Abstract
Extrafine beclometasone dipropionate/formoterol 100/6 and 200/6 μg fixed-dose combination (FDC) is available in the EU as a pressurized metered-dose inhaler (Fostair®, Foster®, Formodual®, Innovair®, Inuvair®, Inuxair®, Combair®) and a dry-powder inhaler (Fostair NEXThaler®, Foster NEXThaler®, Formodual NEXThaler®, Innovair NEXThaler®, Inuvair NEXThaler®). It is an effective, convenient and well-tolerated option for maintenance, or maintenance and reliever therapy in patients with asthma, as well as for the symptomatic treatment of patients with severe chronic obstructive pulmonary disease (COPD). Extrafine beclometasone/formoterol is generally well tolerated, improves lung function and asthma control and reduces COPD exacerbations in these patients in clinical studies. It is generally more effective than beclometasone and formoterol monotherapies in clinical studies and generally provided better asthma control, reduced the use of rescue medication and improved health-related quality of life compared with large-particle FDCs in real-world studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adis evaluation of extrafine beclometasone/formoterol inhalers in asthma and COPD
Combines the inhaled corticosteroid beclometasone with the long-acting β 2-adrenoreceptor agonist formoterol in a single pressurized metered-dose or dry-powder inhaler |
Convenient to use relative to the use of two separate inhalers |
Extrafine drug particle size allows the dose of beclometasone to be lower than that in a large-particle formulation and allows drug deposition in both large and small airways |
Less systemic exposure to beclometasone and greater lung deposition of beclometasone and formoterol than with large-particle formulations |
Effective and well tolerated in the treatment of severe COPD and as maintenance, or maintenance and reliever therapy (MART) in asthma |
What is the rationale for developing extrafine beclomethasone/formoterol?
Asthma and chronic obstructive pulmonary disease (COPD) are obstructive pulmonary diseases affecting millions of people worldwide. Bronchodilators and inhaled corticosteroids (ICS) administered alone or in combination are the mainstay of the treatment of asthma and COPD, with combination therapy allowing for better disease control [1]. According to current treatment guidelines, the main goals in the management of asthma and COPD are the reduction of symptoms and future risk of exacerbations [2–4].
In patients with asthma, combination therapy with an ICS [e.g. beclometasone dipropionate (beclometasone hereafter), fluticasone, budesonide] + a long-acting β 2-adrenoreceptor agonist [LABA; e.g. formoterol fumarate (formoterol hereafter), salmeterol], with short-acting β 2-adrenoreceptor agonist as reliever therapy is recommended for patients who do not achieve adequate symptom control and/or are at risk of exacerbations with ICS therapy [2, 4]. Combination treatment with low-dose ICS and formoterol can be used as both maintenance and reliever therapy in adults with asthma who have exacerbations despite other therapies [2, 4]. ICS/LABA combination therapy is also an option for patients with COPD who have persistent exacerbations with long-acting bronchodilator therapy [LABA, or long-acting antimuscarinic agent (e.g. aclidinium bromide, tiotropium)] [3]. Compared with administration via separate inhalers, the use of ICS and LABA fixed-dose combination (FDC) inhalers is more convenient and cost effective, and may potentially improve adherence to therapy [4, 5].
As both asthma and COPD are associated with inflammation in the airways, including in the small airways, optimized drug deposition because of extrafine particle size is expected to result in improved clinical benefits, providing a rationale for developing extrafine drug formulations [6]. Extrafine beclometasone/formoterol (100/6 and 200/6 μg) is a FDC inhaler containing the ICS beclometasone and the LABA formoterol delivered via a pressurized metered-dose inhaler (pMDI) [7, 8] (Fostair®, Foster®, Formodual®, Innovair®, Inuvair®, Inuxair®, Combair®) or a dry-powder inhaler (DPI) [9, 10] (Fostair NEXThaler®, Foster NEXThaler®, Formodual NEXThaler®, Innovair NEXThaler®, Inuvair NEXThaler®). These beclometasone/formoterol inhalers have been formulated with an extrafine particle size [i.e. with a mass median aerodynamic diameter (MMAD) of <2 µm; see Table 1] and a beclometasone dose 2.5-fold lower than in a large-particle formulation [11–13]. This results in lower systemic exposure to beclometasone than with a large-particle formulation, while maintaining the amount of beclometasone and formoterol reaching the lungs [11–13]. A large amount of an inhaled dose of extrafine beclometasone/formoterol is deposited in the lungs with the pMDI (31–34%) [14] and DPI (42%) [9, 10], both in large and small airways, with low variability between healthy subjects and patients with asthma or COPD [14]; lung deposition with extrafine-particle formulations is greater than with large-particle formulations (10–20% with conventional pMDIs and 15–25% with DPIs [6]). Only a small amount of beclometasone/formoterol is exhaled [2.8–3.4% of a nominal dose with pMDI [14] and 1.2–2.5% with DPI [15]].
How do the individual drugs in the combination work?
ICS and LABAs act on different and complementary aspects of the pathophysiology of asthma and COPD, providing a rationale for combination therapy [16]. The inhaled glucocorticoid beclometasone has anti-inflammatory effects in the lungs, resulting in reduced symptoms and exacerbations of asthma and less adverse events than corticosteroids administered systemically [7]. The pharmacological effects of beclometasone are attributed largely to its active metabolite, beclometasone-17-monopropionate, which binds to the glucocorticoid receptor with high affinity [13]. The LABA formoterol acts as a bronchodilator by relaxing bronchial smooth muscles in patients with reversible airways obstruction [7]. The bronchodilatory effects of formoterol are observed rapidly within 1–3 min of inhalation of a single dose and lasts for a duration of 12 h [7]. When administered together, beclometasone and formoterol have additive effects in terms of reduction in asthma and COPD symptoms [7].
Extrafine beclometasone/formoterol 100/6 μg had rapid bronchodilatory [17–20] and dose-dependent anti-inflammatory effects [17] in clinical studies in patients with asthma [17, 18, 20] and COPD [19]. The speed of reverting methacholine-induced bronchoconstriction with extrafine beclometasone/formoterol 100/6 μg was non-inferior to that with salbutamol 200 μg pMDI (3.66 vs 2.15 min), indicating that it may be a good alternative for rescue medication [18]. In patients with COPD, extrafine beclometasone/formoterol improved lung function parameters and hyperinflation [21], reduced air trapping and dyspnoea [22] and reduced moderate/severe exacerbations [23, 24].
