Abstract
Introduction
OnabotulinumtoxinA (OnabotA) appears to be an effective prophylactic treatment for chronic migraine (CM), but its use in patients with episodic migraine (EM) has been little explored. We set out to assess the clinical efficacy of OnabotA in a real-life setting, focusing particularly on EM patients.
Methods
This is a longitudinal, prospective, single-center analysis of 115 migraine patients [70 with CM, 21 with low-frequency EM (LFEM), and 24 with high-frequency EM (HFEM)] who received OnabotA in routine clinical practice in 2005–2015. In this study, the dosage regimen, the number of headaches, impact on quality of life (HIT-6 impact test) and the adverse events were among the parameters assessed.
Results
OnabotA treatment significantly reduced the number of headaches experienced by CM and EM patients, also increasing the number of responders (> 50% reduction in headache days). The quality of life of EM patients improved significantly, reducing the HIT-6 scores in both LFEM and HFEM patients. Adverse events were reported in 40% of patients, always mild and transient.
Conclusions
OnabotA is an effective and safe prophylactic treatment for migraine in routine clinical practice. It significantly improves a patients’ quality of life, particularly that of those suffering from CM and HFEM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a common neurological disorder with an estimated global prevalence of 14.7%. It represents the leading cause of disability among neurological disorders [1] and, according to the World Health Organization (WHO), it is the 19th global cause of disability and the sixth most disabling disease [2]. Migraine affects three times more women than men; mostly individuals aged 35–45 years [3], imposing significant stress on patients, families, employers and society. Indeed, migraine sufferers experience a substantial loss in functioning and productivity, which deteriorates their quality of life (QoL), and produces a substantial economic burden on healthcare systems and employers [2, 4, 5]. Accordingly, with associated costs amounting to €27 billion in the EU [4], migraine is clearly an important public-health concern that possibly exerts the strongest economic burden among neurological disorders.
Migraine treatment involves acute and preventive therapy. Acute treatment aims to reverse attacks, reducing the associated pain and disability, whereas preventive treatment should reduce the frequency, duration and severity of migraine attacks. Effective migraine management should involve both acute and preventive treatment, particularly when attacks are frequent and/or disabling, or when acute treatment fails. Several drugs are available for acute migraine treatment and there are numerous preventative therapies, including ß-blockers, amitriptyline and anti-epileptic drugs (e.g., topiramate and valproate). Other treatments are generally less efficacious or produce worse adverse effects (AEs: e.g., selective serotonin reuptake inhibitors, calcium channel antagonists, gabapentin and herbal medicines). Such treatments must be tailored to the individual based on their clinical features and any co-morbidities, also taking into consideration AEs and drug efficacy.
Episodic (EM) and chronic (CM) migraine can be considered clinically distinct entities. CM is defined as a headache on ≥ 15 days per month for ≥ 3 months, of which ≥ 8 days meet the criteria of migraine without aura and/or respond to specific treatment [6]. EM can be classified as low (LFEM) or high-frequency EM (HFEM) depending on the headache days suffered: LFEM (1–9 headache days/month), HFEM (10–14 headache days/month [7]). Some patients progress from EM to CM (2.5% per annum), changing from LFEM to HFEM and eventually to CM [8, 9], while CM often remits to EM (2-year transition rate 26%) [10]. Compared with EM, CM patients are more likely to have a worse health-related (HR) QOL, less capacity to work and greater healthcare resource use [11,12,13]. Moreover, co-morbidities are more prevalent among CM patients [11, 12, 14]. Notably, the characteristics of HFEM were recently considered to be more similar to CM than to LFEM, with the emotional and functional impact of HFEM potentially as disabling as that of CM [7].
