Abstract
Amphetamine extended-release oral suspension (Adzenys ER™) and extended-release orally disintegrating tablets (Adzenys XR-ODT®) are easy-to-administer, long-acting, and convenient CNS stimulant options for treating attention-deficit hyperactivity disorder (ADHD) in children aged ≥ 6 years, adolescents, and adults. The bioavailability of d- and l-amphetamine with Adzenys ER™ suspension and XR-ODT® is equivalent to that of a corresponding dose of the reference product [i.e. mixed amphetamine salt extended-release capsules (MAS ER)], which has well-established efficacy, tolerability, and safety profiles. As Adzenys ER™ suspension/XR-ODT® contain both immediate- and extended-release amphetamine particles, plasma concentrations of d- and l-amphetamine increase rapidly, remain relatively stable for several hours, then slowly decline, allowing for once-daily administration. The use of Adzenys ER™ suspension/XR-ODT® may be of particular benefit in individuals who require a rapid onset and prolonged reduction in ADHD symptoms, as well as those who have difficulty swallowing tablets or capsules whole (neither formulation requires swallowing whole tablets/capsules, and both may be taken without regard to food).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bioequivalent to a corresponding dose of MAS ER, which has well-established efficacy, tolerability and safety profiles |
Adzenys ER™ suspension/XR-ODT® 3.1, 6.3, 9.4, 12.5, 15.7, and 18.8 mg are equivalent to MAS ER 5, 10, 15, 20, 25, and 30 mg, respectively |
Provides rapid and sustained increases in d- and l-amphetamine plasma concentrations, allowing for once-daily administration |
Both orange-flavored formulations are easy and convenient to administer, and may be taken with or without food |
How is attention-deficit hyperactivity disorder treated?
Attention-deficit hyperactivity disorder (ADHD) is a chronic neurobehavioral disorder that begins in childhood and may persist throughout adulthood in some patients [1,2,3,4,5,6]. Pharmacologic treatment and behavioral therapy, alone or together, are used to treat ADHD symptoms, such as inattention, hyperactivity, and impulsivity [1,2,3,4,5,6].
The pharmacologic treatment of ADHD generally involves the use of CNS stimulants, with various formulations of amphetamine and methylphenidate (all of which have comparable effectiveness, tolerability, and safety profiles) being commonly used [4,5,6,7,8]. Other, generally less commonly used, options include atomoxetine, extended-release guanfacine, and extended-release clonidine [5, 7, 8]. The mechanism of action of amphetamines in the treatment of ADHD is thought to involve restoring the imbalance in dopamine and norepinephrine (noradrenaline) levels in the areas of the brain involved in cognition and emotion; however, their exact mode of therapeutic action is not yet known [6, 9,10,11]. By blocking the re-uptake of dopamine and norepinephrine from the synaptic cleft into the presynaptic neurons, amphetamines increase the levels of these monoamine neurotransmitters in the synaptic cleft, thereby leading to an improvement in the clinical symptoms of ADHD.
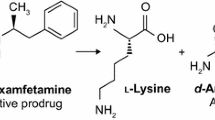
To provide once-daily, long-acting options to treat ADHD in school-aged children, adolescents, and adults, Neos Therapeutics (Grand Prairie, TX) have developed the following two extended-release formulations of amphetamine: an orange-flavored oral suspension (Adzenys ER™ suspension) [9] and orange-flavored orally disintegrating tablets (Adzenys XR-ODT®) [10]. Adzenys ER™ oral suspension and XR-ODT® contain a 3:1 ratio of d- and l-amphetamine loaded onto a nearly equivalent mixture of immediate-release and polymer-coated extended-release resin particles. The immediate-release particles are uncoated and are designed to be absorbed in the stomach, whereas the extended-release particles have a pH-dependent, acid-resistant coating that is designed to dissolve in the alkaline environment of the intestinal tract, thereby prolonging the release and activity of amphetamine. The tradenames of these formulations are used hereafter to avoid confusion with other extended-release formulations of amphetamine.
For whom are Adzenys ER™ suspension/XR-ODT® indicated?