For whom are extrafine beclometasone/formoterol inhalers indicated?
In the EU, extrafine beclometasone/formoterol is indicated as maintenance [7–10], or maintenance and reliever therapy (MART) [7] in patients with asthma, and for the symptomatic treatment of patients with severe COPD and a history of repeated exacerbations [7, 9]. The EU prescribing information for extrafine beclometasone/formoterol is summarized in Table 1. Consult local prescribing information for further details.
What is the clinical efficacy of extrafine beclometasone/formoterol inhalers in asthma?
In patients with uncontrolled asthma
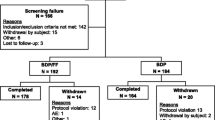
Several randomized, double-blind studies assessed the efficacy of extrafine beclometasone/formoterol as maintenance [20, 27–29], or maintenance and reliever [30] therapy for adults with uncontrolled asthma. The studies enrolled patients with moderate-to-severe asthma inadequately controlled by moderate to high doses of ICS, a forced expiratory volume in 1 s (FEV1) of 40–80% [27, 29], 50–80% [20, 28] or ≥60% [30] of predicted normal values and a positive reversibility test after salbutamol [20, 27, 29, 30]; in the study assessing extrafine beclometasone/formoterol as maintenance and reliever, patients were also required to have had at least one severe exacerbation in the 12 months before study entry (but not in the past month) [30].
Patients were randomized to receive maintenance treatment with:
-
two inhalations twice daily of extrafine beclometasone/formoterol 100/6 μg pMDI, two inhalations twice daily of beclometasone 250 μg pMDI + one capsule twice-daily of formoterol DPI 12 μg, or two inhalations twice daily of beclometasone 250 μg pMDI (Table 2) [27];
-
two inhalations twice daily of high-strength, extrafine beclometasone/formoterol 200/6 μg pMDI or four inhalations twice daily of beclometasone 100 μg (Table 2) [29];
-
two inhalations twice daily of extrafine beclometasone/formoterol 100/6 μg pMDI or two inhalations twice daily of fluticasone/salmeterol 125/25 μg (Table 2) [20];
-
two inhalations twice daily of extrafine beclometasone/formoterol 100/6 μg pMDI or two inhalations twice daily of budesonide/formoterol 200/6 μg DPI (Table 2) [28].
In the study assessing extrafine beclometasone/formoterol as maintenance and reliever, 2079 patients entered a 2-week run-in period during which they received one inhalation twice daily of extrafine beclometasone/formoterol 100/6 μg pMDI, and salbutamol 100 μg pMDI as needed for the relief of symptoms [30]. Following the run-in period, patients with uncontrolled asthma continued regular treatment with twice-daily extrafine beclometasone/formoterol 100/6 pMDI μg and were randomly assigned to receive extrafine beclometasone/formoterol 100/6 μg pMDI (intent-to-treat n = 852) or salbutamol 100 μg pMDI (n = 849) as reliever therapy for 48 weeks [30].
Maintenance treatment
Maintenance treatment with extrafine beclometasone/formoterol (100/6 or 200/6 μg) pMDI improved lung function and reduced asthma symptoms and exacerbations in patients with moderate-to-severe disease [20, 27–29].
After 24 weeks treatment, extrafine beclometasone/formoterol 100/6 μg was noninferior to beclometasone and formoterol administered via separate inhalers (referred to as free combination hereafter) and superior to beclometasone alone in improving lung function, as assessed by morning pre-dose peak expiratory flow (mPEF; primary endpoint) and FEV1 (Table 2) [27]. Patients receiving extrafine beclometasone/formoterol relative to those receiving beclometasone + formoterol free combination or beclometasone monotherapy also had significantly (p < 0.05) greater proportions of days (36 vs 29 and 24%), nights (37 vs 30 and 27%) and complete days free from asthma symptoms (26 vs 20 and 15%) and days with asthma control (i.e. no nocturnal awakenings, no asthma symptoms or no rescue medication use; 23 vs 15.4 and 12.3%; post hoc analysis; some values estimated from graphs) [27]. In addition, extrafine beclometasone/formoterol significantly (p < 0.05) reduced the overall number of exacerbations (1.3 vs 2.0), mild-to-moderate exacerbations (0.9 vs 1.2) and severe exacerbations (0.5 vs 0.8) per patient, relative to beclometasone alone; there were also significant differences between the FDC and free-combination for these outcomes (1.3 vs 1.6, 0.9 vs 0.9, and 0.5 vs 0.7, respectively) [27].
High-strength extrafine beclometasone/formoterol 200/6 μg was also superior to beclometasone alone in terms of improving lung function [assessed by mPEF (primary endpoint) and FEV1] (Table 2) [29]. However, the two treatment groups did not differ significantly in symptom parameters, including the mean average use of rescue medication (1.7 vs 1.9 puffs/day; baseline 2.7 and 2.9 puffs/day) and the improvement from baseline in the asthma control questionnaire (ACQ) scores (both groups had more than the minimal clinical reduction from baseline of 0.5 points; all p < 0.001 vs baseline) [29]. The proportion of patients with exacerbations and the corresponding number of exacerbations with extrafine beclometasone/formoterol were 2.2% and 4 and with beclometasone alone were 3.4% and 7 [29].
When compared with other FDC inhalers, extrafine beclometasone/formoterol 100/6 μg was noninferior to fluticasone/salmeterol [20] and budesonide/formoterol [28] in terms of its effects on lung function, with all three combinations significantly improving mPEF (primary endpoint) and FEV1 from baseline following 12 weeks treatment (Table 2). Asthma symptoms and exacerbations also improved to a generally similar extent with extrafine beclometasone/formoterol 100/6 μg and the other FDCs [20, 28]. For example, in the extrafine beclometasone/formoterol and budesonide/formoterol treatment groups, the daytime scores (mean change from baseline −0.93 vs −0.86) and night-time scores (−0.73 vs −0.66) decreased significantly from baseline to the last 2 weeks of treatment (all p < 0.001 vs baseline); the daily use of rescue medication decreased significantly from 2.16 puffs/day at baseline to 0.76 puffs/day with extrafine beclometasone/formoterol and from 2.28 to 0.87 puffs/day with budesonide/formoterol (p values not available) [28]. Asthma exacerbations occurred in 15.9% of extrafine beclometasone/formoterol and 11.0% of budesonide/formoterol recipients, with no significant between-group difference (BGD) in the time to first exacerbation (median 29 vs 24 days) [28]. Compared with fluticasone/salmeterol, extrafine beclometasone/formoterol had a significantly faster onset of bronchodilation, as indicated by significantly greater mean changes from baseline in FEV1 with extrafine beclometasone/formoterol at all timepoints from 5 to 60 min post-dose (all p < 0.05) [20].
