Abstract
Rozanolixizumab (rozanolixizumab-noli; RYSTIGGO®), a humanized IgG4 monoclonal antibody with a high affinity and specificity for human neonatal Fc receptor (FcRn; which plays a vital role in the transport, distribution and persistence of IgG), is an effective and generally well tolerated treatment option in adults with acetylcholine receptor (AChR) or muscle-specific kinase (MuSK) autoantibody-positive generalized myasthenia gravis (gMG). Administered subcutaneously once weekly for 6 weeks, with subsequent treatment cycles based on clinical evaluation, it is approved for the treatment of adults with gMG in the EU, Japan and the USA. In the pivotal, multinational, phase 3 MycarinG study, one 6-week cycle of rozanolixizumab ≈ 7 mg/kg or ≈ 10 mg/kg improved multiple disease-related outcomes versus placebo, with the benefits sustained following repeated treatment cycles according to a pooled analysis of data from the phase 3 study and two phase 3 extension studies. While increased infection susceptibility could be a consequence of the transient reduction in IgG levels with rozanolixizumab therapy, no severe or serious infections were reported in either rozanolixizumab group in the pivotal study.
Plain Language Summary
Myasthenia gravis (MG) is a disease in which an individual’s own IgG antibodies damage the communication between nerves and voluntary muscles, thereby weakening the muscles. Eye movements, speech, swallowing, breathing and use of the limbs are affected. A cure is not yet available, with treatment (based on changing or suppressing the immune system) aimed at easing symptoms and improving well-being. Rozanolixizumab (rozanolixizumab-noli; RYSTIGGO®) is an IgG4 antibody that targets a receptor that plays a vital role in the transport, distribution and persistence of IgG. It is administered under the skin once weekly for 6 weeks, with further treatment based on the initial response to rozanolixizumab. In adults with generalized MG, the administration of rozanolixizumab once weekly for 6 weeks improved various disease-related outcomes versus placebo, with the benefits sustained following repeated treatment. Rozanolixizumab was generally well tolerated by these patients, with repeated treatment associated with an acceptable safety profile. Headache was the most frequently reported treatment-emergent adverse event. Thus, rozanolixizumab is an effective and generally well tolerated treatment option in adults with generalized MG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Q&A can be found at https://doi.org/10.6084/m9.figshare.25843852. |
Humanized IgG4 monoclonal antibody for human FcRn |
Administered subcutaneously once weekly for 6 weeks, with subsequent treatment cycles based on clinical evaluation |
Improves multiple disease-related outcomes versus placebo, with benefits sustained following repeated cycles |
Generally well tolerated, with repeated cycles associated with an acceptable safety profile |
What is the rationale for using rozanolixizumab in generalized myasthenia gravis (gMG)?
Myasthenia gravis (MG) is a rare, chronic, antibody-mediated autoimmune disorder resulting from impaired communication between nerves and skeletal muscles at the neuromuscular junction [1,2,3,4]. Most (up to 85%) patients with MG have IgG autoantibodies directed against the postsynaptic acetylcholine (ACh) receptor (AChR) [2, 5, 6]. Autoantibodies against other proteins are less common, with muscle-specific kinase (MuSK; a transmembrane component of the postsynaptic neuromuscular junction that mediates the clustering of AChR and anchors acetylcholinesterase during the formation of the neuromuscular junction) and low-density lipoprotein receptor-related protein 4 (LRP4) autoantibodies seen in just 6% and 1–2% of patients with MG [2,3,4,5,6]. AChR autoantibodies impede the binding of ACh to its receptor, thus inhibiting ACh-dependent signalling; accelerate receptor degradation by activating complement, which results in reduced receptor density on the postsynaptic membrane; and cross-link AChRs, resulting in their endocytosis and destruction [2,3,4,5,6]. MuSK autoantibodies prevent AChR clustering by masking the site of normal MuSK–LRP4 interaction, and LRP4 autoantibodies prevent the interaction of LRP4 with neural agrin, thereby inhibiting its activation of MuSK [2].