The treatment of acute migraine attacks is essentially identical for both CM and EM patients, with several therapeutic agents currently available [15]. However, the tolerance and therapeutic efficacy of acute and preventive treatments are often less than optimal. Indeed, as most preventive treatments are ineffective for CM [16], there is a clear need to search for new effective and safe therapies. onabotulinumtoxinA (OnabotA: Allergan, Inc., Irvine, CA) was first approved by the US Food and Drug Administration (FDA) for therapeutic indications in 1989, and its use has since extended to encompass a variety of neurological, cosmetic and urological indications, including CM [17, 18]. It has now been approved in more than 85 countries, and the clinical safety and efficacy of OnabotA has been established in over 60 randomized placebo-controlled trials. OnabotA is thought to provoke headache prophylaxis by inhibiting peripheral signals to the central nervous system, blocking neuropeptide and neurotransmitter release from primary sensory neurons, thereby preventing neurogenic inflammation. Accordingly, the sensitization of central pain pathways is dampened indirectly, which manifests as a reduction in the symptoms associated with chronic pain [19]. In Spain, OnabotA was approved to treat symptoms of CM in adults who do not respond adequately or who are intolerant to prophylactic migraine drugs in 2012. OnabotA was shown to be effective, safe and well-tolerated in clinical studies on adults with CM (PREEMPT; Phase III REsearch Evaluating Migraine Prophylaxis Therapy [20,21,22,23]). Yet there is inconclusive data as to whether it is also effective in other headache disorders or in EM [24, 25]. Indeed, the American Academy of Neurology considered OnabotA unlikely to be effective in the treatment oft EM [26].
Here we present data from 115 patients with different types of migraine who were treated with OnabotA in routine clinical practice. The results from patients with EM were compared to those obtained in patients with CM to investigate whether OnabotA benefits both types of patients as prophylactic treatment for migraine in routine clinical practice.
Methods
Study design
A longitudinal, prospective, single-center study was carried out on 115 adult migraine patients (aged 18–76 years) who attended the Neurology Service at Cruces University Hospital (Bilbao, Spain) in 2005–2015. Only patients with complete data and who had received OnabotA doses ≥ 70 U were included in the cohort. Patients were categorized as CM or EM (as described in the Introduction), and the latter were further subgrouped as having LFEM or HFEM. All the patients in the cohort had previously failed to respond to, or experienced contraindications, to three or more standard preventive treatments. Patients were injected with OnabotA at doses of 70–155 U, using three different infiltration paradigms (see below). Those treated before publication of the PREEMPT study were administered lower doses and at different sites to those indicated in the PREEMPT paradigm. Once the PREEMPT study results were published, establishing the treatment paradigm to be used in clinical practice, all patients were infiltrated accordingly. The Clinical Research Ethics Committee of the “Hospital Universitario Cruces” approved the study and all the patients provided written informed consent before their inclusion in the study.
Study measurements
Patient and disease characteristics were recorded (Table 1) and the patients provided information on the number of days on which they had experienced migraine in the month prior to injection. After treatment, the patients maintained a headache diary for 3 months to evaluate the efficacy and safety of the treatment. The primary efficacy endpoint was the change in the frequency of headache days relative to the baseline in the 3 months after treatment. The secondary efficacy endpoint was the responder rate, with responders defined as patients that achieved a reduction in the number of headache days > 50% relative to the baseline following treatment.
The number of headache days and AEs were assessed from the patient diaries, and the responder rate was calculated based on a > 25 and > 50% reduction in the frequency of headache days relative to the baseline following OnabotA treatment.
Changes in QoL were measured using the 6-item Headache Impact Test (HIT-6), recording the scores prior to treatment, on the day of treatment and during the 3-month follow-up. The HIT-6 questionnaire yields scores from 36 to 78, in which scores < 50, 50–55, 56–59 and > 60, respectively, indicate no, mild, substantial and strong impact.
Statistical analysis
Standard summary statistics were employed, and the descriptive data are presented as the mean ± standard deviation (SD). The outcomes of the treatment were assessed with a paired t test. As such, the average post-treatment measurements in the 3-month follow-up period were compared with values before treatment. The mean absolute change is also shown. The SPSS statistical analysis package (Ver. 21: IBM Corp., Armonk, NY, USA) was used for all statistical analyses and a p value < 0.001 was defined as significant.
