic review studies have attempted to shed light on the preferences of people with lung cancer [20, 25]. Blinman et al. [20] reported that there was a wide variation in the minimum survival benefits judged sufficient to provide chemotherapy. The report showed that the treatment choices are dependent on cancer metastases/localization, drug toxicities, and the region of the study (e.g., North America vs. Japan) and that the level of dependency varies according to age, presence of dependent families, educational attainment of at least a college degree, and the baseline QoL. Schmidt et al. [25] found that people with lung cancer consider specific attributes more important for drug selection than others, depending on patient characteristics such as age.
From the variety of patient preference methods, the quantitative approach aims to clarify the relative importance of each attribute of treatment while considering the trade-offs. The results are further applicable to quantitative benefit–risk assessments, such as multicriteria decision analysis. In this analysis, the relative importance of each attribute is used to put weight on the attributes of drugs [9]. However, limitations and concerns regarding traditional quantitative methods have also been noted, including the validity of the experimental design, influence of responders’ experience, and cognitive and/or statistical appropriateness of the number of attributes [14, 26]. The studies by Blinman et al. and Schmidt et al. mentioned above did not focus on or discuss quantitative methods for considering relative importance [20, 25].
In this study, we conducted a systematic review of quantitative patient preference studies (i.e., studies that investigated the relative importance of attributes) for people with lung cancer to understand the trade-offs between attributes for the patients. We examined trends in the design and results of previous studies and discussed limitations and concerns about quantitative preference research in general. We also examined whether the importance of specific attributes among different studies is consistent or if they fluctuate depending on the characteristics of the population being studied.
2 Methods
We identified relevant literature on June 3, 2020, by an exhaustive search of five electronic databases, including MEDLINE, CINAHL, EMBASE, PLOS, and SpringerLink, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27]. With reference to Marshall et al. [14], we used the following English term combinations to search for quantitative preference research literature: “lung neoplasms” AND (“conjoint analysis” OR “choice behavior” OR “stated preference” OR “discrete choice” OR “latent class analysis”). The specific search term inputs in each database are shown in the appendix. To focus on recent literature, we narrowed our search to English-language literature published from January 1, 2000, to April 30, 2020. We applied the following inclusion criteria to obtain studies that met the purpose of this research:
-
a.
Studies in which preferences were stated by people with lung cancer or healthy individuals who are assumed to have lung cancer (hereinafter, we call all these respondents ‘patients’). Studies of caregivers, relatives and physicians were excluded. If the studies had patients with lung cancer and other cancers, they were included.
-
b.
Studies in which the preference for attributes of treatment (i.e., overall survival [OS], fatigue, frequency of administration, etc.) was explicitly stated. Studies of preferences for screening tests, preferences for specific treatment, preferences for prevention methods, studies of physicians’ explanations about drug choices such as shared decision making, studies of the medical economic evaluation of drugs, and studies of status transition such as Markov models were excluded.
-
c.
Studies in which the preference was stated quantitatively and the relative importance between attributes was investigated. We excluded studies with only qualitative interviews for each attribute and methodological studies without patient surveys. Articles showing the relative importance of attributes were included. Relative importance was presented as either continuous values or ordinal numbers.
-
d.
Original research articles. Systematic reviews and abstracts of conference presentations were excluded.
The systematic review was conducted by two independent reviewers, and any disagreements were resolved by discussion.
From the studies that satisfied the criteria above, the research year, country, sample size, therapy attribute and its importance, background of research population, disease status, and funding organization were extracted and summarized. We categorized the treatment attributes into benefit attributes (e.g., OS, progression-free survival [PFS], QoL), risk attributes (e.g., fatigue, nausea, rash), and other attributes (e.g., mode of administration, financial burden). We used the ten-item checklist proposed by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) to confirm the quality of the individual papers [26]. Each article was rated based on whether each item was explained, and we considered the possible impact of the quality of the articles on the results. To compare the relative importance of the attributes that were obtained as continuous values between studies, the following standardization approach was used. The weight of the relative importance of the most important attribute within each study was 10.0, and the weight of the relative importance of the other attributes was calculated by linear conversion (i.e., the weight of the relative importance of each attribute in each study was divided by the weight of the relative importance of the most important attribute and multiplied by 10.0). In cases where the weights of the relative importance for each level of an attribute were available, we considered the difference between the highest and lowest levels as the weight of that attribute.
