Abstract
Augmentation with intravenous infusions of human α1-proteinase inhibitor [Respreeza® (EU); Zemaira® (USA)] slows the progression of emphysema in patients with severe α1-antitrypsin (AAT)-deficiency emphysema. In the randomized, double-blind RAPID trial in patients with severe AAT-deficiency emphysema, Respreeza®/Zemaira® significantly reduced the mean annual rate of lung density loss based on computed tomography imaging at total lung capacity (TLC), but not at functional residual capacity (FRC) or combined TLC + FRC measurements. Respreeza®/Zemaira® is generally well tolerated, with a generally similar tolerability profile to that of placebo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adis evaluation of intravenous Respreeza ® /Zemaira ® in α 1 -antitrypsin deficiency emphysema
Slows the loss of lung density, thereby showing the progress of emphysema |
Preliminary evidence shows treatment should be commenced early when indicated |
Generally well tolerated |
Highly purified formulation that undergoes viral inactivation/removal processes; but, as with any product made from human plasma, carries a very low risk of transmitting infective agents |
What is the rationale for using human α1-proteinase inhibitor?
α1-Antitrypsin (AAT) deficiency is an inherited disorder that predisposes individuals to liver disease and early-onset emphysema [1–4]. AAT (also known as α1-proteinase inhibitor) belongs to the family of serine protease inhibitors and is thought to be the major anti-protease in the lower respiratory tract, where it inhibits neutrophil elastase (NE) [1–4]. NE is produced by neutrophils activated in response to events such as respiratory infection and smoking. In people with normal levels of AAT, NE activity is controlled, thereby preventing undesirable proteolysis of lung tissue. However, in individuals with AAT deficiency, activity of NE is poorly controlled, resulting in elastin damage and contributing to the development of emphysema [1–4].
AAT deficiency is thought to be underdiagnosed, and is considered one of the most prevalent inherited disorders among people of European descent [5]. The American Thoracic Society and European Respiratory Society recommend that clinicians consider a diagnosis of AAT deficiency in all emphysema patients with onset aged ≤45 years and in those with no known risk factors [6]. Individuals with AAT deficiency are most likely to present with pulmonary disease as early as the third to the fourth and fifth decades of life, with the risk of early disease onset being higher in smokers [7–10]. Where suspicion of AAT deficiency exists, diagnosis can be confirmed by measuring serum ATT levels and phenotyping [6]. Most common mutations in AAT deficiency show deficient alleles homo- or heterozygous for the ‘S’ or ‘Z’ variants, with null variants being much less common [2].
Although estimates of prevalence vary, one report based on genetic epidemiological surveys that included 93 countries found that out of a population of ≈5.2 billion, ≈182,000 (0.003 %) individuals carried the proteinase inhibitor genotype ZZ (PiZZ), which is associated with severe AAT deficiency [11]. The proportion of these individuals who will develop emphysema is unknown, but it is thought that the majority remain unaffected [10].
Augmenting serum levels of α1-proteinase inhibitor in individuals with severe AAT-deficiency emphysema represents a rational approach to treatment [3, 12]. Intravenous (IV) infusions of human α1-proteinase inhibitor are the only specific approved therapy for AAT deficiency that are currently available [13, 14]. Disease progression [as assessed by rates of decline of forced expiratory volume in 1 second (FEV1), gas transfer and lung density (a potential surrogate measure for survival) and other outcomes] has been shown to vary markedly in both in smokers and non-smokers with AAT deficiency. As a result, some, but not all, patients with AAT-deficiency emphysema will require augmentation with an α1-proteinase inhibitor. A personalized, risk-based approach to patient management should be implemented [15, 16], with augmentation therapy being initiated only when there is evidence of progressive disease subsequent to optimal standard therapy (including smoking cessation) [3].
α1-Proteinase inhibitor is produced from the plasma of human donors. This article focuses on one such agent approved in the EU in 2015 (Respreeza®) [13] and in the USA in 2003 (Zemaira®) [14]; henceforth referred to as Respreeza®.
How does Respreeza® work?
In patients with AAT-deficiency emphysema, IV infusions of α1-proteinase inhibitor increase plasma levels beyond the protective threshold (11 µM) [2]. This leads to increased inhibition of NE in the lung, thereby controlling NE-induced destruction of lung elastin and alveolar walls [2]. In a randomized, crossover, 24-week trial (double-blind for the first 10 weeks) in patients with AAT-deficiency emphysema, once steady-state was achieved, weekly infusions of Respreeza® 60 mg/kg produced mean trough serum levels of α1-proteinase inhibitor above the therapeutic threshold [4]. Furthermore, steady-state mean trough serum levels from week 7 to 11 (period of comparison selected based on expectation that all subjects would achieve steady-state trough serum levels after 6 doses of Respreeza®) were bioequivalent to those observed after weekly infusions of Prolastin® (another human α1-proteinase inhibitor) [4].