MG typically manifests as generalized or localized skeletal muscle weakness that affects eye movements, speech, swallowing, breathing and use of the limbs [1, 4]. A definitive cure for MG is not yet available, with treatment (aimed at alleviating symptoms and improving health-related quality of life) based on immunosuppressive or immunomodulatory mechanisms, which range from the wide and non-targeted action of conventional drugs to the high selectivity of target-specific biological agents [2, 3]. Current treatment strategies include reducing the production or increasing the clearance of IgG [4]. As the long half-life of pathogenic IgG permits target organ damage, interfering with its half-life and/or reducing its retention time may be beneficial. The neonatal Fc receptor (FcRn) plays a vital role in the transport, distribution and persistence of IgG [4]: blocking it prevents the recycling of IgG and subsequently reduces its half-life, thereby reducing circulating IgG levels [2]. Moreover, the high selectivity of FcRn inhibitors spares other off-target blood components [2].
Rozanolixizumab (rozanolixizumab-noli; RYSTIGGO®) is a humanized IgG4 monoclonal antibody directed against human FcRn that has been approved for the treatment of adults with generalized MG (gMG) in the EU [7], Japan [8] and the USA [9]. Its dosing regimen varied across the gMG clinical development programme, with body weight-based dosing employed in early phase 1 and 2 studies and body weight-tiered dosing utilized in phase 3 studies [10, 11]. The doses of rozanolixizumab approved in the EU (Table 1) are based on the lower of the two doses (≈ 7 mg/kg and ≈ 10 mg/kg) used in the phase 3 studies; please see the EU assessment report [10] for further details on the dose selection process in the EU. The doses approved in the USA (Table 1) are based on a three-fixed dose regimen derived from pharmacokinetic/pharmacodynamic modelling and simulations that estimated the performance of doses in terms of exposure, total IgG reduction, disease-specific autoantibody reduction, and Myasthenia Gravis Activities of Daily Living (MG-ADL) response. Please see the US FDA integrated review [11] for further details on the dose selection process in the USA. Efficacy and tolerability results for the rozanolixizumab ≈ 10 mg/kg dose are included in this article for completeness.
How does rozanolixizumab work?
Rozanolixizumab has a high affinity (dissociation constant 23 pmol/L and 34 pmol/L at pH 6.0 and pH 7.4; surface plasmon resonance analysis) and specificity for human FcRn [12]. By binding to FcRn, it blocks the binding of IgG to FcRn, thereby accelerating IgG catabolism and reducing circulating IgG levels [5, 7, 9, 12].
In vitro, rozanolixizumab dose dependently inhibited the human FcRn-mediated recycling of human IgG (half-maximal inhibitory concentration 0.41 nmol/L) and did not induce cytokine release [12]. In vivo, a single dose of rozanolixizumab induced a rapid reduction in plasma IgG levels but had no effect on plasma albumin levels (albumin binds to a different site on FcRn [7]) [12].
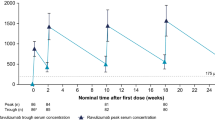
In healthy adults participating in a first-in-human, phase 1 dose-escalating study, single ascending doses of intravenous or subcutaneous rozanolixizumab resulted in sustained dose-dependent reductions (of up to 50%) in total serum IgG levels [13]. Maximal reductions were attained by days 7–10, with levels gradually returning to baseline by day 57 [13].
In adults with AChR or MuSK autoantibody-positive gMG who participated in a multinational phase 3 study, reductions in total IgG and anti-AChR antibody levels were seen as early as day 8 and 15, respectively, with rozanolixizumab (≈ 7 mg/kg or ≈ 10 mg/kg once weekly) therapy, with levels gradually increasing to approach baseline by day 99 [14]. In the rozanolixizumab ≈ 7 mg/kg, rozanolixizumab ≈ 10 mg/kg and placebo groups, the median maximum reductions from baseline were 73%, 79% and 9%, respectively, in total IgG levels and 73%, 82% and 25%, respectively, in anti-AChR antibody levels [14]. There was no difference in the reduction in total IgG levels between patients who were neutralising antibody-positive and those who were antidrug antibody (ADA)-negative [7]. Reductions in anti-MuSK antibody levels followed a similar pattern to that seen with anti-AChR antibody levels [9], with median maximum reductions of 37%, 58% and 17% in the rozanolixizumab ≈ 7 mg/kg, rozanolixizumab ≈ 10 mg/kg and placebo groups, respectively (mostly driven by patients with baseline MuSK levels of > 20 nmol/L) [14]. No clinically relevant reductions over time in mean albumin levels were seen, suggesting that albumin salvage remains intact during rozanolixizumab therapy [14].
What are the pharmacokinetic properties of rozanolixizumab?