3 Results
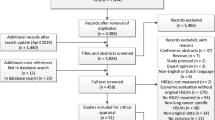
The sample selection flowchart is shown in Fig. 1. The initial search yielded 542 articles, and after excluding duplicates, 474 articles remained. These articles were further screened by reviewing the titles and abstracts, and a full-text review was conducted for 52 articles. As a result, 15 articles met the inclusion criteria. The excluded articles are detailed in the electronic supplementary material.
Table 1 summarizes the basic information of the 15 studies of patients with lung cancer, and Table 2 shows the detailed information of each study. Nine studies focused on people with lung cancer only, four studies focused on people with cancer, including lung cancer, and two studies focused on healthy individuals. In the studies of people with cancer, subgroup analyses of people with lung cancer were not performed, and the results of all people with cancer are shown in Table 2 and hereafter. The number of attributes ranged from three to 14, with an average of 6.5. In terms of the research methodology, respondents were recruited online in recent studies (n = 5/15). The method of explaining attributes and levels were face-to-face interviews in five out of 15 studies. The discrete choice experiment was most frequently adopted (n = 10/15). In two studies, the results of responder selection from hypothetical treatment options were simply summarized by cross tabulation, and these are shown as “Selection” in Table 2. The conditional logit model was most frequently adopted (n = 5/15). The benefit attributes related to treatment, survival, tumor progression/recurrence/metastasis, tumor shrinkage, symptoms due to cancer, and QoL (including symptoms due to cancer) were adopted. Regarding the benefit attributes, the most frequently adopted attributes were symptoms due to cancer (n = 4/15). Regarding the risk attributes, fatigue, nausea, vomiting, diarrhea, hair loss, and rash were adopted in two or more studies, and the most frequently adopted attribute was nausea (n = 8/15). Regarding other attributes, financial concerns, route of administration, annual number of operations at the institution, waiting time, driving time to the hospital, presence or absence of explanations for the diseases and medications, being a burden to family or friends, staying away from intensive medical care, and staying away from regular visits to doctors and hospitals were adopted.
Regarding the characteristics of the study population, the mean or median age was not available in all publications, and in many studies, the mean age was approximately 60 years. The sex distributions varied among the studies, with male proportions ranging from 29 to 98%. Some of the studies described employment status (n = 9/15) and educational background (n = 8/15), showing that 14–67% of the respondents were working full- or part-time and 7–87% attained a university degree or higher. Most of the articles described the stage of cancer (n = 11/13), and in those papers, the rate of stage I–III disease ranged from 0 to 100%. Eight out of 15 studies were funded by private companies.
Figure 2 shows the relative weight of each attribute in the ten studies that investigated preference related to pharmacotherapy for which the relative importance of attributes was obtained as a continuous variable. The quality ratings of these studies were more than 6 out of 10 (see the Electronic Supplementary Material), which were considered to allow us to summarize the results of these studies. The relative importance of each attribute is visualized in Fig. 2. According to their standardized weights of relative importance, higher-importance attributes are colored black and less important attributes are colored white. For each category, the attributes shown in Fig. 2 are placed in the order of relative importance.
For the attributes reflecting benefits, the standardized weights of the relative importance of OS and PFS were all more than 7.7. Regarding the QoL aspects, specific symptoms, general symptoms, and physical domains were consistently cited as more important than other domains of QoL. The standardized relative importance of risk attributes varied between studies, but most showed fatigue, nausea and vomiting, and diarrhea to be more important than hair loss and rash. Financial burden was evaluated very highly (10.00) in one study that did not include benefit attributes. Other attributes related to pharmacotherapy (i.e., mode of administration, waiting time, and guide) had low importance, with weights of less than 5.30.
4 Discussion
In the first systematic review to focus on quantitative patient preference for lung cancer treatment, we revealed that patients with lung cancer tend to place higher weight on benefit attributes, including QoL, than on other attributes. As expected, OS was found to be the most important attribute. Patients tended to place lower importance on adverse drug reactions than they did for attributes reflecting benefits from therapies. Clinical utility, such as route of administration, was not considered important in general.