Maintenance and reliever treatment
Extrafine beclometasone/formoterol 100/6 μg as maintenance and reliever treatment was more effective than extrafine beclometasone/formoterol as maintenance treatment + as-needed salbutamol for reducing exacerbations during 48 weeks’ therapy in adults with uncontrolled asthma [30]. The primary endpoint was the time to first severe exacerbation, which was defined as deterioration in asthma resulting in admission to hospital or a visit to the emergency department or requiring systemic steroids for ≥3 days [30].
Extrafine beclometasone/formoterol as MART significantly prolonged the time to first severe exacerbation by 75 days compared with extrafine beclometasone/formoterol as maintenance + salbutamol as reliever (209 vs 134 days), corresponding to a 36% reduction in the risk of severe exacerbation [hazard ratio (HR) 0.64; 95% CI 0.49–0.82; p = 0.0005] [30]. During 48 weeks’ treatment, 326 severe exacerbations were reported in 251 patients (99 and 152 patients in the respective treatment groups). Significant reductions were seen in the group receiving extrafine beclometasone/formoterol as reliever relative to those receiving salbutamol as reliever both in the rate of exacerbations requiring a visit to the emergency department or admission to hospital because of asthma (HR 0.67; 95% CI 0.54–0.84; p = 0.0003) and in the rate of exacerbations requiring the use of systemic corticosteroids for ≥3 days (HR 0.65; 95% CI 0.54–0.80; p < 0.0001). The Kaplan–Meier estimated probability of severe exacerbations (12 vs 18%; p = 0.0003), the yearly rate of severe exacerbations (14.8 vs 22.4 events/100 patients/year; HR 0.66; 95% CI 0.55–0.80; p < 0.0001) and the yearly rate of mild exacerbations (56.0 vs 65.1 days/patient/year; HR 0.86; 95% CI 0.76–0.98; p = 0.021) were also significantly reduced in the as-needed extrafine beclometasone/formoterol group relative to the as-needed salbutamol group; no significant BGD was seen for the time to first mild exacerbation [30].
There were no significant BGDs in other asthma control measures, with both treatments significantly improving asthma symptoms (mean change −1.59 in the as-needed extrafine beclometasone/formoterol group vs −1.44 in the as-needed salbutamol group; baseline 6.6 and 6.4), the proportion of asthma control days (9.5 vs 10.9%; baseline 8.3 vs 8.9%) and the use of reliever therapy (−0.29 vs −0.27 inhalations/24 h; baseline 0.98 and 0.97 inhalations/24 h; both p < 0.0001 vs baseline) from the run-in period to week 48 (p values reported where available). Lung function parameters also improved in the two groups, with no significant BGDs in parameters, including the mean change from baseline in FEV1 (0.90 with beclometasone/formoterol as reliever vs 0.90 L with salbutamol as reliever; baseline 2.21 and 2.27 L) and mPEF (−9.07 and −12.75 L/min; baseline 365.1 and 364.4 L/min)] [30].
In patients with controlled asthma
Switching from fluticasone/salmeterol
In a randomized, double-blind, phase 4 study (FACTO), extrafine beclometasone/formoterol was as effective as fluticasone/salmeterol in maintaining lung function and asthma control in patients with previously controlled asthma, indicating that patients can effectively switch from fluticasone/salmeterol to extrafine beclometasone/formoterol therapy [31]. Adults with asthma controlled with fluticasone propionate 500 μg + salmeterol 100 μg daily (via a DPI or pMDI, or via separate inhalers) were randomized to receive two inhalations twice daily of extrafine beclometasone/formoterol 100/6 μg pMDI or one inhalation twice daily of fluticasone/salmeterol DPI 500/100 μg for 12 weeks [31].
At week 12, the pre-dose morning FEV1 (primary endpoint) in patients receiving extrafine beclometasone/formoterol was equivalent to that in patients receiving fluticasone/salmeterol; mPEF was also similar between the two groups (Table 2). However, the mean changes from baseline in the FEV1 at 5, 15 and 30 min post dose, and the change in FEV1 area under the curve (AUC) from time 0 to 1 h post dose were significantly (p < 0.05) greater with extrafine beclometasone/formoterol than with fluticasone/salmeterol at the start (baseline) and at the end (week 12) of treatment. There were no significant BGDs with regard to asthma control measures, including the mean change from baseline in the ACQ-7 score (0.03 with extrafine beclometasone/formoterol vs 0.02 with fluticasone/salmeterol; baseline ≈0.33), mean daytime symptom score (0.16 vs 0.13), mean night-time symptom score (0.11 vs 0.08) and the proportion of complete days without asthma (88.5 vs 88.8%) in the last 4-week treatment period [31].
Stepping down from high-dose fluticasone/salmeterol
Extrafine beclometasone/formoterol 100/6 μg pMDI was as effective as fluticasone/salmeterol 250/50 μg DPI in maintaining lung function and asthma control in patients stepping down from the highest recommended dose of an ICS/LABA combination in a 24-week, randomized, multinational, open-label phase 3 study (FORTE) [32]. Adults with asthma controlled with 1000 μg fluticasone propionate + 100 μg salmeterol were randomized to receive two inhalations twice daily of extrafine beclometasone/formoterol 100/6 μg pMDI or one inhalation twice daily of fluticasone/salmeterol 250/50 μg DPI. At study end, the mean change from baseline in the morning PEF (primary endpoint) in patients receiving beclometasone/formoterol was equivalent to that in patients receiving fluticasone/salmeterol (Table 2). There were also no significant BGDs in other outcomes, including FEV1 (Table 2), mean daytime symptom scores (1.37 vs 1.32), night-time symptom scores (1.33 vs 1.46) and the proportion of patients with controlled asthma (90.0 vs 85.2%; baseline ≈98%) in the last 4-week treatment period [32].