According to a population pharmacokinetic (PPK) analysis, the absolute bioavailability of rozanolixizumab following subcutaneous administration was ≈ 70% [7]. Rozanolixizumab exhibits nonlinear pharmacokinetics typical for a monoclonal antibody undergoing target-mediated drug disposition [7]; over a 1–20 mg/kg dose range, its exposure (following subcutaneous administration) increased in a greater than dose-proportional manner [9]. In healthy individuals, peak plasma levels of rozanolixizumab following subcutaneous administration were reached after ≈ 2 days [7, 9], with levels undetectable in the plasma within 1 week after dosing [7]. It is expected that rozanolixizumab will be degraded into small peptides and amino acids via catabolic pathways (in a manner similar to endogenous IgG) [7, 9].
According to a PPK analysis, the pharmacokinetics of rozanolixizumab were not affected to a clinically relevant extent by age, sex or race [7, 9]. In addition, neither renal nor hepatic impairment is expected to affect the pharmacokinetics of rozanolixizumab [7, 9]. Clinical drug interaction studies on rozanolixizumab have not been performed [9]. Rozanolixizumab is not metabolized by cytochrome P450 (CYP) enzymes; thus, interactions with concomitant medications that are substrates, inducers or inhibitors of CYP are unlikely. However, rozanolixizumab may reduce the concentrations of compounds that bind to human FcRn; thus, patients receiving such medications concomitantly with rozanolixizumab should be closely monitored [9].
What is the efficacy of rozanolixizumab in gMG?
Subcutaneous rozanolixizumab is effective in improving disease-related outcomes in adults with AChR or MuSK autoantibody-positive gMG, as demonstrated in a pivotal, randomized, double-blind, placebo-controlled, multinational phase 3 study (MG0003; MycarinG) [14].
A two-stage adaptive design was utilized: stage one consisted of a 6-week treatment period, with a prespecified interim analysis conducted at its end, while stage two consisted of an 8-week observation period [14]. Enrolled patients had a documented diagnosis of gMG and Myasthenia Gravis Foundation of America class II–IVa disease; confirmed autoantibodies against AChR or MuSK; an MG-ADL score of ≥ 3 (with ≥ 3 points for non-ocular symptoms); a Quantitative Myasthenia Gravis (QMG) scale score of ≥ 11; had been considered for additional treatment (e.g. intravenous Ig, plasma exchange); and had a body weight of ≥ 35 kg. Patients with severe oropharyngeal or respiratory weakness, a clinically relevant active infection or recent serious infection, or a total IgG concentration of ≤ 5.5 g/L were among those excluded [14].
The rozanolixizumab dosing regimen comprised fixed doses (280 mg, 420 mg, 560 mg, 840 mg and 1120 mg) across four body weight tiers (35 to < 50 kg, 50 to < 70 kg, 70 to < 100 kg, or ≥ 100 kg), resulting in the assessment of doses ranging from 5.7 to 8.4 mg/kg (i.e. equivalent to ≈ 7 mg/kg) and from 8.1 to 12.0 mg/kg (i.e. equivalent to ≈ 10 mg/kg)] [10, 11]. The assignment of patients to randomized (1:1:1) treatment arms (rozanolixizumab ≈ 7 mg/kg, rozanolixizumab ≈ 10 mg/kg or placebo) was stratified by the presence of AChR or MuSK autoantibodies [14].
Patients with disease worsening could receive rescue therapy (intravenous Ig or plasma exchange) [14]. Those who received rescue therapy during the treatment period completed the period without receiving further study medication before moving to the observation period while those who received it during the observation period discontinued MycarinG and were ineligible for extension studies [14]. Patients who completed the observation period or whose disease severity worsened (according to the investigator) during the observation period were eligible for enrolment in two randomized, open-label, multinational, phase 3 extension studies: MG0004 or MG0007, with those who completed ≥ 6 treatment visits in MG0004 eligible to enter the observation period of MG0007 and subsequently continue therapy [14, 15]. Baseline patient demographics and clinical characteristics in MycarinG were generally similar between the treatment groups; 90% and 11% of 200 patients were AChR or MuSK autoantibody positive, the mean MG-ADL score was 8.3 and the mean QMG scale score was 15.6. Analyses were conducted in the intent-to-treat population [14].