In the USA, Adzenys ER™ oral suspension [9] and XR-ODT® [10] are indicated for the treatment of ADHD in children and adolescents aged 6‒17 years, and adults (Table 1). The dosage should be individualized based on the response and needs of the patient (Table 1). Doses of 3.1, 6.3, 9.4, 12.5, 15.7 and 18.8 mg of Adzenys ER™ suspension/XR-ODT® are equivalent to 5, 10, 15, 20, 25, and 30 mg of mixed amphetamine salts (MAS) extended-release capsules (MAS ER).
What evidence supports the US approval of Adzenys ER™ suspension/XR-ODT®?
As Adzenys ER™ suspension [12] and XR-ODT® [13] have been shown to bioequivalent to the reference product MAS ER (Adderall XR®) in healthy adults, clinical trials of Adzenys ER™ suspension and XR-ODT® were not required prior to approval by the US FDA [9, 10]. Instead, their approval was based on the clinical evidence for the efficacy and tolerability of MAS ER in the treatment of ADHD in randomized, double-blind trials in treatment-naïve or treatment-experienced children [14, 15], adolescents [16], and adults [17].
The following sections summarize the results of single-dose bioequivalence and other pharmacokinetic studies of Adzenys ER™ suspension [12, 18, 19] and XR-ODT® [13, 20, 21], and of the clinical trials [14,15,16,17] supporting the use of these amphetamine formulations.
Evidence of bioequivalence to mixed amphetamine salts extended-release capsules (MAS ER)
The bioequivalence of a single 18.8-mg dose of Adzenys ER™ suspension [12] and XR-ODT® [13] to an equivalent single 30-mg dose of MAS ER was established in two single-dose, randomized, open-label crossover studies in healthy adults (completer n = 42 [12] and 39 [13]).
-
Adzenys ER™ suspension [12] Exposure to d- and l- amphetamine following administration of 18.8 mg of Adzenys ER™ suspension was bioequivalent to that of 30 mg of MAS ER. The 90% confidence intervals (CIs) for the Adzenys XR-ODT®: MAS ER least-squares geometric mean (LSM) ratios for maximum plasma concentration (Cmax), area under the concentration–time curve (AUC) from time zero to 5 h (AUC0–5), AUC from 5 h to last quantifiable concentration (AUC5–last), and AUC from time zero to infinity (AUC0–∞) were within the standard bioequivalence CIs of 80–125% for both d- and l- amphetamine.
-
Adzenys XR-ODT® [13] Administration of 18.8 mg of Adzenys XR-ODT® resulted in exposure to d- and l- amphetamine that was bioequivalent to that of 30 mg of MAS ER. The 90% CIs for Adzenys XR-ODT®: MAS ER LSM ratios for Cmax, AUC0–last, AUC0–∞, and AUC5–last were within the standard 90% bioequivalence CIs of 80–125% for both d- and l- amphetamine.
Pharmacokinetic profiles
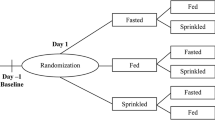
Randomized, open-label studies of the pharmacokinetics of Adzenys ER™ suspension [18, 19] and XR-ODT® [13, 20] have been conducted in children aged 6–12 years with ADHD [18, 20] and healthy adults aged 20–70 years [13, 19] (Table 2). Unless otherwise noted, participants received a single 18.8 mg dose of Adzenys ER™ suspension [18, 19] or XR-ODT® [13, 20] under fasting conditions.
The overall pharmacokinetic profiles of Adzenys ER™ suspension and XR-ODT® allow once-daily administration with or without food, with bodyweight appearing to be the primary determinant of apparent age-related differences in some parameters [9, 10]. The threefold higher exposure to d-amphetamine than to l-amphetamine (Table 2) is in keeping with the 3: 1 ratio of d-amphetamine and l-amphetamine in these formulations [13, 18,19,20].
In children
Following administration of Adzenys ER™ suspension or XR-ODT® in children aged 6–12 years, plasma concentrations of d- and of l-amphetamine increased rapidly, followed by broad AUC peak, then by a slow decline (Table 2), in keeping with extended-release profile of these formulations [18, 20].