Results
Demographic and baseline headache characteristics
Data from a clinical cohort of 115 adult patients with migraine (70 with CM, 21 with LFEM and 24 with HFEM) treated with OnabotA between 2005 and 2015 were analyzed; their clinical characteristics at baseline are shown in Table 1. The mean age of the cohort was 47.09 (± 12.25: range 18–76), with 13.9% males and 86.1% females. The mean age at onset was 17.48 (± 9.61), and the mean age at chronification and/or the occurrence of high-impact migraine was 35.21 (± 15.14). The mean time from the occurrence of high-impact migraine to OnabotA treatment was 11.56 (± 11.34) years, the mean number of headache days per month was 20.09 (± 8.25) and the mean HIT-6 score at baseline before OnabotA treatment was 68.98 (± 5.77).
Treatment regimens and efficacy
Patients received a mean dose of 127.83 (± 32.17) U of OnabotA (range 70–155 U). The OnabotA injections were administered following one of three infiltration paradigms: bilaterally into the frontal, glabellar and temporal muscle (24 patients); into these regions and the posterior cervical region (22 patients); or according to the PREEMPT paradigm (69 patients) [27]. A significant mean reduction of 9.88 days/month in the number of headache days per month (primary endpoint) was observed in all groups after the 3-month follow-up relative to the baseline (Fig. 1 and Table 2). In terms of the different patient groups, OnabotA treatment produced a mean reduction of 12.9 headache days per month in CM patients and of 5.15 days per month in EM patients. In the latter, the mean reduction was 7.3 and 2.7 days per month for patients in the HFEM and LFEM subgroups, respectively.
Responder rates
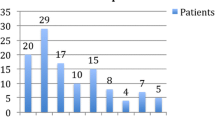
With regard to the secondary efficacy endpoint, 49.6% of individuals were responders (i.e., > 50% reduction in headache days), while a further 26.1% reported a reduction of 25–50%, and 24.3% a reduction < 25% (Fig. 2). When analyzed by patient group, a responder rate of 57.1% was evident in patients with CM and a responder rate of 54.2% in HFEM patients, while 19% of the LFEM patients responded to the treatment. Interestingly, among the patients reporting a > 25% reduction in headache frequency, there was a greater improvement in HFEM patients (95.9%) than in CM patients (74.2%), while a milder improvement was evident in the LFEM patients (57.1%: Fig. 2).
Impact on quality of life
Total HIT-6 scores significantly improved in CM and EM patients after treatment and even in LFEM patients, with a decrease of at least 10 points in mean scores in each patient group (Fig. 3 and Table 2). Furthermore, significant differences were observed when the scores for each of the 6 items (pain, role functioning, social functioning, energy/fatigue, cognitive functioning and emotional distress) in the HIT-6 questionnaire were compared before and after treatment (data not shown, p < 0.001).
Safety
AEs were consistent with the established safety profile of OnabotA injection. Although 40.9% of patients reported at least one AE, all were mild and transient. Of the patients that reported AEs, 34.3% had CM, 62.5% had HFEM and 38.1% had LFEM. The most typical AEs were neck weakness/pain (23.5%), palpebral ptosis (10.4%) and frontal weakness (8.7%: Table 3).
Discussion
This prospective study focuses on the response to OnabotA treatment in a real-world clinical population of migraine patients in Spain. The data confirm the efficacy and tolerability of this treatment, as well as the improvement in QoL, not only in patients with CM but also in patients suffering from EM, with particular benefits for HFEM patients.
The patient cohort was representative of migraine patients with demographic characteristics similar to those reported elsewhere for CM patients [22, 28,29,30,31]. The baseline clinical characteristic are also consistent with the PREEMPT trial and other studies, although our CM patients appear to be more severely affected than those in the PREEMPT trial given their failure to respond to prior treatments and the number of headache days prior to treatment. Conversely, medication overuse prior to treatment was lower in our cohort than in the PREEMPT study.
In our real-world population of CM and EM patients, OnabotA effectively reduced the number of headache days per month and improved patient QoL, as reported elsewhere. While there is a stronger reduction in patients with CM, it is still considerable in HFEM patients, indicating that OnabotA offers similar benefits to both migraine groups. Indeed, the responder rate was similar for CM and HFEM patients, although somewhat lower in LFEM patients. Indeed, when a reduction of > 25% in the number of headache days was considered, a particularly strong improvement was evident in HFEM patients, above that in CM patients and supporting the beneficial use of OnabotA in HFEM.