The mean half-life of α1-proteinase inhibitor following weekly IV Respreeza® 60 mg/kg was estimated at 6.8 days (consistent with weekly administration) based on a population pharmacokinetic analysis using data from the RAPID trial [17]. At this dosage, the model predicted a mean steady-state trough α1-proteinase inhibitor level of 16.2 µM. The analysis found that pharmacokinetic parameters were not significantly influenced by age, gender, bodyweight or baseline serum α1-proteinase inhibitor levels [17].
During the manufacturing process of Respreeza®, the product is highly purified and undergoes viral inactivation/removal processes [13]. A pilot study that compared each of the α1-proteinase inhibitors commercially available in the USA found clear differences in the purity profiles, with Respreeza® having greater purity than Aralast® and Prolastin® [18]. Thus, Respreeza® would be expected to deliver a lower non-therapeutic protein load than the other two agents.
For whom is Respreeza® indicated?
Respreeza® is indicated for the maintenance treatment of emphysema in adults with confirmed severe AAT-deficiency [13, 14]. The EU prescribing information further specifies that patients are to be receiving optimal pharmacological and non-pharmacological treatment and have evidence of progressive lung disease (e.g. FEV1 predicted, impaired walking capacity or an increased number of exacerbations) [Table 1] [13].
What is the clinical efficacy of Respreeza®?
As indicated by a meta-analysis [19], results of observational and cohort studies in individuals with AAT-deficiency emphysema indicated that human α1-proteinase inhibitor (usually Prolastin®) augmentation therapy achieved a slower rate of FEV1 decline than that observed with placebo. Furthermore, one study demonstrated a potential survival benefit with α1-proteinase inhibitor augmentation in patients with severe pulmonary disease [20]. However, no randomized, placebo-controlled trials have demonstrated a reduction in emphysema progression with α1-proteinase inhibitor therapy using established endpoints such as FEV1 [12]. Because the decline in FEV1 in emphysema patients is gradual over many years and because of the rare nature of AAT deficiency, the size and cost of a well-controlled trial using accepted endpoints is considered prohibitive. Therefore, computed tomography (CT)-measured lung density, which is a more sensitive measure of disease progression in AAT-deficiency emphysema than spirometry, has been used to evaluate efficacy in clinical trials [21].
The RAPID trial
In the double-blind RAPID trial, 180 patients with emphysema (FEV1 35–70 % of predicted value) secondary to severe AAT deficiency (serum AAT level ≤11 μM) were randomized to receive weekly IV infusions of Respreeza® 60 mg/kg or placebo for 24 months [21]. Ninety-three percent of patients were the ZZ genotype, which is associated with severe AAT deficiency. Patients who had smoked in the previous 6 months were excluded.
The trial used CT imaging to evaluate the clinical efficacy of human α1-proteinase inhibitor augmentation therapy [21]. CT imaging was selected because it is a more sensitive measure of lung disease progression than spirometry, thereby facilitating a randomized, controlled trial in fewer patients over a shorter time period. Spiral CT scans at total lung capacity (TLC) and functional residual capacity (FRC) were performed at baseline and at 3, 12, 21 and 24 months to evaluate the annual rate of decrease in lung density, which was calculated from the shift of the 15th percentile of lung density (primary analysis) [21].
The mean annual rate of lung density loss based on TLC was significantly lower in patients receiving Respreeza® than in those receiving placebo (Table 2) [21]. However, the annual rate of lung density loss based on FRC and a combined assessment of TLC and FRC did not differ between the Respreeza® and placebo groups. The lack of a significant treatment effect with Respreeza® with regard to CT imaging at FRC is likely due to the greater variation in CT measured density at FRC than at TLC [21].
At 24 months, the mean increase from baseline in α1-proteinase inhibitor levels was significantly (p = 0.02) greater with Respreeza® than with placebo (secondary endpoint) [21]. However, there were no significant differences between the Respreeza® and placebo groups with regard to other secondary efficacy endpoints, including spirometry, exacerbations, St George’s Respiratory Questionnaire scores and shuttle walk distance. As the RAPID trial was not designed with sufficient power to detect changes in these conventional pulmonary function and clinical endpoints, the lack of significant between-group differences is not surprising [21].