For both Adzenys ER™ suspension and XR-ODT®, the 95% CIs for geometric mean weight-normalized clearance (CL/F/kg) and weight-normalized volume of distribution (Vd/F/kg) for d- and l-amphetamine usually fell within the target range of 60–140% for each age group (i.e. 6–7, 8–9, and 10–12 years) [18, 20]. The only exception was CL/F/kg in children aged 6–7 years in the study of Adzenys ER™ suspension [18].
Overall, the pharmacokinetic profile of d- and l-amphetamine was more variable in children aged 6–7 years than in the older age groups (Table 2) [18, 20]. In addition, mean values for the CL/F/kg of d- and l-amphetamine increased as age increased from 6–7 to 10 − 12 years, whereas corresponding values for mean Vd/F/kg decreased as age increased [18, 20].
Mean elimination half-life (t½) values for d- and l-amphetamine also decreased as children aged (Table 2). The decrease in exposure with increasing age in these studies may have been caused by the use of a fixed-dose across all body weights from 20 to 56 kg, leading to lower doses/kg in older/heavier children, and the slight increase in clearance of amphetamine with increased age [18, 20]. Overall, when doses are weight normalized, systemic exposure to amphetamine was 30% lower in children than in adults [9, 10].
In adults
The overall pharmacokinetic profile of Adzenys ER™ suspension and XR-ODT® over time in adults is comparable to that in children and adolescents (i.e. a rapid increase in plasma concentrations of d- and l-amphetamine, followed by a relatively broad AUC peak and a slow decline) [9, 10]. Following administration of Adzenys ER™ suspension and XR-ODT® in adults, Cmax of d- and l-amphetamine were achieved in ≈ 5 h, with a mean t½ of ≈ 12 − 14 h (Table 2) [19].
Potential for food and drug interactions
Adzenys ER™ suspension and XR-ODT® may be taken with or without food [9, 10].
Relative to when a single 18.8 mg dose of Adzenys ER™ suspension or XR-ODT® was administered to adults in the fasted state, administration 30 min after a high-fat meal was associated with decreases in mean Cmax values for d-amphetamine of 11% (Adzenys ER™ suspension) [19] and 19% (Adzenys XR-ODT®) [13]. Median tmax values for d- and l-amphetamine were ≈ 0.5–1 h (Adzenys ER™ suspension) or ≈ 0.2–2.5 h (Adzenys XR-ODT®) shorter in the fed state than in the fasted state [13, 19]. However, these pharmacokinetic differences were not considered to be clinically relevant [9, 10, 13, 19].
Certain classes of drugs [i.e. gastrointestinal acidifying and alkalizing agents, monoamine oxidase inhibitors (MAOIs), serotonergic drugs, and tricyclic antidepressants (TCAs)] have the potential to interact with Adzenys ER™ suspension/XR-ODT®, and may result in clinically important outcomes (Table 3) [9, 10]. The concomitant use of Adzenys ER™ suspension/XR-ODT® is contraindicated with MAOIs, is not advised with GI alkalinizing agents or requires precautionary measures with serotonergic drugs and TCAs [9, 10].
As the consumption of alcohol may result in a more rapid release of amphetamine from Adzenys ER™ suspension/XR-ODT®, patients taking Adzenys ER™ suspension/XR-ODT® should be advised to avoid alcohol [9, 10]. The release of amphetamine did not increase in the presence of 5, 10 or 20% alcohol, but substantially increased in the presence of 40% alcohol in an in vitro alcohol-induced dose dumping study [9, 10]. However, in a study in which 32 healthy adults received a single 18.8 mg dose of Adzenys XR-ODT® followed by consumption of 240 mL of 4, 20 or 40% alcohol within 30 min, exposure to d- and l-amphetamine did not differ to a significant extent relative to intake of the same volume of deionized water [21].
Efficacy evidence supporting the US approval of Adzenys ER™/XR-ODT®
The 3- [15] - and 4-week [16, 17] multicenter trials used as evidence to support the US approval of Adzenys ER™ suspension/XR-ODT® enrolled patients who met DSM-IV criteria for ADHD. These trials started with a washout period, followed by randomization to the treatment groups. In groups receiving MAS ER, patients receiving the lowest-dose of MAS-ER in the trial for the first week, followed by forced titration to achieve the final dosage of MAS ER to which the patient had been randomized (i.e. the daily dose of MAS ER was increased by increments of 10 mg at the beginning of each week until the final assigned dosage was reached).