OnabotA treatment improves the QoL of all migraine patients, reflected by the significant reduction in the mean HIT-6 score, a test validated for EM and CM patients [32]. Moreover, improved QoL was observed in all 6 domains assessed by the HIT-6 questionnaire, indicating the treatment produces clinically meaningful benefits in migraine patients. Although the mean change from baseline was not compared between groups, the improvement in QoL appeared to be similar in CM and HFEM patients, yet smaller in LFEM patients. This is further evidence that OnabotA treatment provides similar benefits to CM and HFEM patients.
OnabotA was well-tolerated in our mixed cohort of migraine patients, producing only mild and transient AEs. The two most common AEs (neck weakness/pain and palpebral ptosis) were consistent with previous studies of OnabotA in the treatment of migraine. Indeed, the AEs identified here were also reported elsewhere and they were consistent with the safety profile established for OnabotA injection, particularly to head and neck muscles. Hence, the safety profile of OnabotA makes it the best option when other prophylactic treatments fail or are not well-tolerated.
Other pharmacological agents may effectively prevent EM [33], although their efficacy is not optimal, they have safety issues and their use needs to be validated in large, well-designed, placebo-controlled trials. OnabotA is not approved for EM, but is currently the only preventive treatment specifically indicated for CM. Topiramate is approved in many countries to prevent migraine in adults and it has been shown to be effective against CM, supporting its use in clinical practice [34, 35]. Recently, monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) ligand (galcanezumab, eptinezumab and fremanezumab) or the CGRP receptor (erenumab) completed phase 3 trials, demonstrating their efficacy as preventative treatment for migraine. These treatments are now pending commercialization. In addition, there is preliminary data suggesting that neurostimulation may be an effective treatment for migraine [36]. While the mechanism of action of OnabotA is not yet well understood, it can modulate the inflammatory mediators in the trigeminal ganglion [37] and decrease interictal CGRP plasma levels in patients with CM [38], thereby preventing migraine attacks.
A detailed comparison of our data and previous clinical trials is complicated by the different patient groups and study designs involved. The current cohort is a mix of both CM and EM patients, whereas previous studies focused on either EM or CM patients alone (PREEMPT trial), or alternatively, specific subgroups of migraine patients were not identified. Moreover, in clinical trials, the cohorts differ in terms of the number of prior treatments, analgesic overuse and follow-up time, as well as the OnabotA dose and the infiltration protocol used. Nevertheless, the results obtained here in a clinical setting are comparable to the data from Phase III clinical trials and particularly, the PREEMPT trial. Furthermore, cohorts of patients seen in clinical practice include those that may be excluded from clinical trials, better reflecting the general population of migraine patients. Therefore, similar outcomes can be expected through OnabotA treatment of CM and EM patients that failed previous preventive treatments at other clinical centers. Indeed, based on the Spanish Society of Neurology’s latest guidelines in clinical practice for headache [39], OnabotA should be offered to CM patients that fail to achieve relief with two or more preventive treatments and it may also be considered for HFEM patients requiring migraine prophylaxis.
The long-term outcome of patients treated with OnabotA is currently unclear. There are data from CM patients that received treatment for 1–3 years [40,41,42] and a 108-week CM OnabotA Prolonged Efficacy open Label (COMPEL) study is ongoing, which will enhance our understanding of the long-term effects of prophylactic OnabotA treatment in adult CM patients [43]. In addition, we continue to follow-up the patients included in this study, which in the future will shed further light on the long-term benefits on the use of OnabotA therapy to treat migraine in a clinical setting. Our study did not include a control group or other treatment options, although all patients had failed at least three previous standard treatment options, and they had a relatively high-frequency of headache days and high HIT-6 scores. Nevertheless, OnabotA treatment had a favorable outcome and improved these clinical characteristics, providing benefits similar to those observed in Phase II clinical trials.