In adults with AChR or MuSK autoantibody-positive gMG, subcutaneous rozanolixizumab ≈ 7 mg/kg and ≈ 10 mg/kg (administered once weekly for 6 weeks) were associated with statistically significant and clinically meaningful improvements from baseline in the primary endpoint (Table 2) [14]. Improvements from baseline in MG-ADL scores were seen as early as day 8 and throughout the treatment period. Sensitivity analyses demonstrated that the results of the primary analysis were robust, with the benefits of rozanolixizumab over placebo in this endpoint consistent across prespecified subgroups based on sex (male or female) and autoantibody status (AChR or MuSK) [14].
In terms of secondary endpoints, statistically significant least-squares mean (LSM) between-group differences favouring both rozanolixizumab ≈ 7 mg/kg and ≈ 10 mg/kg over placebo were seen for the LSM change from baseline to day 43 in the MG-Composite (MG-C) scale score, the QMG scale score and the Myasthenia Gravis Symptoms Patient-Reported Outcome (PRO) scale scores of muscle weakness fatigability, physical fatigue and bulbar muscle weakness (Table 2). Improvements from baseline in MG-C, QMG and Myasthenia Gravis Symptoms PRO scale scores were seen as early as day 8 and throughout the treatment period [14]. Furthermore, clinically meaningful improvements of ≥ 2 points for MG-ADL (secondary endpoint; Table 2) and ≥ 3 points for MG-C (60.9% and 74.2% vs 40.6%) and QMG (54.7% and 72.6% vs 39.1%) were achieved at day 43 by approximately half to three-quarters of patients in the rozanolixizumab ≈ 7 mg/kg and ≈ 10 mg/kg groups and approximately one-third to two-fifths of those in the placebo group. Minimal symptom expression (defined as an MG-ADL score of 0 or 1 at any time up to and including day 43) was achieved by 25.8% and 28.4% of rozanolixizumab ≈ 7 mg/kg and ≈ 10 mg/kg recipients and 3.0% of placebo recipients. Rescue therapy was not required by any patient in either of the rozanolixizumab groups [14].
Are the benefits of rozanolixizumab sustained over the longer term?
Repeated cycles of subcutaneous rozanolixizumab (≈ 7 mg/kg and ≈ 10 mg/kg once weekly) resulted in sustained benefits across multiple disease-related outcomes, according to a pooled analysis of data from adults with ≥ 2 symptom-driven cycles (up to six 6-week cycles) who participated in MycarinG, the first 6 weeks of MG0004, or MG0007 (interim analysis; data cutoff date 8 July 2022) [15, 16].
In MG0004, patients received up to 52 weeks of weekly rozanolixizumab, which was followed by an 8-week observation period [15, 16]. In MG0007, they received an initial 6-week rozanolixizumab cycle, which was followed by a 16-week observation period, with subsequent cycles administered at the investigator’s discretion based on symptom worsening (e.g. increase in MD-ADL scores of ≥ 2 points, increase in QMG scale scores of ≥ 3 points). Patients in MG0007 without symptom worsening at the end of the observation period entered a nontreatment period until symptoms emerged and subsequent treatment cycles were required. Of the 127 patients in the pooled analysis, 69 received rozanolixizumab ≈ 7 mg/kg and 58 received rozanolixizumab ≈ 10 mg/kg [15, 16].
In cycles 1–6, the mean changes from baseline to day 43 in the MG-ADL score were − 3.7, − 3.9, − 3.4, − 3.8, − 3.9 and − 4.5 points, respectively (n = 127, 127, 98, 75, 51 and 32), and the mean changes from baseline to day 43 in the QMG scale score were − 5.4, − 4.7, − 4.7, − 5.1, − 4.5 and − 6.3 (n = 127, 125, 97, 74, 51 and 32) [15]. Reductions in MG-C scale score were also consistent across the cycles, as were MG-ADL, MG-C, QMG and minimal symptom expression responder rates [15, 16]. Moreover, the benefits of rozanolixizumab in the change from baseline in MG-ADL score were maintained across repeated cycles regardless of age, autoantibody status, disease duration, disease severity and thymectomy status [16].
What is the tolerability profile of rozanolixizumab?
Subcutaneous rozanolixizumab ≈ 7 mg/kg and ≈ 10 mg/kg once weekly were generally well tolerated in adults with AChR or MuSK autoantibody-positive gMG participating in the pivotal MycarinG study [14]. However, across all TEAE categories, the incidence of TEAEs was higher in the rozanolixizumab ≈ 10 mg/kg group than the rozanolixizumab ≈ 7 mg/kg group, with the incidence of infections and severe headache in the ≈ 10 mg/kg group of concern [10].