Real-world experience
Real-world data supported the efficacy of extrafine beclometasone/formoterol for the treatment of asthma [33–40]. For example, in the 12-month prospective phase of the large observational PRISMA study (n = 1017), significantly more patients receiving extrafine beclometasone/formoterol pMDI (n = 301) than receiving budesonide/formoterol DPI (n = 145) had fully controlled asthma (26 vs 8%; p < 0.001) and an improvement of 3 points in the asthma control test score (89 vs 80%; p = 0.022) [33]. Relative to patients receiving budesonide/formoterol DPI, those receiving extrafine beclometasone/formoterol pMDI also had a higher probability of having full asthma control (OR 3.8; 95% adjusted CI 1.73–8.4) and significantly (p < 0.001) higher health-related quality of life (HR-QOL), as assessed by the European Quality of Life-5 Dimension Questionnaire (EQ-5D) and EQ-5D visual analogue scale scores (VAS) [33]. There were no significant differences between extrafine beclometasone/formoterol pMDI and fluticasone/salmeterol DPI or pMDI (n = 123) recipients for these outcomes, apart from significantly (p < 0.001) higher EQ-5D VAS scores in the extrafine beclometasone/formoterol pMDI group [33]. Generally similar results were also observed in the cross-sectional phase of the PRISMA study (n = 2853) [34].
In another real-world, cross-sectional study, extrafine beclometasone/formoterol pMDI (n = 53) demonstrated better asthma control than large-particle fluticasone/salmeterol (n = 25) or budesonide/formoterol (n = 33) DPIs [39]. Significantly more patients receiving beclometasone/formoterol than receiving fluticasone/salmeterol or budesonide/formoterol had asthma control (57 vs 36%; p = 0.031). The mean daytime symptom score (1.40 vs 2.33 with the two DPIs), rescue medication use score (1.81 vs 2.60) and asthma control total score (5.83 vs 8.47) were also significantly lower (indicating improvement) with beclometasone/formoterol (all p < 0.05), as was the daily usage of ICS (321 μg with beclometasone/formoterol vs 720 and 715 μg with fluticasone/salmeterol and budesonide/formoterol; p < 0.0001 vs the two DPIs) [39].
The retrospective, observational REACH study (n = 1528) showed that patients with asthma may be switched from the fluticasone/salmeterol FDC to the extrafine beclometasone/formoterol FDC at an equivalent or lower ICS dosage, with no loss of clinical effectiveness [40]. At the time of review when the physician either continued fluticasone/salmeterol treatment or switched the patient to beclometasone/formoterol, the majority of patients (71.2%) were receiving a fluticasone dosage of 500 μg/day, 11.5% were receiving 200–250 μg/day, and 17.3% were receiving 1000 μg/day. These proportions did not change in patients continuing treatment with fluticasone/salmeterol. In patients switching to extrafine beclometasone/formoterol, 306 (80.1%) patients were receiving a beclometasone dosage of 400 μg/day and 76 (19.9%) patients a dosage of 200 μg/day, which were equivalent to the most recent fluticasone/salmeterol dosage in 296 (77.5%) patients and represented a 50% reduction in the ICS dosage in the remaining 86 (22.5%) patients. During the outcome year (i.e. 12 months after the review date), beclometasone/formoterol was noninferior to fluticasone/salmeterol in terms of the incidence of severe exacerbations (adjusted BGD difference in the proportions of patients with no exacerbations 0.02; 95% CI −0.03 to 0.07). However, the odds of achieving overall asthma control (i.e. no asthma-related hospitalisations, bronchial infections or acute oral steroids; salbutamol ≤200 μg/day) was significantly higher (adjusted odds ratio 1.56; 95% CI 1.14–2.14) and patients had significantly lower daily SABA usage (0.74; 95% CI 0.60–0.91) and significantly lower average daily ICS dosage (mean −130 μg/day fluticasone equivalents; p < 0.001) than with fluticasone/salmeterol. In addition, the mean asthma-related healthcare costs were significantly (p < 0.001) reduced by ₤93.63/patient/year with beclometasone/formoterol relative to fluticasone/salmeterol [40].
Another prospective, observational, multicentre, real-life study (n = 213) showed that extrafine beclometasone/formoterol 100/6 FDC improved asthma control and symptoms in patients categorized according to smoking habits, disease duration and air trapping [36]. In the overall population, the proportion of well-controlled patients increased significantly after 12 weeks’ beclometasone/formoterol therapy (from 6.1 to 66.3%; p < 0.001), with a corresponding decrease in the proportion of uncontrolled patients (from 70.3 to 10.0%; p < 0.001). When stratified according to phenotypes related to small airways, similar improvements were seen in all subgroups; the proportion of well-controlled patients increased significantly and the proportion of uncontrolled patients decreased significantly in smokers, ex-smokers and nonsmokers, in patients with asthma for ≤10 or >10 years, and in patients with baseline FVC <80 or ≥80% of predicted normal (all p < 0.01) [36].
What is the clinical efficacy of extrafine beclometasone/formoterol inhalers in COPD?
Two pivotal, 48-week, randomized, double-blind, phase 3 studies compared the efficacy of extrafine beclometasone/formoterol 100/6 μg pMDI (two inhalations twice daily) with that of budesonide/formoterol 200/6 μg DPI (two inhalations twice daily) [23] and/or formoterol 12 μg DPI (one inhalation twice daily) [23, 24] in adults with severe, stable COPD [23] or severe COPD with a history of exacerbations (FORWARD) [24]. The studies enrolled patients aged ≥40 [23] or >40 [24] years with a post-bronchodilator FEV1 of 30–50% of the predicted normal [23, 24] (and an absolute value of ≥0.7 L [23]) and pre-dose FEV1/FVC ratio of ≤0.7 [23] or post-salbutamol FEV1/FVC ratio of <0.7 [24]. Patients had to have a smoking history of ≥20 years and symptomatic COPD for >2 years [23], or current or former smokers (≥10-pack smoking history) with a documented history of ≥1 exacerbation in the previous year [24].