It should be noted that in the study carried out here, the patients studied were not prevented from continuing their ongoing medication for migraine and they were not subjected to any washout period. While this feature of the study may open the way to interactions between the OnabotA therapy and the patients’ ongoing medicine, we feel this is unlikely to have influenced the results of the study. In the first place, the patients were unresponsive to the actual medicine and thus, the effects observed are likely to be due to the administration of OnabotA (alone or in conjunction with the habitual medicine). In addition, the response to OnabotA was similar to that seen in more controlled clinical trial situations in which the subjects were submitted to a washout period. Moreover, the subjects were using distinct medications in an attempt to control their migraines and thus, it is unlikely that OnabotA would be interacting with each of these in the same way. While we cannot formally rule out an influence of any interactions between the medications that the patients had been using until they began to use the OnabotA therapy, we continue to study these patients and their continued use of this therapy. As such, we will collect data from these patients over a longer follow-up period in which they will have been using only OnabotA to control their migraines and these data will indicate whether any interactions with other medications are necessary to achieve its beneficial effects.
Further studies will be necessary to determine how to identify patients that may best respond to OnabotA and in particular, those who will eventually be able to cease this treatment. In addition, the optimal dose of OnabotA and the optimal duration of treatment must be defined based on patient characteristics, as well as the position of OnabotA in the therapeutic sequence to treat migraine and the possible efficacy of concomitant oral prophylactic treatments. Such data will guide and support future decision-making in the treatment of migraine.
Conclusions
OnabotA appears to be a useful, effective and safe alternative for prophylactic migraine treatment in routine clinical practice, where it matches the effects observed in clinical studies. The evidence supports the use of OnabotA in CM and EM patients in a routine clinical setting, significantly improving patients’ quality of life and particularly, that of those suffering from CM and HFEM.
References
Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. J Headache Pain. 2013;14:1. https://doi.org/10.1186/1129-2377-14-1.
World Health Organization. headache disorders fact sheet no. 277, 2016. http://www.who.int/mediacentre/factsheets/fs277/en/.
World Health Organization. Lifting The burden, atlas of headache disorders and resources in the world 2011. http://www.who.int/mental_health/management/atlas_headache_disorders/en/.
Stovner LJ, Colette A. On behalf of the Eurolight Steering Committee. Impact of headache in Europe: a review for the Eurolight project. J Headache Pain. 2008;9:139–46.
Berg J. Economic evidence in migraine and other headaches: a review. Eur J Health Econ. 2004;5:s43–54.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia. 2013;33:629–808.
Torres-Ferrús M, Quintana M, Fernandez-Morales J, et al. When does chronic migraine strike? A clinical comparison of migraine according to the headache days suffered per month. Cephalalgia. 2017;37:104–13.
Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157–68.
Bigal ME. The paradoxical effects of analgesics and the development of chronic migraine. Arq Neuropsiquiatr. 2011;69:544–51.
Manack A, Buse DC, Serrano D, et al. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. 2011;76:711–8.
Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16:86–92.
Blumenfeld A, Varon S, Wilcox T, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31:301–15.
Bigal ME, Serrano D, Reed M, et al. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–66.
Buse DC, Manack A, Serrano D, et al. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428–32.
Goadsby PJ, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol. 2010;9:285–98.
Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:103–22.
BOTOX® (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use. Allergan, Irvine. 2013.
Gooriah R, Ahmed F. Therapeutic uses of botulinum toxin. J Clin Toxicol. 2015;5:225. https://doi.org/10.4172/2161-0495.1000225.
Aoki KR, Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Relat Disord. 2011;17:S28–33.
Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–73.
Aurora S, Dodick D, Turkel C, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803.
Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache J Head Face Pain. 2010;50:921–36.
Diener H, Dodick D, Aurora S, Turkel C, DeGryse R, Lipton R, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–14.
Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: a meta-analysis. JAMA. 2012;307:1736–45.
Robertson C, Garza I. Critical analysis of the use of onabotulinumtoxinA (botulinum toxin type A) in migraine. Neuropsychiatr Dis Treat. 2012;8:35–48.
Naumann M, So Y, Argoff CE, et al. Assessment: botulinum neurotoxin in the treatment of autonomic disorders and pain (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2008;70:1707–14.