Among the attributes that show benefit-side aspects, survival was the most important attribute in the studies that included survival as an option (Table 2). The importance of survival was also shown in an earlier review [25]. It should be noted, however, that only five out of 15 studies included survival as a benefit. This probably reflects the background objectives of these studies because all five studies were not funded by private drug companies. Most of the ten studies that did not include survival were funded by private drug companies and aimed to examine preferences for the typical profile of drugs, some of which were currently available and/or under development. The objectives of these preference studies were not necessarily to explore the overall structure of benefit and risk, but to obtain useful clues for product differentiation and positioning in markets. This may lead to seemingly insufficient attention to survival as an option of benefit.
Tumor shrinkage, tumor progression/recurrence/metastasis, symptoms due to cancer, and the physical domains of QoL tended to follow survival in the rank of importance. Compared to risk attributes, benefit attributes were considered important in most of the studies (Fig. 2). Although there were four studies that compared tumor-related symptoms or QoL with risk items, the tumor-related symptoms and QoL items, including the physical, social, and role domains, were more important than any risk attribute in those studies [29, 32, 34, 42]. This finding is in line with the recent tendency to emphasize QoL in the clinical evaluation of anticancer drugs [43]. The duration of response, which has often been used as a clinical endpoint in recent cancer immunotherapy trials, was not tested in the studies.
The rank of importance of an adverse event varied between the studies. Among the adverse events investigated in the studies, fatigue tended to have the highest importance, and the development of rashes tended to have the lowest importance. The relative importance of each adverse event may differ according to the severity of lung cancer, as suggested by the subgroup analysis or latent class analysis of several publications [33, 34, 41], but there has been insufficient evidence that enables further discussion. Just one study [39] focused on molecular targeted agents, and most of the studies regarding pharmacotherapy in our sample actually focused on many adverse events observed in conventional chemotherapy. However, a particular type of adverse event that is more likely to appear in a specific mode of drug therapy has attracted attention. For example, hypertension, gastrointestinal perforation, and interstitial pneumonia are frequently observed with molecularly targeted agents, and cytokine syndrome is often observed with cancer immunotherapy. These adverse events were not examined in the research articles. Further preference studies are needed to elicit patients’ preferences and needs for such updated drug therapies.
There are some attributes of clinical interventions that are complicated to ascribe to benefit or risk. We condensed several attributes (i.e., financial burden, mode of administration, waiting time, and treatment guide) investigated in the preference studies into “other attributes.” These multifaceted attributes reflect several aspects of treatment, including both direct and indirect costs, access, and public acceptance. In one study about pharmacotherapy, financial burden was considered more important than risk attributes (Table 2 and Fig. 2) [33]. The other attributes related to pharmacotherapy were generally of low importance, but operative methods and the experience of surgeons in the hospital were highly weighed in surgery and radiotherapy [36]. This finding seems reasonable because of the correct assumption of patients that the experience of surgeons is directly associated with treatment success [44]. The importance of other attributes also depends on the prognosis of the disease. For example, it is interesting that other attributes (e.g., route of administration) tend to be highly emphasized in chronic diseases where the prognosis is not as poor as that of lung cancer [45]. In disease areas with poor prognosis, including lung cancer, the other attributes of drug therapies may be less relevant. With the skyrocketing costs of some new drug therapies in recent years, it is highly expected that attributes related to financial burden would be given an even higher priority in patient preference, not only in lung cancer but also in other serious disease areas.