In the FORWARD study, extrafine beclometasone/formoterol significantly reduced the rate of exacerbations and improved lung function and HR-QOL relative to formoterol alone in patients with severe COPD and a history of exacerbations [24]. The adjusted mean rate of exacerbations per patient per year was significantly lower (Table 3; coprimary endpoint; adjusted rate ratio 0.72; 95% CI 0.62–0.84), and the time to first exacerbation was significantly longer (hazard ratio 0.80; 95% CI 0.68–0.95; p = 0.01) with extrafine beclometasone/formoterol than with formoterol [24]. A subgroup analysis showed that the rate of moderate/severe exacerbations was significantly (p = 0.002) reduced with beclometasone/formoterol relative to formoterol alone both in patients receiving and in those not receiving tiotropium at baseline (28 and 29% reduction, respectively) [41].
The mean changes from baseline in pre-dose morning FEV1 at week 12 (coprimary endpoint; Table 3) and at all post-randomization visits, as well as the average FEV1 change over the treatment period (adjusted mean BGD 0.062 L; p < 0.001), were significantly greater with extrafine beclometasone/formoterol than with formoterol [24]. In addition, the St. George’s respiratory questionnaire (SGRQ) score decreased (i.e. improved) to a significantly greater extent with beclometasone/formoterol than with formoterol, indicating improvement in HR-QOL (Table 2) [24].
The second pivotal study also demonstrated the superiority of extrafine beclometasone/formoterol over formoterol alone and similarity between the extrafine beclometasone/formoterol and budesonide/formoterol in terms of the mean change from baseline in pre-dose morning FEV1 (coprimary endpoint; Table 3) [23]. However, unlike results from FORWARD, there was no significant difference between extrafine beclometasone/formoterol and formoterol with regard to the rate of exacerbations per patient per year (coprimary endpoint); the extrafine beclometasone/formoterol and budesonide/formoterol groups also did not differ significantly in terms of the exacerbation rate (Table 3). A likely explanation for the lack of BGDs could be the low rate of exacerbations in this study, which enrolled patients who had stable disease and were, therefore, less likely to experience exacerbations during the study. SGRQ scores decreased from baseline in all three treatment groups, with no significant BGDs [23].
In addition to these studies, a 12-week, randomized, double-blind study in patients (aged ≥40 years) with moderate/severe COPD (post-bronchodilator FEV1 <60% of predicted and FEV1/FVC <0.7) showed that extrafine beclometasone/formoterol pMDI 100/6 μg (two inhalations twice daily; n = 211) provided equivalent improvement in dyspnoea and a faster onset of action than fluticasone/salmeterol DPI 500/50 μg (one inhalation twice daily; n = 207) [19]. At week 12, the adjusted mean transition dyspnea index (TDI; coprimary endpoint) score with extrafine beclometasone/formoterol was equivalent to that with fluticasone/salmeterol (1.32 vs 1.15) and the adjusted mean change in the AUC from time 0 to 30 min in FEV1 (coprimary endpoint) was significantly greater with extrafine beclometasone/formoterol than with fluticasone formoterol (0.18 vs 0.11 L; p < 0.001). FEV1 improved significantly (p < 0.001) from baseline at 5, 15 and 30 min in both treatment groups, with greater (p < 0.001) improvements seen in beclometasone/formoterol recipients at all timepoints. Both treatment groups significantly (p < 0.05) improved the 6-min walk distance from baseline (mean change from baseline 27.5 m with beclometasone/formoterol and 15.4 m with fluticasone/salmeterol), with no significant BGD. Similarly, the SGRQ total score and the single domain scores decreased significantly (p < 0.001) from baseline in both treatment groups (mean change in SQRQ total score −5.8 vs −3.8 with fluticasone/salmeterol), with no significant BGDs [19].
What is the clinical efficacy of the dry-powder inhaler?
An 8-week, randomized, double-blind phase 3 study (Neptune) showed that extrafine beclometasone/formoterol 100/6 μg DPI was as effective as extrafine beclometasone/formoterol 100/6 μg pMDI in patients with asthma who needed regular treatment [42]. Patients with controlled asthma (FEV1 >80% of predicted normal) after wash out of inhaled bronchodilators and on treatment with regular, medium-dose ICS (≤1000 μg large-particle beclometasone or equivalent) or an FDC of an ICS and LABA (fluticasone/salmeterol ≤500/100 μg or equivalent) were switched to one inhalation twice daily of extrafine beclometasone/formoterol 100/6 pMDI in a 4-week run-in period. Patients were then randomized to one inhalation twice daily of either extrafine beclometasone/formoterol 100/6 μg DPI, extrafine beclometasone/formoterol 100/6 pMDI or large-particle beclometasone 100 μg DPI [42].
At week 8, extrafine beclometasone/formoterol DPI was noninferior to extrafine beclometasone/formoterol pMDI, and both FDCs were superior to beclometasone DPI in terms of the mean change from baseline in mPEF (primary endpoint; Table 2) [42]. The extrafine beclometasone/formoterol DPI and the extrafine beclometasone/formoterol pMDI also did not differ significantly in terms of other lung function parameters (including FEV1; Table 2) and asthma control measures. Extrafine beclometasone/formoterol DPI and pMDI inhalers were significantly (p < 0.05) more effective than beclometasone DPI with regard to several measures, including the mean change from baseline in FEV1 (Table 2), the proportion of days without rescue therapy (5.2 and 6.1 vs 1.9%) and the ACQ-7 score (−0.026 and −0.028 vs −0.058); the proportion of asthma-control days was higher with extrafine beclometasone/formoterol pMDI than with beclometasone DPI (9.6 vs 5.6%; p < 0.05) [42].
In a small, randomized, crossover trial in 66 adults with asthma with previous use of a pMDI, but no previous use of a DPI, the usability of the NEXThaler® was significantly (p < 0.001 for all endpoints) better than that of two other DPIs (Diskus® and Turbohaler®) [43]; the proportion of participants completing a successful inhalation without any errors (critical or any errors) was also significantly higher with the NEXThaler® (p < 0.001). Of these three DPIs, the NEXThaler® was considered by patients to be the easiest to use and the most preferred inhaler to own (p < 0.001) [43].