In children
The clinical efficacy of Adzenys ER™ suspension and XR-ODT® in children aged 6–12 years with ADHD was established based on the results of the following trials of oral MAS ER 10, 20, and 30 mg capsules administered once daily in the morning [14, 15].
-
Classroom analog study [14] Following a 1- week run-in period with MAS ER 20 mg/day to assess individual tolerability, 51 children with ADHD were randomized in a crossover design to each of the 5 treatment weeks [i.e. once-daily placebo, MAS 10 mg (active control), or MAS ER 10, 20, or 30 mg]. Children were assessed at intervals of ≈ 1.5 h, over 12 h, on seven consecutive Saturdays. In the intent-to-treat (ITT) population (n = 49), MAS ER 10, 20, and 30 mg/day provided better efficacy than placebo and was at least as effective as MAS 10 mg/day, as assessed by Swanson, Alger, M-Flynn and Pelham attention and deportment scores, and Permanent Product Measure of Performance scores, which assess academic performance. The improvement in ADHD symptoms and the duration of response were dependent on the dose of MAS ER.
-
Multicenter 3-week trial [10, 15] Children (n = 584) were randomized to receive once-daily placebo, or once-daily MAS ER force-titrated to 10, 20, or 30 mg [15]. In the ITT population (n = 563), ADHD Rating Scale-IV (ADHD-RS-IV) scores based on attention and hyperactivity levels as assessed by teachers improved from baseline to a significantly greater extent with MAS ER 10, 20, and 30 mg than with placebo (all p < 0.001) [10]. These significant improvements in ADHD-RS-IV scores were shown in both the morning and afternoon assessments, and during all 3 weeks [10]. All three MAS ER dosages were also significantly (p < 0.05), more effective than placebo as assessed by improvements from baseline in Connors Global Index Scale for parents, the physician-assessed Clinical Global Impressions Scale for improvement (CGI-I), and the Parent’s Global Assessment for improvement [15]. For all endpoints, the 30-mg/day dosage provided the greatest improvements from baseline [15].
In an open-label extension of the placebo-controlled trials [14, 15], improvements in behavioral symptoms with MAS ER were consistently maintained during treatment for up to 2 years [22]. MAS ER was also effective in improving ADHD behavior in the community-practice setting in an open-label study in 2968 school-aged children with ADHD [23].
In adolescents
Evidence of the clinical efficacy of Adzenys ER™ suspension and Adzenys XR-ODT® in adolescents with ADHD was provided by the results of a 4-week multicenter trial of once-daily MAS ER in patients aged 13–17 years [16]. Adolescent patients (n = 287) were randomized to receive once-daily placebo or forced titration to once-daily MAS ER 10, 20, 30 and 40 mg [16].
In the ITT population (n = 278), ADHD Rating Scale-IV (ADHD-RS-IV) scores improved from baseline to a significantly (p < 0.001) greater extent in all four MAS ER treatment groups than in the placebo groups at the end of each week [16]. All MAS ER groups showed significant improvements from baseline for both the ADHD-RS-IV inattentive and hyperactive-impulsive subscale scores (p < 0.001 vs placebo). MAS ER 10, 20, 30 and 40 mg/day were also significantly (p < 0.01) more effective than placebo with regard to CGI-I scores at endpoint [16]. Of note, evidence was not adequate to conclude that MAS ER dosages > 20 mg/day conferred additional benefit in this patient population [10].
In adults
In adults, the efficacy of Adzenys ER™ suspension/XR-ODT® was established based on a 4-week trial of randomized, forced-titrated treatment with once-daily MAS ER 20, 40, or 60 mg versus placebo in 255 patients with ADHD aged ≥ 18 years [17].
Improvements from baseline in ADHS-RS scores were significantly (p ≤ 0.001) better with MAS ER 20, 40, and 60 mg/day than with placebo at week 4 [17]. A 2-h duration of treatment was indicated by the significant (p < 0.05) improvements from baseline in Conners’ Adult ADHD Rating Scale Short-Version Self-Report Scores at 4- and 12-h after administrations. ADHD symptoms improved within the first week of treatment [17]. As in the trial in adolescents [16], available evidence is not sufficient to conclude that MAS ER dosages > 20 mg/day conferred additional benefit in adults [10].