The consideration of trade-offs between benefit and risk is always at the center of discussions in optimized drug therapies. However, our research clarified that there has not been sufficient evidence regarding patient preference for such trade-offs in the field of lung cancer. As a result of the literature review, the weight of the relative importance of the trade-off between OS and risk attributes was confirmed in only two studies on pharmacotherapy. One of the studies showed that OS was three times more important than major risk attributes [38], and the other study showed that OS was 1.1 times more important than major risk attributes [42]. There were three studies in which the trade-offs between PFS and risk attributes were evaluated. One study showed that nausea and vomiting were more important than PFS [39], and the other two studies showed that PFS was approximately 1.8–2.5 times more important than the most important risk attributes of fatigue and nausea and vomiting [34, 41]. There were three studies in which the trade-offs between symptoms related to cancers and risk attributes were evaluated. They showed that cancer-related symptoms were approximately 1.1–2 times more important than the most important risk attributes of fatigue and nausea and vomiting [32, 34, 42]. Regarding the trade-offs between the domains of QoL and risk attributes, it was reported that the most important QoL domain, the physical domain, was 1.7 times as highly weighed as risk attributes [29]. Our results suggest that benefit components are given high priority in most patient preference research projects for which the target population included patients with lung cancer. However, as discussed above, this result may vary depending on the nature of the disease (e.g., prognosis, acute vs. chronic) [45]. Caution should be taken in interpreting the weights of importance across these studies, since the investigated populations, prespecified definitions, and levels of attributes were different among the studies.
Since the demographic characteristics of the patients surveyed in the studies were not fully published, overall associations between demographic characteristics and the preferences of patients were not examined in this research. Several previous studies clearly showed that preference depended on the demographics of the patients. For example, age, disease severity (e.g., Eastern Clinical Oncology Group [ECOG] performance status, disease stage, and disease symptoms), and educational background were shown to be associated with the relative importance of each attribute [31,32,33, 35, 38, 41]. These associations were also reported in some previous systematic review articles [20, 25]. It would be useful to examine possible associations in a pooled sample. However, each study presented different patient demographic information in somewhat inconsistent ways, which makes it difficult to further explore unknown associations. The sample sizes of some studies were 100 or less (n = 7/15) and were not considered sufficient to analyze subgroups.
We confirmed in this systematic review that a majority of the studies were funded by private companies. The dominance of private funding in patient preference studies was also reported in other disease areas [45]. It is possible that some studies in grey literature, such as government reports, were overlooked in this type of literature search. Preference studies by private companies that are implemented for market research purposes are not routinely published. Thus, it is difficult to present a real picture of funding, but it is likely that the results of preference studies have been used for the purposes of product differentiation explicitly or implicitly and will be used in the future as long as the funding situation remains unchanged. The dominance of funding from private companies may have a substantial impact on the design and implementation of preference studies. For example, the attributes and levels of a conjoint analysis inevitably depend on the features of specific drug products assumed in the scenario, which is mostly reflective of the funding company’s objective. As a result, such studies may focus more on attributes that have potential impact on business objectives and may not necessarily address concerns important for patients and/or clinical decisions. To prevent this from happening, it is recommended that patients participate in the design phases of preference studies, advising decisions on the attributes, levels, and descriptions of the questionnaire. Such efforts have already been made, but not specifically for lung cancer [46,47,48].
Finally, we should note several limitations of this study, most of which are inevitable in systematic literature reviews and analyses using pooled samples. The definitions of attributes were different between studies, which makes it difficult to interpret the quantitative assessment results. It is natural that the revealed preference for an adverse event differs depending on the assumptions made in the questionnaire about how severe it is, how often it occurs, and how long it lasts. We were not able to align variations in the assumptions for comparison between the studies in Fig. 2, but considerations of the assumptions in different scenarios and/or options would be necessary, especially when direct and rigorous comparisons are made. For example, in a study by Bridges et al. [32], the severity level of nausea and vomiting was defined as “none: no nausea and vomiting” and “moderate: loss of appetite and eating less than normal; dehydration; vomits 2–5 times per day.” Muhlbacher et al. [34] defined it as “mild: 1 time in 24 h” and “severe: more than 6 times in 24 h.” The differences between these assumptions presented to patients seem to lead to different importance levels placed on nausea and vomiting; that is, nausea and vomiting had the highest importance among the risk attributes in the study by Muhlbacher et al., which presented higher severity assumptions, while their relative importance was lower in the study by Bridges et al. Miller et al. examined nausea and vomiting separately, and thus the assumptions on the probability, duration, and severity were presented separately [33]. As a result, the duration of vomiting was evaluated as the most important item, and the probability of vomiting was the least important item. Definitions and assumptions regarding severity may be similar in studies in the same period because they usually assume similar products and refer to the same guidelines. However, heterogeneities in definitions and assumptions would have a critical impact on preference studies, as the mode of action and standard-of-care therapy shift over time.