In MycarinG, TEAEs occurred in 81%, 83% and 67% of patients in the rozanolixizumab ≈ 7 mg/kg, rozanolixizumab ≈ 10 mg/kg and placebo groups, respectively (n = 64, 69 and 67) [14]. Most TEAEs were mild to moderate, with severe TEAEs reported in 5%, 19% and 4% of patients in the respective groups. TEAEs occurring in ≥ 10% of patients in any group were headache (45%, 38% and 19% with rozanolixizumab ≈ 7 mg/kg, rozanolixizumab ≈ 10 mg/kg and placebo, respectively), diarrhoea (25%, 16% and 13%), pyrexia (13%, 20% and 1%) and nausea (8%, 12% and 7%) [14]. Headache occurred most frequently after the first infusion and within 1 to 4 days after infusion [7, 14]. Severe headache was considered an adverse event (AE) of special monitoring and occurred in one rozanolixizumab ≈ 7 mg/kg recipient and six rozanolixizumab ≈ 10 mg/kg recipients; no placebo recipient reported experiencing a severe headache [14]. For the most part, severe headache was well managed with non-opioid analgesics, and all patients fully recovered with no sequelae; severe headache recurrence was not observed in any patient. In terms of other AEs of special monitoring (severe vomiting, severe diarrhoea, severe abdominal pain and opportunistic infection), severe vomiting occurred in 2% of rozanolixizumab ≈ 7 mg/kg recipients only and severe diarrhoea in 3% of rozanolixizumab ≈ 10 mg/kg recipients only; no placebo recipients experienced severe vomiting or diarrhoea. Moreover, no opportunistic infections were reported in either rozanolixizumab group [14].
Infections (with an increased risk of infection potentially resulting from the transient reduction in circulating IgG levels [11]) and hypersensitivity reactions are among the regulatory warnings associated with the use of rozanolixizumab (Table 3). In MycarinG, 16%, 30% and 19% of patients in the rozanolixizumab ≈ 7 mg/kg, rozanolixizumab ≈ 10 mg/kg and placebo groups, respectively, developed an infection, with nasopharyngitis (2%, 7% and 4% of patients), oral herpes (0%, 4% and 0%) and upper respiratory tract infection (3%, 1% and 1%) the most frequently reported infections [14]. No severe or serious infections were reported in either rozanolixizumab group; one serious infection (COVID-19 pneumonia) occurred in the placebo group. No serious hypersensitivity or anaphylactic reactions were described [14].
In the rozanolixizumab ≈ 7 mg/kg, rozanolixizumab ≈ 10 mg/kg and placebo groups, respectively, treatment-related TEAEs were reported in 50%, 57% and 33% of patients and serious TEAEs in 8%, 10% and 9% of patients [14, 17]. All serious TEAEs occurring in > 1 patient in each group were related to the disease under investigation (MG worsening or MG crisis). A serious TEAE of worsening MG requiring hospital admission was seen in one rozanolixizumab ≈ 7 mg/kg recipient during the treatment period and two rozanolixizumab ≈ 10 mg/kg recipients during the observation period. MG crises were not reported in either rozanolixizumab group but did occur in two placebo recipients. One rozanolixizumab ≈ 10 mg/kg recipient had a severe headache that was classified as serious but did not result in a change in the dose, or the interruption or discontinuation of treatment. Treatment discontinuation due to TEAEs were reported in two rozanolixizumab ≈ 7 mg/kg recipient (arthralgia and headache), four rozanolixizumab ≈ 10 mg/kg recipients (diarrhoea, upper abdominal pain, vomiting, oral herpes, metastatic squamous cell carcinoma, pruritus and deep vein thrombosis; some patients reported > 1 event) and two placebo recipients (MG and MG crisis). One rozanolixizumab ≈ 7 mg/kg recipient discontinued therapy owing to a severe headache. No deaths occurred [14].
Injection-site reactions occurred in 6% of rozanolixizumab ≈ 7 mg/kg recipients, 6% of rozanolixizumab ≈ 10 mg/kg recipients and 3% of placebo recipients [14]. Apart from injection-site rash, which occurred in two rozanolixizumab ≈ 7 mg/kg recipients (one of which was considered to be treatment related), no injection-site reaction TEAEs were seen in > 1 patient. No serious injection-site reactions were reported [14]. Local reactions at the administration site were observed within 1–3 days following the most recent administration of rozanolixizumab [9].
