Abstract
Background
For older adults with relapsing-onset multiple sclerosis (MS), limited information is available to inform if, or when, disease-modifying drugs (DMDs) may be safely discontinued.
Objective
The aim of this study was to project the outcomes of DMD discontinuation among older adults with relapsing-onset MS.
Methods
We projected the 10-year outcomes of discontinuation of a DMD (interferon-β, fingolimod, or natalizumab) among older adults (aged 55 or 70 years) who were relapse-free for 5 or more years and had not reached an Expanded Disability Status Scale (EDSS) score of 6. Outcomes included the percentage of people who had at least one relapse or reached EDSS 6, and quality-adjusted life-years (QALYs), which incorporated both relapses and disability. We used a simulation modeling approach. With increased age, relapses decreased and the effectiveness of DMDs for disability outcomes also decreased.
Results
We found lower projected benefits for DMD continuation at 70 years of age than at 55 years of age. Compared with discontinuation, the projected benefit of DMD continuation ranged from 0.007 to 0.017 QALYs at 55 years of age and dropped to 0.002–0.006 at 70 years of age. The annual projected benefits of DMD continuation (0.1–3.0 quality-adjusted life-days) were very low compared with typical patient preferences regarding treatment burden.
Conclusion
The benefits of DMDs may not be substantial among older adults with relapsing-onset MS. Direct clinical evidence remains limited and the decision of whether to discontinue a DMD should also take into account patient preferences. It is important to gain a better understanding of how age-related changes in the trajectory of relapsing-onset MS affect treatment effectiveness among older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We projected the results of discontinuation of disease-modifying drugs among older adults with multiple sclerosis using evidence-anchored simulations. |
The projected benefits of drug continuation were relatively low, compared with typical patient preferences regarding the burden of taking medication. |
1 Introduction
Multiple sclerosis (MS) is a chronic immune-mediated and neurodegenerative disease of the brain and spinal cord. In recent decades, the prevalence of MS and the mean age of people with MS have increased, representing approximately 800,000 people living with MS in North America, with an average age of 55–64 years [1, 2]. The first disease-modifying drugs (DMDs) for MS became available in the 1990s and required regular injections (the β-interferons) [3]. In 2004, natalizumab, a monthly infusion, was approved in the US, and, in 2010, the first oral drug, fingolimod, became available [4, 5]. Today there are over ten different DMDs licensed for use in MS; the majority are specifically for treating the relapsing form of MS. Once a DMD is initiated, the optimal duration of time that a person should take the drug is unknown. Treatment guidelines have predominantly focused on when to initiate DMDs and how to optimize adherence, and there is a paucity of recommendations specifically for older adults [6].
DMDs, such as interferon-β, fingolimod, or natalizumab, can be burdensome for people with relapsing-onset MS and their families. Treatment burdens may include adverse events (e.g. flu-like symptoms, serious infections, cardiovascular problems, and visual disturbances), invasive drug delivery methods or laboratory monitoring, and high costs [3,4,5, 7]. DMDs are generally considered more effective in people who are younger, with a shorter time since disease onset, and who have higher disease activity, such as frequent relapses or greater lesion burden based on magnetic resonance imaging (MRI) [8, 9]. People over 55 years of age were typically excluded from the key clinical trials of DMDs [10]. Disease activity decreases with age, and a threshold level of disease activity may be necessary for the benefits of DMDs to outweigh the treatment burden for older people with relapsing-onset MS [11,12,13,14,15]. People who discontinue medication when they are older are less likely to relapse compared with younger people [16,17,18], but few studies of DMD discontinuation focused on older adults have been completed [14, 19].
Our objective was to project the outcomes of DMD discontinuation among older adults with relapsing-onset MS. Outcomes included relapses, disability, and quality-adjusted life-years (QALYs), which captured both relapse and disability outcomes. We incorporated an age-related decrease in relapses and underlying disease heterogeneity (subgroups with different rates of relapse and disability progression) to reflect the reduced relapse activity among older adults with relapsing-onset MS. We quantified the loss of clinical benefit from discontinuing a DMD (prior to consideration of the burden of taking the DMD) and compared these results with quantitative estimates of treatment burden derived from the literature.