The number of studies extracted for our purposes was small, which casts some doubt on the applicability and generalizability of our findings. Patients who responded to the questionnaire differed from study to study, and they were not a random sample in any sense. Therefore, it is inevitably difficult to determine how consistent the observed preferences would be in populations that have different basic characteristics, including sex, age, severity of disease, and race. In addition, the attributes of new modes of drug therapies (e.g., molecularly targeted agents and immunotherapies) were not sufficiently included in the studies that we reviewed. To consider the current treatment options, efforts to examine these additional attributes will be needed in the future.
5 Conclusions
This systematic review of quantitative preference studies of patients with lung cancer confirmed that these patients tend to consider benefit attributes, including QoL, to be more important than other attributes and considered OS to be the most important. Adverse events followed the benefit attributes. Clinical utility, including route of administration, was not given high priority. The possible impact of sociodemographic factors was inconclusive because of the limited number of studies available and the divergence in study designs. Further studies are needed to clarify the relationship between those factors and patients’ preferences in more rigorous ways. It may also be necessary for researchers to make efforts to harmonize the ways in which they present attributes to responders (i.e., patients).
Availability of Data and Material
All data generated or analyzed during this systematic review study are included in this published article.
References
FDA, Patient-Focused Drug Development. https://www.fda.gov/drugs/developmentapprovalprocess/ucm579400.htm. Accessed 2 Jan 2020.
EMA, Patients and Consumers. https://www.ema.europa.eu/en/partners-networks/patients-consumers. Accessed 2 Jan 2020.
DIA, Patient Engagement. https://www.diaglobal.org/en/resources/areas-of-interest/patient-engagement. Accessed 2 Jan 2020.
Zwitter M. Toxicity and quality of life in published clinical trials for advanced lung cancer. Support Care Cancer. 2018;26:3453–9.
ICH, Revision of M4E Guideline on Enhancing the Format and Structure of Benefit-Risk Information in ICH. June 2016. https://www.ich.org/products/ctd/ctdsingle/article/m4er1-efficacy.html. Accessed 2 Jan 2020.
FDA, Benefit-risk assessment in drug regulatory decision-making, Draft PDUFA VI Implementation Plan (FY2018-2022). 30 March 2018. https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM602885.pdf. Accessed 2 Jan 2020.
Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American Society of Clinical Oncology Statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–77.
Coplan PM, Noel RA, Levitan BS, Ferguson J, Mussen F. Development of a framework for enhancing the transparency, reproducibility and communication of the benefit-risk balance of medicines. Clin Pharmacol Ther. 2011;89:312–5.
Postmus D, Richard S, Bere N, van Valkenhoef G, Galinsky J, Low E, et al. Individual trade-offs between possible benefits and risks of cancer treatments: results from a stated preferences study with patients with multiple myeloma. Oncologist. 2018;23:44–51.
MDIC, Medical Device Innovation Consortium (MDIC) Patient Centered Benefit-Risk Project Report: A framework for incorporating information on patient preferences regarding benefit and risk into regulatory assessments of new medical technology. 2015. http://mdic.org/wp-content/uploads/2015/05/MDIC_PCBR_Framework_Web.pdf. Accessed 2 Jan 2020.
Gonzalez JM, Johnson FR, Levitan B, Noel R, Peay H. Symposium title: preference evidence for regulatory decisions. Patient. 2018;11:467–73.
Janssens R, Russo S, van Overbeeke E, Whichello C, Harding S, Kubler J, et al. Patient preferences in the medical product life cycle: what do stakeholders think? Semi-structured qualitative interviews in Europe and the USA. Patient. 2019;12:513–26.
Benz HL, Lee TJ, Tsai JH, Bridges JFP, Eggers S, Moncur M, et al. Advancing the use of patient preference information as scientific evidence in medical product evaluation: a summary report of the patient preference workshop. Patient. 2019;12:553–7.