In addition, two small studies in patients with asthma [44, 45] or COPD [44] showed that the beclometasone/formoterol DPI inhaler can be used and activated in a wide population, regardless of age or disease severity [44] or asthma control [45]. In a multicentre, open-labelled, placebo-controlled study in patients with asthma (n = 68) or COPD (n = 21), all patients were able to activate the breath-actuated mechanism (BAM) multidose-reservoir and no patient had a problem in using the DPI correctly [44]. The mean peak inspiratory flow (PIF) value was greater than the threshold set for BAM activation and was not influenced by age or disease severity (104.4 and 97.9 L/min in patients with asthma and COPD, respectively) [44]. In another study (n = 40), inhalation flows triggering the activation of the BAM were consistent between inhalations and independent of the level of asthma control [45]. All patients were able to use the device effectively, as indicated by high PIF values in patients with controlled asthma (n = 20) or poorly controlled/uncontrolled asthma (n = 20) and significantly higher than the flow required to trigger the BAM (e.g. PIF was 58.8 after the first and 63.0 L/min after the second inhalation in patients with poorly controlled/uncontrolled asthma) [45]. Initial acceleration (an additional critical factor in determining effective powder de-aggregation) was also high, consistent between inhalations and independent of asthma control (e.g. 123.5 after the first and 134.4 L/min/s after the second inhalation in patients with poorly controlled/uncontrolled asthma) [45].
What is the tolerability profile of extrafine beclometasone/formoterol inhalers?
Extrafine beclometasone/formoterol (100/6 or 200/6 μg via pMDI or DPI) FDC inhalers are generally well tolerated in patients with asthma or COPD. For example, in patients with asthma, adverse drug reactions were reported in 12.3% of patients receiving extrafine beclometasone/formoterol 100/6 μg pMDI compared with 17.3% of patients receiving beclometasone + formoterol free combination and 15.0% of patients receiving beclometasone; one serious adverse reaction (moderate intensity oesophageal candidiasis) was reported with the FDC and none were reported with the free combination or beclometasone alone [27]. No patient in the extrafine beclometasone/formoterol or beclometasone monotherapy groups and two patients in the beclometasone + formoterol group discontinued treatment because of adverse reactions [27].
The nature and severity of adverse reactions with the FDC were as expected with the individual agents, with no additional adverse reactions reported with combined use of beclometasone and formoterol [7, 8]. Adverse reactions usually associated with beclometasone include oral fungal infections, oral candidiasis, dysphonia and throat irritation, and adverse reactions associated with formoterol include hypokalaemia, headache, tremor, palpitations, cough, muscle spasms and prolongation of the corrected QT interval [7, 8].
Commonly occurring (incidence ≥1/100 and <1/10) adverse reactions with the extrafine beclometasone/formoterol 100/6 or 200/6 μg FDC administered via:
-
pMDI (or with the individual single agents) were pharyngitis [7, 8], oral candidiasis [7, 8], pneumonia (in patients with COPD) [7], headache [7, 8] and dysphonia [7]
-
DPI were tremor [9, 10] and pneumonia (in patients with COPD) [9].
There is an increased risk of pneumonia (including that requiring hospitalization) in patients with COPD receiving ICS therapy [7]. In the FORWARD study, pneumonia was reported in 3.8% of extrafine beclometasone/formoterol 100/6 μg pMDI recipients compared with 1.8% of formoterol recipients [24]. A post hoc analysis of the FORWARD study assessing the risk–benefit balance of treatment with extrafine beclometasone/formoterol found that the number of incident pneumonia cases relative to the number of exacerbations was small [46]. Following treatment, moderate/severe exacerbations decreased from 535 to 410 (125 fewer cases), while confirmed cases of pneumonia increased from 11 to 24 (increase of 13 cases) [46]. The number of patients with at least one exacerbation decreased from 294 to 264 (decrease of 30 events) and patients with at least one pneumonia event increased from 11 to 21 (10 more events) [46]. Although the number of incidents of pneumonia is small, patients receiving extrafine beclometasone/formoterol 100/6 μg pMDI or DPI should be monitored for the development of pneumonia [7]. Smoking, older age, low body mass and severe COPD increase the risk of developing pneumonia [7].
As with other inhalation therapy, paradoxical bronchospasm may occur with extrafine beclometasone/formoterol, with an increase in wheezing and shortness of breath immediately after dosing. Patients with bronchospasm should be treated immediately with a fast-acting inhaled bronchodilator; extrafine beclometasone/formoterol treatment should be discontinued and alternative therapy started if necessary [7–10].
The tolerability profile of the extrafine beclometasone/formoterol FDC was generally similar to that of budesonide/formoterol [28] and fluticasone/salmeterol [20] FDCs, with no significant BGDs in the incidence of treatment-emergent adverse events.
What is the current clinical positioning of extrafine beclometasone/formoterol?
Inhalers containing FDCs of extrafine beclometasone/formoterol provide a useful option for patients with asthma or COPD who require ICS and LABA combination therapy. These FDC inhalers are indicated for the treatment of asthma (i.e. regular treatment of patients with asthma not adequately controlled with an ICS + ‘as required’ inhaled SABA, as well as in patients who are adequately controlled with an ICS + a LABA) and for the treatment of COPD (i.e. symptomatic treatment of patients with severe COPD and a history of repeated exacerbations despite regular therapy with long-acting bronchodilators). In the EU, extrafine beclometasone/formoterol FDCs are available as two dosage strengths (100/6 or 200/6 μg) and as two types of inhaler (a pMDI and a NEXThaler® DPI), which enables prescribers to tailor ICS dose to the level of symptoms and to select the type of inhalation device that is preferred and/or most suitable for the individual. The use of individualized pharmacological therapy and adjustment of treatment is recognized as an important element of respiratory disease management in order to improve symptom control and reduce future risk of exacerbations [2, 3].
The role of small airways in the pathophysiology of asthma and COPD, and the impact of small airways disease on health status is being increasingly recognized [47, 48]. Studies have shown high prevalence of small airways disease in asthma and COPD of all disease severity [47, 49]. The extrafine formulation of beclometasone/formoterol delivers the drugs throughout the bronchial tree (including the small and large airways) and results in greater lung deposition than with large-particle formulations. In clinical studies, extrafine beclometasone/formoterol reduced symptoms and exacerbations and improved lung function and/or HR-QOL in patients with asthma (as maintenance, and as MART) or COPD. In real-world studies, extrafine beclometasone/formoterol generally provided better asthma control, reduced the use of rescue medication and improved HR-QOL compared with large-particle FDC formulations. Extrafine beclometasone/formoterol was generally well tolerated, with adverse reactions as expected with the individual components. Improved lung deposition with the extrafine formulation of the inhaler allows for administration of a lower dose of ICS, which is expected to lower the risk of systemic corticosteroid-related adverse effects.