In an extension of this trial, improvements in ADHD symptoms were sustained when MAS ER treatment was continued for up to 24 weeks [24].
Tolerability evidence supporting the US approval of Adzenys ER™/XR-ODT®
The tolerability profiles of Adzenys ER™ suspension/XR-ODT® are based on the tolerability of MAS ER in the treatment of ADHD in the randomized, double-blind trials in treatment-naïve or -experienced children [15], adolescents [16], and adults [17]. The overall tolerability profile of MAS-ER, and hence of Adzenys ER™ suspension/XR-ODT®, are comparable to those of other long-acting formulations of amphetamine, with most adverse reactions being mild or moderate in intensity. In common with other CNS stimulants, there is a potential for misuse, abuse, and dependence with Adzenys ER™ suspension/XR-ODT®, and precautions should be followed to ameliorate their risk (Table 3).
Table 4 provides a summary of adverse reactions that were reported in ≥ 5% of MAS ER recipients and at an incidence ≥ 2% higher than in placebo recipients in the randomized trials of MAS ER that were used as the basis of the US FDA approval of Adzenys ER™ suspension/XR-ODT®. As expected in clinical trials, rates of adverse reactions, as well as the type of adverse reaction, varied between these trials. Across all age groups, the most commonly reported adverse reactions were the loss of appetite and weight, insomnia, and various digestive order symptoms (i.e., nausea, abdominal pain, dyspepsia, vomiting) [Table 4].
The adverse reactions leading to the discontinuation of MAS ER treatment also varied between the MAS ER trials. In the trial in children, 2.4 and 2.9% of MAS ER and placebo recipients discontinued treatment because of an adverse reaction; corresponding rates in the trial in adolescents were 2.1 and 0%, and in the trial in adults were 12.0 and 1.6%. The most common drug-related adverse reaction leading to the discontinuation of MAS ER at a rate at least twice that with placebo was insomnia (1.3% of adolescents and 5.2% of adults) [insomnia was not reported in children]. No other drug-related adverse reactions led to treatment discontinuation in ≥ 1% of adolescents. In adults, 2.1% of patients discontinued treatment because of anxiety, 1.6% each discontinued due to nervousness, dry mouth, anorexia, tachycardia, and headache, and 1% discontinued due to asthenia.
Longer-term treatment with MAS ER was also well tolerated in the open-label extension studies in school-aged children [22] and adults [24], as well as in school-aged children in the community-practice setting [23].
Tachycardia was reported in adults [17], but not in children [15] or adolescents [16] (Table 4). According to an assessment of the short- and long-term cardiovascular effects of MAS ER in children in the placebo-controlled trial and its extension, MAS ER ≤ 30 mg/day had minimal CV effects in otherwise healthy children with ADHD [25]. Precautions should be followed to minimize the risk of adverse CV-related outcomes, as well as the risk of psychiatric and other adverse outcomes, in at-risk patients (Table 3).
What conclusions can be made regarding Adzenys ER™ suspension/XR-ODT®?
Adzenys ER™ suspension and XR-ODT® are easy-to-administer, long-acting, and convenient options for treating ADHD in children aged ≥ 6 years, adolescents, and adults. They may be especially useful in patients in whom a rapid onset and prolonged reduction in ADHD symptoms is required, and those who have difficulty swallowing whole tablets or capsules. The bioavailability of these amphetamine formulations is equivalent to that of the MAS ER formulation, for which efficacy, tolerability, and safety are well-established.
As the effectiveness, tolerability, and safety profiles of various formulations of amphetamine and methylphenidate are comparable, a number of other factors should be considered when selecting the most appropriate CNS stimulant to treat the individual child, adolescent, or adult with ADHD [5,6,7]. The use of a formulation that more closely matches the preferences of the individual may improve treatment adherence, thereby potentially improving treatment outcomes [8].