An estimate of time to terminal respiratory failure (either death or lung transplantation) based on RAPID data was estimated at 18.1 and 12.3 years for Respreeza® and placebo recipients, respectively [21]. The time to terminal respiratory failure was extrapolated from two lung density values (i.e. average lung density at study exit for the five patients who experienced a terminal event and at baseline for all enrolled patients) and the rates of annual lung density decrease at TLC [21].
Extension phase
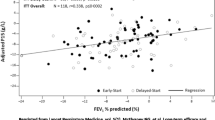
Results of a 2-year open-label extension phase of the RAPID trial suggest that early treatment with human α1-proteinase inhibitor in patients with severe AAT-deficiency emphysema may be more beneficial than later treatment [21, 22]. After 4 years, patients who started Respreeza® at the beginning of the RAPID trial had numerically lower levels of lung density decline than those who commenced Respreeza® at the beginning of the extension phase. While lung density loss was slowed with the switch from placebo to Respreeza®, lung parenchyma loss during the placebo period was not recovered.
Post-hoc analyses
Post-hoc per-protocol analyses found that lung density correlated with pulmonary function, and that the annual rate of lung density decline was inversely proportional to trough serum α1-proteinase inhibitor levels [21]. As a result, further investigation of optimum dosage regimens may be needed [21]. During active treatment, trough serum α1-proteinase inhibitor levels tended to be higher in patients with a higher bodyweight and in those with higher pretreatment levels of serum α1-proteinase inhibitor.
An analysis of CT data indicated that the reduction in the loss of lung density in Respreeza®-treated patients was associated with a reduction in the loss of lung mass, indicative of a reduction in the rate of emphysema progression [23]. Further analysis of CT data indicated that the reduction in lung density decline observed with Respreeza® therapy was greater in the basal and mid-lung regions than in the apical region. Although the clinical significance of this finding is not clear, emphysema in patients with AAT deficiency is predominantly basal in distribution [24].
What is the tolerability profile of Respreeza®?
Respreeza® is generally well tolerated and, in the RAPID trial, the incidence of adverse events with Respreeza® was generally similar to that with placebo [21]. Ninety-nine percent of patients in both the Respreeza® (n = 93) and placebo (n = 87) groups reported at least one treatment-emergent adverse event, with 27 and 31 % in each group, respectively, experiencing at least one severe adverse event. Adverse events in Respreeza and placebo groups that were considered to be related to study treatment occurred in 23 and 24 % of patients, respectively. A single treatment-emergent adverse event led to treatment withdrawal in one patient receiving Respreeza® and 10 treatment-emergent adverse events led to treatment withdrawal in four patients receiving placebo. The time to the first exacerbation of disease was not different between groups [21]. During the 2-year trial, one (1 %) patient in the Respreeza® group died (respiratory failure) and three (3 %) patients in the placebo group died (sepsis, pneumonia and metastatic breast cancer).
The most common treatment-related adverse events associated with Respreeza® were headache and dizziness, according to the results of an integrated safety analysis of six clinical trials (221 patients received Respreeza®) [25]. Five serious adverse events were considered to be at least possibly related to Respreeza® (enterocolitis, pancreatitis, back pain, pulmonary fibrosis and undefined). There was no evidence of antibody formation or viral transmission in patients treated with Respreeza® [25].
In the randomized, crossover trial that evaluated biochemical efficacy in patients with AAT-deficiency emphysema, treatment-related adverse events were reported in 2 (7 %) of 30 patients treated with Respreeza® and 3 (21 %) of 14 patients treated with Prolastin® [4].
What is the current clinical positioning of Respreeza®?
Augmentation with Respreeza®, a highly purified human α1-proteinase inhibitor, is a valuable option in the maintenance treatment of emphysema in patients with severe AAT-deficiency. IV infusions of Respreeza® slow the rate of loss of lung density, thereby slowing the progression of emphysema, and are generally well tolerated. When indicated, it may be beneficial to commence treatment with Respreeza® relatively early in the course of the disease; treatment later in the course of the disease slows lung density loss, but does not recover lung parenchyma lost prior to treatment. These findings underscore the importance of an early diagnosis in this under-recognized condition.
References
Juvelekian GS, Stoller JK. Augmentation therapy for α1-antitrypsin deficiency. Drugs. 2004;64(16):1743–56.
Chotirmall SH, Al-Alawi M, McEnery T, et al. Alpha-1 proteinase inhibitors for the treatment of alpha-1 antitrypsin deficiency: safety, tolerability, and patient outcomes. Ther Clin Risk Manag. 2015;11:143–51.
Stockley RA. α1-Antitrypsin deficiency: what has it ever done for us? Chest. 2013;144(6):1923–9.
Stocks JM, Brantly M, Pollock D, et al. Multi-center study: the biochemical efficacy, safety and tolerability of a new α1-proteinase inhibitor, Zemaira. COPD. 2006;3(1):17–23.