Blumenfeld A, Silberstein SD, Dodick DW, et al. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50:1406–18.
Pedraza MI, de la Cruz C, Ruiz M, et al. OnabotulinumtoxinA treatment for chronic migraine: experience in 52 patients treated with the PREEMPT paradigm. SpringerPlus 2015 [cited 2016 May 11];4. https://doi.org/10.1186/s40064-015-0957-z. Available at: http://www.springerplus.com/content/4/1/176.
Khalil M, Zafar HW, Quarshie V, et al. Prospective analysis of the use of onabotulinumtoxinA (BOTOX) in the treatment of chronic migraine; real-life data in 254 patients from Hull, UK. J Headache Pain. 2014;15:54. https://doi.org/10.1186/1129-2377-15-54.
Ahmed F, Zafar HW, Buture A, et al. Does analgesic overuse matter? Response to onabotulinumtoxinA in patients with chronic migraine with or without medication overuse. SpringerPlus. 2015;4:589. https://doi.org/10.1186/s40064-015-1386-8.
Negro A, Curto M, Lionetto L, et al. A two years open-label prospective study of onabotulinumtoxinA 195 U in medication overuse headache: a real-world experience. J Headache Pain. 2015;17:1. https://doi.org/10.1186/s10194-016-0591-3.
Yang M, Rendas-Baum R, Varon SF, et al. Validation of the headache impact test (HIT-6) across episodic and chronic migraine. Cephalalgia. 2011;31:357–67.
Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: table 1: report of the quality standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–45.
Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache J Head Face Pain. 2009;49:1153–62.
Diener H-C, Bussone G, Van Oene J, et al. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814–23.
Mitsikostas DD, Rapoport AM. New players in the preventive treatment of migraine. BMC Med. 2015;13:279. https://doi.org/10.1186/s12916-015-0522-1.
Edvinsson J, Warfvinge K, Edvinsson L. Modulation of inflammatory mediators in the trigeminal ganglion by botulinum neurotoxin type a: an organ culture study. J Headache Pain. 2015;16:555. https://doi.org/10.1186/s10194-015-0555-z.
Cernuda-Morollón E, Ramón C, Martínez-Camblor P, et al. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. 2015;156:820–4.
Ezpeleta D, Rosich P, editors. Guía oficial de práctica clínica en cefaleas 2015. Guías Oficiales de la Sociedad Española de Neurología. 5th ed. Madrid: Luzán; 2015.
Lipton RB, Rosen NL, Ailani J, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine over one year of treatment: pooled results from the PREEMPT randomized clinical trial program. Cephalalgia. 2016;36:899–908.
Rothrock JK, Scanlon C, Weibelt S. OnabotulinumtoxinA for the treatment of chronic migraine: long-term outcome [abstract]. In: American Headache Association Meeting; 2011.
Cernuda-Morollon E, Ramon C, Larrosa D, et al. Long-term experience with onabotulinumtoxinA in the treatment of chronic migraine: what happens after one year? Cephalalgia. 2015;35:864–8.
Blumenfeld AM, Aurora SK, Laranjo K, et al. Unmet clinical needs in chronic migraine: rationale for study and design of COMPEL, an open-label, multicenter study of the long-term efficacy, safety, and tolerability of onabotulinumtoxinA for headache prophylaxis in adults with chronic migraine. BMC Neurol. 2015;15:100. https://doi.org/10.1186/s12883-015-0353-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Writing and editorial assistance was provided by Dr Mark Sefton (BiomedRed SL) and funded by Allergan S.A. All authors met the ICMJE authorship criteria and received neither honoraria nor payments for authorship. None of the authors have any conflict of interest to declare.
Ethical approval
All procedures involving human participants were performed in accordance with institutional and/or national research committee ethical standards and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the participants in the study.
Rights and permissions
About this article
Cite this article
Velasco-Juanes, F., Gómez-Esteban, J.C., Fernández-Valle, T. et al. Clinical treatment of chronic and episodic migraine with onabotulinumtoxinA in a real-world setting. Drugs Ther Perspect 34, 335–343 (2018). https://doi.org/10.1007/s40267-018-0511-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-018-0511-5