Factors to consider when selecting a CNS stimulant include the preferred length of ADHD coverage and the number of times per day the formulation needs to be administered [5,6,7]. The pharmacokinetic profiles of Adzenys ER™ suspension and XR-ODT®, which allow for once-daily administration with or without food, may be potentially advantageous for certain patients with ADHD (e.g. those requiring a rapid onset and prolonged reduction in ADHD symptoms throughout and beyond the school/working day). Adzenys ER™ suspension/XR-ODT® include both immediate- and extended-release d- and l-amphetamine particles, resulting in a rapid increase in plasma concentrations, followed by relatively stable concentrations for several hours, then a slow decrease in concentrations (Table 2). Due to this prolonged activity, additional amphetamine doses do not need to be taken at school or work (thereby maintaining the privacy of the patient), and coverage is provided for post-school and –work activities. Long-acting formulations of CNS stimulants that are administered once daily may result in better adherence to treatment relative to immediate-release formulations that require administration more often [3, 8, 26, 27].
The ability of the patient to swallow tablets or capsules and the ease of administration are other factors that should be considered when selecting the most appropriate formulation for the individual [5,6,7]. Some patients, especially children, may have difficulty swallowing tablets or capsules whole. Adzenys ER™ suspension and XR-ODT® are both relatively easy to administer; the orange-flavored suspension does not require reconstitution prior to administration, and the orange-flavored XR-ODT® dissolves rapidly on the tongue, followed by swallowing of the disintegrated particles with saliva without the need for water (Table 1). Adzenys ER™ suspension/XR-ODT® may be taken with or without food.
Other factors to consider when choosing a CNS stimulant are the potential risks of drug dependence, abuse, and diversion [5,6,7]. Due to their slower onset and sustained rate of drug delivery, extended-release formulations of CNS stimulants may reduce the risk of abuse relative to immediate-release formulations, which rapidly reach Cmax. For example, in a US study in patients in an ADHD treatment center, abuse of prescription stimulants was reported by 14.3% of patients; in these patients, the rate of abuse of immediate-release stimulants was much higher than that of extended-release formulations (79.8 vs 17.2%) [28]. The once-daily administration of Adzenys ER™/XR-ODT® avoids the need for taking or storing controlled substances at school or work, thereby limiting the likelihood of diversion or theft in these settings. Appropriate precautions should be followed to minimize the risks of dependence, misuse, and abuse (Table 3).
Change history
12 October 2018
Page 416, column 2, from the beginning of the sentence on the last line to the end of the sentence on page 417, column 1, lines 1–4: the following text, which previously read.
References
Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–22.
Danielson ML, Visser SN, Chronis-Tuscano A, et al. A national description of treatment among United States children and adolescents with attention-deficit/hyperactivity disorder. J Pediatr. 2018;192(240–246):e1.
Jain R, Jain S, Montano CB. Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017. https://doi.org/10.4088/PCC.17nr02153.
Banaschewski T, Becker K, Döpfner M, et al. Attention-deficit/hyperactivity disorder. Dtsch Arztebl Int. 2017;114(9):149–59.
National Collaborating Centre for Mental Health (UK). Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. NICE guideline no. 87. Leicester (UK): British Psychological Society; 2018.
Santosh P. Stimulant medication to treat attention-deficit/hyperactivity disorder. BMJ. 2017;358:j2945. https://doi.org/10.1136/bmj.j294.
Mattingly GW, Wilson J, Rostain AL. A clinician’s guide to ADHD treatment options. Postgrad Med. 2017;129(7):657–66.
Jain R, Katic A. Current and investigational medication delivery systems for treating attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2016. https://doi.org/10.4088/PCC.16r01979.
Adzenys ER (amphetamine) extended-release oral suspension: US prescribing Information. Grand Prairie (TX): Neos Therapeutics, Inc.; 2017.
Adzenys XR-ODT (amphetamine) extended-release orally disintegrating tablets, CII: US prescribing Information. Grand Prairie (TX): Neos Therapeutics, Inc.; 2017.
Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J Clin Psychopharmacol. 2008;28(3 Suppl 2):S39–45.
Sikes C, Stark JG, McMahen R, et al. A single-dose, two-way crossover, open-label bioequivalence study of an amphetamine extended-release oral suspension in healthy adults. J Atten Disord. 2017:1087054717743329. https://doi.org/10.1177/1087054717743329.