Repeated cycles of subcutaneous rozanolixizumab ≈ 7 mg/kg and ≈ 10 mg/kg once weekly were associated with an acceptable safety profile, according to a pooled analysis of data from adults with ≥ 1 cycle who participated in MycarinG, the first 6 weeks of MG0004, or MG0007 (interim analysis) [15, 16]. At least one TEAE was reported in 89.9% of 188 patients; most TEAEs were mild to moderate in severity [15, 16]. There was no increase in the incidence of headache with repeated cyclic treatment [7]. Infections were reported by 48% of 196 patients in the pooled analysis, with the most common (frequency ≥ 5%) being upper respiratory tract infections (17%), COVID-19 infection (14%) urinary tract infections (9%) and herpes simplex (6%) [9]. Serious infections occurred in 4% of patients, with infections leading to treatment discontinuation reported in 3%. Three patients died due to an infection (pneumonia caused by COVID-19 infection and pneumonia caused by an unknown pathogen, n = 2 and 1) [9]. A serious AE of drug-induced aseptic meningitis occurred in one patient in the phase 3 extension study MG0007 following the first infusion of rozanolixizumab ≈ 10 mg/kg [7, 9, 11]. It was considered to be severe and related to treatment, and it resulted in hospitalization and treatment withdrawal, with the patient subsequently recovering without sequelae after study discontinuation [11]. The prescribing information for rozanolixizumab therefore carries a warning for aseptic meningitis (Table 3).
Following one treatment cycle (i.e. six weekly doses) of rozanolixizumab, ADAs and neutralizing antibodies were detected in 37% and 21% of 133 patients, respectively, at the end of the observation period in MycarinG, and in 27.1% and 10.3% of 155 patients in a pooled analysis of data from MycarinG, MG0004 and MG0007 [7, 9]. After treatment reinitiation, ADAs and neutralizing antibodies were detected in 65% and 50% of 20 patients after five treatment cycles [7]. While neutralising antibody development was associated with a 24% reduction in overall plasma rozanolixizumab exposure and there was an up to 60% reduction in trough rozanolixizumab concentrations in ADA-positive vs -negative patients, ADAs (including neutralizing antibodies) appeared to have no impact on efficacy or safety [7, 9].
What is the current clinical position of rozanolixizumab in gMG?
Rozanolixizumab, a humanized IgG4 monoclonal antibody directed against human FcRn, is an effective and generally well tolerated treatment option for adults with AChR or MuSK autoantibody-positive gMG. It binds to human FcRn with a high affinity and specificity, thereby blocking the FcRn-mediated recycling of human IgG and thus reducing circulating IgG levels. Rozanolixizumab ≈ 7 mg/kg and ≈ 10 mg/kg offer a clinically meaningful improvement from baseline versus placebo in the MG-ADL score regardless of patients’ sex or autoantibody status. It also improves other disease-related outcomes versus placebo: the MG-C scale score, the QMG scale score, and the Myasthenia Gravis Symptoms PRO scale scores of muscle weakness fatigability, physical fatigue, and bulbar muscle weakness. The benefits of rozanolixizumab are additionally sustained across multiple disease-related outcomes following repeated cycles (interim analysis). Rozanolixizumab is generally well tolerated, with repeated cycles associated with an acceptable safety profile. While ADAs and neutralizing antibodies were detected during therapy with rozanolixizumab, neither appear to have an impact on efficacy or safety.
The international consensus guidance for management of MG [18] published prior to the approval of rozanolixizumab recommends the acetylcholinesterase inhibitor pyridostigmine as part of the initial therapy regimen for most patients, followed by corticosteroid and/or nonsteroidal (e.g. azathioprine, cyclosporine, mycophenolate mofetil and tacrolimus) immunosuppressive drugs for those patients who did not meet their treatment goals with an adequate trial of pyridostigmine. For patients with refractory MG, the guidance recommends immunosuppressive agents, chronic intravenous Ig and chronic plasma exchange, cyclophosphamide, rituximab, methotrexate, and eculizumab [18].