Marshall D, Bridges JF, Hauber B, Cameron R, Donnalley L, Fyie K, Johnson FR. Conjoint analysis applications in health—how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3:249–56.
Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37:201–26.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9.
Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2017;35:3484–515.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–237.
Blinman P, Alam M, Duric V, McLachlan SA, Stockler MR. Patients’ preferences for chemotherapy in non-small-cell lung cancer: a systematic review. Lung Cancer. 2010;69:141–7.
Blinman P, McLachlan SA, Nowak AK, Duric VM, Brown C, Wright G, et al. Lung cancer clinicians’ preferences for adjuvant chemotherapy in non-small-cell lung cancer: what makes it worthwhile? Lung Cancer. 2011;72:213–8.
Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthema. Am J Respir Crit Care Med. 2010;181:566–77.
Von Korff M, Katon W, Rutter C, Ludman E, Simon G, Lin E, Bush T. Effect on disability outcomes of a depression relapse prevention program. Psychosom Med. 2003;65:938–43.
Mokhles S, Nuyttens JJME, de Mol M, Aerts JGJV, Maat APWM, Birim O, et al. Treatment selection of early stage non-small cell lung cancer: the role of the patient in clinical decision making. BMC Cancer. 2018;18:79.
Schmidt K, Damm K, Prenzier A, Golpon H, Welte T. Preferences of lung cancer patients for treatment and decision-making: a systematic literature review. Eur J Cancer Care. 2016;25:580–91.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–13.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. J Clin Epidemiol. 2009;62:1006–12.
Kind P, Macran S. Eliciting social preference weights for functional assessment of cancer therapy-lung health states. Pharmacoeconomics. 2005;23:1143–53.
Johnson FR, Hauber AB, Osoba D, Hsu MA, Coombs J, Copley-Merriman C. Are chemotherapy patients’ HRQoL importance weights consistent with linear scoring rules? A stated-choice approach. Qual Life Res. 2006;15:285–98.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
Girones R, Torregrosa D, Gomez-Codina J, Maestu I, Tenias JM, Rosell R. Lung cancer chemotherapy decisions in older patients: the role of patient preference and interactions with physicians. Clin Transl Oncol. 2012;14:183–9.
Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77:224–31.
Miller PJ, Balu S, Buchner D, Walker MS, Stepanski EJ, Schwartzberg LS. Willingness to pay to prevent chemotherapy induced nausea and vomiting among patients with breast, lung, or colorectal cancer. J Med Econ. 2013;16:1179–80.
Muhlbacher AC, Bethge S. Patients’ preferences: a discrete-choice experiment for treatment of non-small-cell lung cancer. Eur J Health Econ. 2015;16:657–70.
Lehman M, Gorayski P, Watson S, Edeling D, Jackson J, Whitty J. Patient preferences regarding prophylactic cranial irradiation: a discrete choice experiment. Radiother Oncol. 2016;121:225–31.
Tong BC, Wallace S, Hartwig MG, D’Amico TA, Huber JC. Patient preferences in treatment choices for early-stage lung cancer. Ann Thorac Surg. 2016;102:1837–44.
Fallowfield LJ, Catt SL, May SF, Matthews L, Shilling VM, Simcock R, et al. Therapeutic aims of drugs offering only progression-free survival are misunderstood by patients, and oncologists may be overly optimistic about likely benefits. Support Care Cancer. 2017;25:237–44.
Schmidt K, Damm K, Vogel A, Golpon H, Manns MP, Welte T, von der Schulenburg GJM. Therapy preferences of patients with lung and colon cancer: a discrete choice experiment. Patient Prefer Adherence. 2017;11:1647–56.
Bridges JF, Cruz M, Pavilack M, Flood E, Janssen EM, Chehab N, et al. Patient preferences for attributes of tyrosine kinase inhibitor treatments for EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2019;15:3895–907.
Sullivan DR, Eden KB, Dieckmann NF, Golden SE, Vranas KC, Nugent SM, et al. Understanding patients’ values and preferences regarding early stage lung cancer treatment decision making. Lung Cancer. 2019;131:47–57.