Greulich T, Nell C, Herr C, et al. Results from a large targeted screening program for alpha-1-antitrypsin deficiency: 2003–2015. Orphanet J Rare Dis. 2016;11:75.
American Thoracic Society/European Respiratory Society Statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900.
Gauvain C, Mornex JF, Pison C, et al. Health-related quality of life in patients with alpha-1 antitrypsin deficiency: the French experience. COPD. 2015;12(Suppl 1):46–51.
Green CE, Vayalpra S, Hampson JA, et al. PiSZ alpha-1 antitrypsin deficiency (AATD): pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPD. Thorax. 2015;70(10):939–45.
Brantly ML, Paul LD, Miller BH, et al. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis. 1988;138(2):327–36.
Horizon Scanning Centre. Alpha-1 antitrypsin (Respreeza) for emphysema associated with alpha-1 antitrypsin deficiency: maintenance therapy. Birmingham: National Institute for Health Research Horizon Scanning Centre; 2014.
de Serres FJ, Blanco I. Prevalence of α1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6(5):277–95.
Tirado-Conde G, Lara B, Miravitlles M. Augmentation therapy for emphysema due to alpha-1-antitrypsin deficiency. Ther Adv Respir Dis. 2008;2(1):13–21.
Respreeza® (powder and solvent for solution for infusion): summary of product characteristics. London: European Medicines Agency; 2015.
Zemaira® [alpha1-proteinase inhibitor (human) for intravenous use (lyophilized powder for reconstitution)]: US prescribing information. Kankakee: CSL Behring GmbH; 2015.
Green CE, Parr DG, Edgar RG, et al. Lung density associates with survival in alpha 1 antitrypsin deficient patients. Respir Med. 2016;112:81–7.
Stockley RA, Edgar RG, Pillai A, et al. Individualized lung function trends in alpha-1-antitrypsin deficiency: a need for patience in order to provide patient centered management? Int J Chron Obstruct Pulmon Dis. 2016;11:1745–56.
Tortorici MA, Vit O, Bexon M, et al. Population pharmacokinetics of A1-PI in patients with alpha-1 antitrypsin deficiency [abstract no. PA1486]. Eur Respir J. 2015;46(Suppl 59).
Cowden DI, Fisher GE, Weeks RL. A pilot study comparing the purity, functionality and isoform composition of alpha-1-proteinase inhibitor (human) products. Curr Med Res Opin. 2005;21(6):877–83.
Chapman KR, Stockley RA, Dawkins C, et al. Augmentation therapy for alpha1 antitrypsin deficiency: a meta-analysis. COPD. 2009;6(3):177–84.
Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of α1-antitrypsin. Am J Respir Crit Care Med. 1998;158:49–59.
Chapman KR, Burdon JG, Piitulainen E, et al. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360–8.
McElvaney NG, Chapman KR, Burdon J, et al. Long-term efficacy of A1-PI therapy in RAPID and RAPID extension trials [abstract no. A285]. Eur Respir J. 2015;46(Suppl 59).
Parr D, McElvaney NG, Chapman KR, et al. The relationship between changes in CT-measured lung density and lung volume in severe alpha-1 antitrypsin deficiency: post-hoc analysis of the RAPID trial [abstract]. Am J Respir Crit Care Med. 2016;193:A1559.
Parr D, McElvaney NG, Chapman KR, et al. The effect of alpha-1 proteinase inhibitor (a1-PI) therapy on changes in regional lung density: a post-hoc analysis of the RAPID trial [abstract]. Am J Respir Crit Care Med. 2016;301:A1558.
Sandhaus R, Chapman KR, Burdon J, et al. Integrated safety across six clinical trials of alpha-1 augmentation therapy [abstract]. Eur Respir J. 2014;44(Suppl 58):950.
Acknowledgments
This manuscript was reviewed by: C.E. Green, Centre for Translational Inflammation Research, University of Birmingham, Birmingham, UK; C. McCarthy, Translational Pulmonary Science Centre, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; R.A. Stockley, Department of Lung Function and Sleep, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK. During the peer review process, the manufacturer of Respreeza® was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
K. McKeage and K.A. Lyseng-Williamson are contracted/salaried employees of Adis/Springer, are responsible for the article content and declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
McKeage, K., Lyseng-Williamson, K.A. Human α1-proteinase inhibitor (Respreeza®) in α1-antitrypsin deficiency emphysema: a guide to its use in the EU. Drugs Ther Perspect 32, 422–427 (2016). https://doi.org/10.1007/s40267-016-0338-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0338-x