Randomized controlled studies directly comparing rozanolixizumab with other treatments for adults with AChR or MuSK autoantibody-positive gMG are not available. Results from a meta-analysis determined that eculizumab, efgartigimod, ravulizumab, rituximab, rozanolixizumab and zilucoplan were each effective versus placebo in terms of overall mean MG-ADL score, with the results homogeneous within each class (i.e. the complement inhibitors eculizumab, ravulizumab and zilucoplan appeared to have similar efficacy as did the FcRn therapies efgartigimod and rozanolixizumab) [19]. A network meta-analysis comparing the efficacy of the various therapies found that efgartigimod had the highest probability of being the best treatment, followed by both doses (≈ 7 mg/kg and ≈ 10 mg/kg) of rozanolixizumab, in terms of the MD-ADL score [19]. Direct comparisons between rozanolixizumab and complement inhibitor or other FcRn therapies would be of use in elucidating the position of rozanolixizumab in the management of gMG.
Final results from the ongoing randomized, open-label, multinational, phase 3 extension studies MG0004 and MG0007 are awaited with interest. Additional studies investigating the use of rozanolixizumab in the real-world setting would be of interest.
References
National Institute of Neurological Disorders and Stroke. Myasthenia Gravis. 2023. https://www.ninds.nih.gov/health-information/disorders/myasthenia-gravis. Accessed 17 May 2024.
Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. 2022;82(8):865–87.
Crisafulli S, Boccanegra B, Carollo M, et al. Myasthenia gravis treatment from old drugs to innovative therapies with a glimpse into the future. CNS Drugs. 2024;38(1):15–32.
Zhu L-N, Hou H-M, Wang S, et al. FcRn inhibitors: a novel option for the treatment of myasthenia gravis. Neural Regen Res. 2023;18(8):1637–44.
Hoy SM. Rozanolixizumab: first approval. Drugs. 2023;83(14):1341–7.
Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis: implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212.
UCB Pharma S.A. Rystiggo 140 mg/ml solution for injection: EU summary of product characteristics. 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/rystiggo. Accessed 17 May 2024.
UCB Japan. RYSTIGGO® for SC injection: Japanese prescribing information. 2023. https://www.pmda.go.jp/. Accessed 17 May 2024.
UCB Inc. RYSTIGGO® (rozanolixizumab-noli) injection, for subcutaneous use: US prescribing information. 2023. https://www.fda.gov/. Accessed 17 May 2024.
European Medicines Agency. Rozanolixizumab (Rystiggo): EU assessment report. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rystiggo. Accessed 17 May 2024.
US FDA Center for Drug Evaluation and Research. Rozanolixizumab: integrated review. 2022. https://www.fda.gov/. Accessed 17 May 2024.
Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111–30.
Kiessling P, Lledo-Garcia R, Watanabe S, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med. 2017;9(414):1–12.
Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383–94.
Bril V, Druzdz A, Grosskreutz J, et al. Long-term efficacy and safety of symptom-driven cyclic rozanolixizumab treatment in patients with generalized myasthenia gravis: a pooled analysis of a phase 3 study and two open-label extension studies [abstract no. P1-5.012 plus poster]. Neurology. 2023;100(17 Suppl 2).
Pascuzzi RM, Mantegazza R, Drużdż A, et al. Efficacy of repeated cycles of rozanolixizumab treatment in subgroups of patients with generalized myasthenia gravis: a pooled analysis of a phase 3 study and two phase 3 open-label extension studies [abstract plus poster]. In: MGFA Scientific Session at AANEM 2023. 2023.
US National Institutes of Health. ClinicalTrials.gov identifier NCT03971422. 2024. https://clinicaltrials.gov/study/NCT03971422. Accessed 5 June 2024.
Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis. Neurology. 2021;96(3):114–22.
Saccà F, Pane C, Espinosa PE, et al. Efficacy of innovative therapies in myasthenia gravis: a systematic review, meta-analysis and network meta-analysis. Eur J Neurol. 2023;30(12):3854–67.
Acknowledgements
The manuscript was reviewed by: D. Menon, Department of Neurology, National Institute of Mental Health and Neurosciences, Bangalore, India; R. M. Pascuzzi, Department of Neurology, Indiana University School of Medicine, Indiana University Health, Indianapolis, IN, USA. During the peer review process, UCB Pharma, the marketing authorization holder of rozanolixizumab, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
Sheridan M. Hoy is a salaried employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoy, S.M. Rozanolixizumab in generalized myasthenia gravis: a profile of its use. Drugs Ther Perspect 40, 203–210 (2024). https://doi.org/10.1007/s40267-024-01077-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-024-01077-6