Asthma and COPD symptoms can have considerable impact on patients’ daily activities and HR-QOL. In the recent REALISE survey of 8000 patients with asthma in Europe, 58% of patients reported experiencing symptoms that interfered with their daily activities and 55% of patients had symptom-related night-time awakenings [50]. Similarly, an internet survey of 803 COPD patients in the USA and Europe showed that mornings were the worst time for COPD symptoms, with shortness of breath (a commonly reported symptom) having the greatest impact on morning routine activities [51]. Extrafine beclometasone/formoterol has a rapid onset of action (within 5 min), which supports its use as maintenance and reliever in asthma [30] and is expected to translate into fast relief of dyspnoea in COPD [52]. Extrafine beclometasone/formoterol as maintenance therapy has a twice-daily administration (i.e. in the morning and in the evening) which can provide relief of both daytime and night-time/early-morning symptoms.
The choice of the inhaler for patients with asthma and COPD is influenced by several factors, including patient preference and the ability to use the device correctly [53, 54]. Unlike products available as suspensions, beclometasone/formoterol pMDI formulation is a solution which does not require shaking; variation in shaking of suspensions and storage in the hands may affect dose uniformity and size distribution [55]. The extrafine formulation in the pMDI inhaler results in the generation of a slow-moving plume over a long duration, which helps to coordinate dose generation with inspiration and reduces oropharyngeal deposition [55]. The NEXThaler® DPI is characterized by a simple three-step operating sequence [15] and was used with fewer errors, including critical errors, as compared to other DPIs [43]. Moreover, the BAM of the NEXThaler provides feedback to the patient after delivery of the dose and not after preparation of the dose, thus ensuring patients and healthcare professionals that the full therapeutic dose is inhaled [15].
References
Miller-Larsson A, Selroos O. Advances in asthma and COPD treatment: combination therapy with inhaled corticosteroids and long-acting beta 2-agonists. Curr Pharm Des. 2006;12(25):3261–79.
Global strategy for asthma management and prevention: updated 2017. Bethesda: Global Initiative for Asthma (GINA); 2017.
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2017. Bethesda: Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2017.
British guideline on the management of asthma. London: British Thoracic Society; 2016.
Barnes PJ. A single inhaler for asthma? Am J Respir Crit Care Med. 2005;171(2):95–6.
Nicolini G, Scichilone N, Bizzi A, et al. Beclomethasone/formoterol fixed combination for the management of asthma: patient considerations. Ther Clin Risk Manag. 2008;4(5):855–64.
Fostair (beclometasone dipropionate/formoterol) 100/6 inhalation solution: summary of product characteristics. Manchester: Chiesi Limited; 2016.
Fostair (beclometasone dipropionate/formoterol) 200/6 inhalation solution: summary of product characteristics. Manchester: Chiesi Limited; 2017.
Fostair NEXThaler (beclometasone dipropionate/formoterol) 100/6 inhalation solution: summary of product characteristics. Manchester: Chiese Limited; 2016.
Fostair NEXThaler (beclometasone dipropionate/formoterol) 200/6 inhalation solution: summary of product characteristics. Manchester: Chiesi Limited; 2016.
Bousquet J, Poli G, Acerbi D, et al. Systemic exposure and implications for lung deposition with an extra-fine hydrofluoroalkane beclometasone dipropionate/formoterol fixed combination. Clin Pharmacokinet. 2009;48(6):347–58.
Fabbri LM, Nicolini G, Olivieri D, et al. Inhaled beclometasone dipropionate/formoterol extra-fine fixed combination in the treatment of asthma: evidence and future perspectives. Expert Opin Pharmacother. 2008;9(3):479–90.
Dhillon S, Keating GM. Beclometasone dipropionate/formoterol: in an HFA-propelled pressurised metered-dose inhaler. Drugs. 2006;66(11):1475–83 (discussion 84–85).
De Backer W, Devolder A, Poli G, et al. Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2010;23(3):137–48.
Corradi M, Chrystyn H, Cosio BG, et al. NEXThaler, an innovative dry powder inhaler delivering an extrafine fixed combination of beclometasone and formoterol to treat large and small airways in asthma. Expert Opin Drug Deliv. 2014;11(9):1497–506.
Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J. 2002;19(1):182–91.
O’Connor BJ, Collarini S, Poli G, et al. Rapid effects of extrafine beclomethasone dipropionate/formoterol fixed combination inhaler on airway inflammation and bronchoconstriction in asthma: a randomised controlled trial. BMC Pulm Med. 2011;11:60.
Singh D, Corradi M, Bindi E, et al. Relief of methacholine-induced bronchospasm with extrafine beclomethasone dipropionate/formoterol in comparison with salbutamol in asthma. Pulm Pharmacol Ther. 2012;25(5):392–8.
Singh D, Nicolini G, Bindi E, et al. Extrafine beclomethasone/formoterol compared to fluticasone/salmeterol combination therapy in COPD. BMC Pulm Med. 2014;14:43.
Papi A, Paggiaro P, Nicolini G, et al. Beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy. 2007;62(10):1182–8.
De Backer J, Vos W, Vinchurkar S, et al. The effects of extrafine beclometasone/formoterol (BDP/F) on lung function, dyspnea, hyperinflation, and airway geometry in COPD patients: novel insight using functional respiratory imaging. J Aerosol Med Pulm Drug Deliv. 2015;28(2):88–99.
Tzani P, Crisafulli E, Nicolini G, et al. Effects of beclomethasone/formoterol fixed combination on lung hyperinflation and dyspnea in COPD patients. Int J Chron Obstruct Pulmon Dis. 2011;6:503–9.
Calverley PM, Kuna P, Monso E, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respir Med. 2010;104(12):1858–68.
Wedzicha JA, Singh D, Vestbo J, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108(8):1153–62.
Lewis DA, Brambilla G, Church T, et al. BDP and formoterol association within a combination HFA solution MDI. Respir Drug Deliv. 2006;3:939–42.