Stark JG, Engelking D, McMahen R, et al. A randomized crossover study to assess the pharmacokinetics of a novel amphetamine extended-release orally disintegrating tablet in healthy adults. Postgrad Med. 2016;128(7):648–55.
McCraken JT, Biedernam J, Greenhill LL, et al. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42(6):673–83.
Biederman J, Lopez FA, Boellner SW, et al. A randomized, double-blind, placebo-controlled, parallel-group study of SLI381 (Adderall XR) in children with attention-deficit/hyperactivity disorder. Pediatrics. 2002;110(2 Pt 1):258–66.
Spencer TJ, Wilens TE, Biederman J, et al. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of attention-deficit/hyperactivity disorder in adolescent patients: a 4-week, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(2):266–79.
Weisler RH, Biederman J, Spencer TJ, et al. Mixed amphetamine salts extended-release in the treatment of adult ADHD: a randomized, controlled trial. CNS Spectr. 2006;11(8):625–39.
Sikes CR, McMahen RL, Stark JG, et al. Pharmacokinetics of a new amphetamine extended-release oral suspension in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2018;28(1):29–35.
Sikes C, Stark JG, McMahen R, et al. Pharmacokinetics of a new amphetamine extended-release oral liquid suspension under fasted and fed conditions in healthy adults: a randomized, open-label, single-dose, 3-treatment study. Clin Ther. 2017;39(12):2389–98.
Stark JG, Engelking D, McMahen R, et al. Pharmacokinetics of a novel amphetamine extended-release orally disintegrating tablet in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27(3):216–22.
Newcorn JH, Stark JG, Adcock S, et al. A randomized phase I study to assess the effect of alcohol on the pharmacokinetics of an extended-release orally disintegrating tablet formulation of amphetamine in healthy adults. Clin Ther. 2017;39(8):1695–705.
McGough JJ, Biederman J, Wigal SB, et al. Long-term tolerability and effectiveness of once-daily mixed amphetamine salts (Adderall XR) in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44(6):530–8.
Ambrosini PG, Salle FR, Lopez FA, et al. A community assessment, open-label study of the safety, tolerability, and effectiveness of mixed amphetamine salts extended release in school-age children with ADHD. Curr Med Res Opin. 2006;22(2):427–40.
Biederman J, Spencer TJ, Wilens TE, et al. Long-term safety and effectiveness of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 2005;10(12 Suppl 20):16–25.
Findling RL, Bierderman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr. 2005;147(3):348–54.
Chavez B, Sopko MA Jr, Ehret MJ, et al. An update on central nervous system stimulant formulations in children and adolescents with attention-deficit/hyperactivity disorder. Ann Pharmacother. 2009;43(6):1084–95.
Rashid MA, Lovick S, Llanwarne NR. Medication-taking experiences in attention deficit hyperactivity disorder: a systematic review. Fam Pract. 2018;35(2):142–50.
Bright GM. Abuse of medications employed for the treatment of ADHD: results from a large-scale community survey. Medscape J Med. 2008;10(5):111.
Acknowledgements
The manuscript was reviewed by: A.C. Childress, Center for Psychiatry and Behavioral Medicine, Las Vegas, NV, USA; R.H. Howland, University of Pittsburgh School of Medicine, and Western Psychiatric Institute and Clinic, Pittsburgh, PA, USA; A.S. Poulton, Sydney Medical School Nepean, The University of Sydney, and Department of Paediatrics, Nepean Hospital, Penrith, NSW, Australia; B.J. Warren, College of Nursing, The Ohio State University, Columbus, OH, USA. During the peer review process, the manufacturer of Adzenys ER™/XR-ODT® was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Funding
The preparation of this review was not supported by any external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.A. Lyseng-Williamson is an employee of Adis/Springer, is responsible for the article content and declares no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A. Amphetamine extended-release oral suspension (Adzenys ER™) and orally disintegrating tablets (Adzenys XR-ODT®) in attention-deficit hyperactivity disorder: a profile of their use. Drugs Ther Perspect 34, 411–419 (2018). https://doi.org/10.1007/s40267-018-0539-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-018-0539-6