Sun H, Wang H, Xu N, Li J, Shi J, Zhou N, et al. Patient preferences for chemotherapy in the treatment of non-small cell lung cancer: a multicenter discrete choice experiment (DCE) study in China. Patient Prefer Adherence. 2019;13:1701–9.
Valenti V, Ramos J, Perez C, Capdevila L, Ruiz I, Tikhomirova L, et al. Increased survival time or better quality of life? Trade-off between benefits and adverse events in the systemic treatment of cancer. Clin Transl Oncol. 2020;22:935–42.
Camps C, del Pozo N, Blasco A, Blasco P, Siera R. Importance of quality of life in patients with non-small-cell lung cancer. Clin Lung Cancer. 2009;10:83–90.
Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J. 2007;83:777–9.
Von Arx LB, Kjeer T. The patient perspective of diabetes care: a systematic review of stated preference research. Patient. 2014;7:283–300.
McCarthy MC, De Abreu Lourenco R, McMillan LJ, Meshcheriakova E, Cao A, Gillam L. Finding out what matters in decision-making related to genomics and personalized medicine in pediatric oncology: developing attributes to include in a discrete choice experiment. Patient. 2020;13:347–61.
Louis E, Ramos-Goni JM, Cuervo J, Kopylov U, Barreiro-de Acosta M, McCartney S, et al. A qualitative research for defining meaningful attributes for the treatment of inflammatory bowel disease from the patient perspective. Patient. 2020;13:317–25.
Hollin IL, Craig BM, Coast J, Beusterien K, Vass C, DiSantostefano R, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient. 2020;13:121–36.
Author information
Authors and Affiliations
Contributions
YS and SO contributed to the study conception and design. Material preparation, data collection, and analysis were performed by YS. The independent systematic review was performed by NS. The first draft of the manuscript was written by YS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
YS and NS are full-time employees at Chugai Pharmaceutical Co., Ltd. All authors have no other relevant conflicts of interest to disclose.
Funding
This work was conducted as part of the authors'employment with their organisations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
The literature search strategies for the databases are detailed below. All searches were conducted on June 3, 2020.
-
a.
MEDLINE
(“conjoint analysis”[All Fields] OR “conjoint analyses”[All Fields] OR “choice behavior”[All Fields] OR “stated preference”[All Fields] OR “discrete choice”[All Fields] OR “latent class analysis”[All Fields] OR “latent class analyses”[All Fields]) AND “Lung Neoplasms”[MeSH] AND (“2000/01/01”[PDAT]: “2020/04/30”[PDAT])
-
b.
CINAHL
Published date ranged from 20000101 to 20200430 and (“conjoint analysis” OR “conjoint analyses” OR “choice behavior” OR “stated preference” OR “discrete choice” OR “latent class analysis” OR “latent class analyses”) AND (“lung neoplasms” OR “lung tumor” OR “lung cancer” OR “lung tumour”)
-
c.
EMBASE
(“conjoint analysis” OR “conjoint analyses” OR “choice behavior” OR “stated preference” OR “discrete choice” OR “latent class analysis” OR “latent class analyses”) AND (“lung tumor”/exp OR “lung tumor”) AND [2000-2020]/py
-
d.
PLOS
Published date ranged from 2000-01-01 to 2020-04-30 and (everything:”conjoint analysis” OR everything:”conjoint analyses” OR everything:”choice behavior” OR everything:”stated preference” OR everything:”discrete choice” OR everything:”latent class analysis” OR everything:”latent class analyses”) AND (everything:”lung neoplasms” OR everything:”lung tumor” OR everything:”lung cancer” OR everything:”lung tumour”)
-
e.
SpringerLink
Published date ranged from 2000 to 2020 and (“conjoint analysis” OR “choice behavior” OR “stated preference” OR “discrete choice” OR “latent class analysis”) AND (“lung neoplasms” OR “lung tumor” OR “lung cancer” OR “lung tumour”).
Rights and permissions
About this article
Cite this article
Sugitani, Y., Sugitani, N. & Ono, S. Quantitative Preferences for Lung Cancer Treatment from the Patients’ Perspective: A Systematic Review. Patient 13, 521–536 (2020). https://doi.org/10.1007/s40271-020-00434-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-020-00434-7