Corradi M, Spinola M, Petruzzelli S, et al. High-dose beclometasone dipropionate/formoterol fumarate in fixed-dose combination for the treatment of asthma. Ther Adv Respir Dis. 2016;10(5):492–502.
Huchon G, Magnussen H, Chuchalin A, et al. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103(1):41–9.
Papi A, Paggiaro PL, Nicolini G, et al. Beclomethasone/formoterol versus budesonide/formoterol combination therapy in asthma. Eur Respir J. 2007;29(4):682–9.
Paggiaro P, Corradi M, Latorre M, et al. High strength extrafine pMDI beclometasone/formoterol (200/6 μg) is effective in asthma patients not adequately controlled on medium-high dose of inhaled corticosteroids. BMC Pulm Med. 2016;16(1):180.
Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013;1(1):23–31.
Barnes N, van Noord JA, Brindicci C, et al. Stepping-across controlled asthmatic patients to extrafine beclometasone/formoterol combination. Pulm Pharmacol Ther. 2013;26(5):555–61.
Papi A, Nicolini G, Crimi N, et al. Step-down from high dose fixed combination therapy in asthma patients: a randomized controlled trial. Respir Res. 2012;13:54.
Terzano C, Cremonesi G, Girbino G, et al. 1-year prospective real life monitoring of asthma control and quality of life in Italy. Respir Res. 2012;13:112.
Allegra L, Cremonesi G, Girbino G, et al. Real-life prospective study on asthma control in Italy: cross-sectional phase results. Respir Med. 2012;106(2):205–14.
Kuna P, Kuprys-Lipinska I, Debowski T. Control of asthma in adults treated with beclomethasone and formoterol in extrafine particle formulation in a real-life setting in Poland: the CASPER noninterventional, observational trial. Pol Arch Med Wewn. 2015;125(10):731–40.
Marth K, Spinola M, Kisiel J, et al. Treatment response according to small airway phenotypes: a real-life observational study. Ther Adv Respir Dis. 2016;10(3):200–10.
Brusselle G, Peche R, Van den Brande P, et al. Real-life effectiveness of extrafine beclometasone dipropionate/formoterol in adults with persistent asthma according to smoking status. Respir Med. 2012;106(6):811–9.
Popov TA, Petrova D, Kralimarkova TZ, et al. Real life clinical study design supporting the effectiveness of extra-fine inhaled beclomethasone/formoterol at the level of small airways of asthmatics. Pulm Pharmacol Ther. 2013;26(6):624–9.
Muller V, Galffy G, Eszes N, et al. Asthma control in patients receiving inhaled corticosteroid and long-acting β2-agonist fixed combinations. A real-life study comparing dry powder inhalers and a pressurized metered dose inhaler extrafine formulation. BMC Pulm Med. 2011;11:40.
Price D, Small I, Haughney J, et al. Clinical and cost effectiveness of switching asthma patients from fluticasone-salmeterol to extra-fine particle beclometasone-formoterol: a retrospective matched observational study of real-world patients. Prim Care Respir J. 2013;22(4):439–48.
Singh D, Corradi M, Spinola M, et al. Extrafine beclometasone diproprionate/formoterol fumarate: a review of its effects in chronic obstructive pulmonary disease. NPJ Prim Care Respir Med. 2016;26:16030.
Kanniess F, Scuri M, Vezzoli S, et al. Extrafine beclomethasone/formoterol combination via a dry powder inhaler (NEXThaler®) or pMDI and beclomethasone monotherapy for maintenance of asthma control in adult patients: a randomised, double-blind trial. Pulm Pharmacol Ther. 2015;30:121–7.
Voshaar T, Spinola M, Linnane P, et al. Comparing usability of NEXThaler® with other inhaled corticosteroid/long-acting β 2-agonist fixed combination dry powder inhalers in asthma patients. J Aerosol Med Pulm Drug Deliv. 2014;27(5):363–70.
Sergio F, Chetta A, Pisi G, et al. A novel NEXT DPI® dry powder inhaler and its use in asthmatic and COPD population [abstract no. P831]. Eur Respir J. 2011;38(Suppl 55):152.
Scuri M, Alfieri V, Giorgio A, et al. Measurement of the inhalation profile through a novel dry powder inhaler (NEXThaler®) in asthmatic patients using acoustic monitoring [abstract no. A1931]. Am J Respir Crit Care Med. 2013;187.
Corradi M, Spinola M, Guasconi A, et al. Association of incident pneumonia and exacerbations with extrafine beclometasone dipropionate/formoterol (BDP/FF) in severe COPD patients: a post-hoc analysis of FORWARD study [abstract no. OA4831]. Eur Respir J. 2016;48.
Crisafulli E, Pisi R, Aiello M, et al. Prevalence of small-airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration. 2017;93(1):32–41.
van den Berge M, ten Hacken NH, Cohen J, et al. Small airway disease in asthma and COPD: clinical implications. Chest. 2011;139(2):412–23.
Usmani OS, Singh D, Spinola M, et al. The prevalence of small airways disease in adult asthma: a systematic literature review. Respir Med. 2016;116:19–27.
Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi:10.1038/npjpcrm.2014.9.
Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25(8):2043–8.
Cazzola M, Beeh KM, Price D, et al. Assessing the clinical value of fast onset and sustained duration of action of long-acting bronchodilators for COPD. Pulm Pharmacol Ther. 2015;31:68–78.
Haughney J, Price D, Barnes NC, et al. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010;104(9):1237–45.
Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–71.
Ganderton D, Lewis D, Davies R, et al. Modulite®: a means of designing the aerosols generated by pressurized metered dose inhalers. Respir Med. 2002;96(Suppl D):S3–8.
Acknowledgements
The manuscript was reviewed by: F. Kanniess, Pulmonary Research Institute Hospital Grosshansdorf, Grosshansdorf, Germany; S. Saluja, Saran Ashram Hospital, Dayalbagh, Agra, India. During the peer review process, the manufacturer of extrafine beclometasone/formoterol FDC inhalers was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
S. Dhillon is a salaried employee of Adis/Springer, is responsible for the article content and declares no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dhillon, S. Extrafine beclometasone dipropionate/formoterol fumarate metered-dose and dry-powder inhalers in asthma and chronic obstructive pulmonary disease: a profile of their use. Drugs Ther Perspect 33, 260–271 (2017). https://doi.org/10.1007/s40267-017-0397-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-017-0397-7