2 Methods
2.1 Study Design
We used a simulation modeling approach to project the 10-year outcomes of discontinuation of a DMD (interferon-β, fingolimod, or natalizumab) among older adults (55 or 70 years of age) who were relapse-free for 5 or more years and had not reached an Expanded Disability Status Scale (EDSS) score of 6. The three drugs were selected for this study because they represent three different routes of administration (injections, oral medication, and intravenous infusion) and different levels of risk and benefit. Relative to other DMDs, typically interferon-β is considered to have lower risk and efficacy, while natalizumab and fingolimod have greater risk; natalizumab offers greater efficacy, while fingolimod is less burdensome in delivery, as an oral medication [3,4,5, 20]. Our outcomes were the percentage of people who experienced at least one relapse or reached EDSS 6 or above within the next 10 years, and QALYs, which captured each simulated person’s relapses and disability over the time period. The EDSS is an ordinal scale ranging from 0 (no neurological abnormality) to 10 (MS-related death); EDSS 6 is equivalent to requiring a cane to walk [21]. The EDSS measure of disability is known to have limitations, but it remains widely used in clinical trials [22]. We anchored the simulation of DMD continuation versus discontinuation at two age-based milestones—55 and 70 years of age. These ages were selected because relapse rates are relatively low in these age groups and an ongoing randomized trial of DMD discontinuation enrolled participants aged 55 years and older [12, 13, 19]. Additionally, an observational study of DMD discontinuation reported only one relapse among adults aged 60 years and older (mean age 68 years) during up to 2 years of follow-up [14], and an autopsy study found that the inflammatory markers associated with pathologically active disease were similar among older people with MS (median age 76 years) and age-matched controls [23].
2.2 Markov Model
We developed a Markov state-transition model to simulate adults with relapsing-onset MS (Fig. 1). We characterized disability by four categories based on the EDSS. Each year, a simulated person could have a relapse(s) or not and progress in disability or not, based on a set of probabilities, including an age-specific background mortality risk (Table 1) [24]. Because advanced disability is associated with increased mortality risk [25,26,27], an additional risk of death was assumed for individuals with EDSS scores of 8.0–9.5 (Fig. 1). The simulation started from MS symptom onset at age 30 years, which is the approximate mean onset age [28]. We used five subgroups to represent heterogeneity in the MS population. The course of MS varies across individuals, with some people experiencing more relapses than others [15], and wide variation in long-term disability outcomes [29, 30]. Simulated individuals had different risk levels for relapses and disability progression, instead of assuming that all simulated persons face the same risks. We used TreeAge Pro 2018, R2.0 software (Tree Age Software Inc., Williamstown, MA, USA).
Schematic representation of the Markov model simulating people with relapsing-onset multiple sclerosis. ARR annualized relapse rate, MS multiple sclerosis, rn,t ARR over time by subgroup n, a annual age-specific decrease in ARR, rn,t0 ARR at onset (age 30 years in the Markov model) by subgroup n, t years post-onset, EDSS Expanded Disability Status Scale, dn relative risk of disability progression by subgroup n, p(EDSS) average annual probability of progression (EDSS scores categorized as 0.0–2.5, 3.0–5.5, 6.0–7.5, 8.0–9.5); r8–9.5 relapse probability for people with EDSS scores of 8–9.5 relative to 0–7.5. bAge-specific background mortality was applied to simulated individuals at all EDSS scores, with an additional risk of death from MS for individuals with EDSS scores of 8.0–9.5
2.3 Clinical Cohort
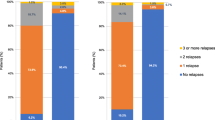
The British Columbia Multiple Sclerosis (BCMS) database in Canada represents one of the largest sources of clinical information collected on people with MS prior to the widespread availability of DMDs [28]. The BC provincial government’s coverage of the first MS DMD (β-interferon-1b) began in 1996. Data were prospectively collected at each visit to one of the four MS clinics in BC, with a clinical history (including early relapses) taken at the first MS clinic visit by the attending neurologist. These data are considered population-based and included an estimated 80% of people who were living with MS in BC during the study period [31]. The overall cohort (n =2203) included adults (age ≥ 18 years at their initial visit) with MS confirmed by an MS specialist neurologist based on the prevailing criteria at the time of diagnosis, a relapsing-onset disease course, and at least two available disability assessments. In order to reduce potential biases due to excluding people who died or moved away, no minimum follow-up time was required. We defined subgroups of people in the BCMS cohort by the number of relapses during the 5 years following the onset of MS symptoms (0–1, 2, 3, 4, and ≥ 5), excluding the onset relapse. When creating these subgroups, we focused on the period prior to widespread availability of DMDs by limiting the subgroup sample (n =934) to patients with an initial clinic visit prior to January 1996. We also required a clinic visit at least 5 years after onset, and hence disease onset prior to January 1991. The full study period was from 1980 to 2009 to enable assessment of disability outcomes. DMD use remained low for several years after 1996 [12], and limited DMD exposure was a strength of our clinical cohort. However, our estimates of heterogeneity in disability progression may be conservative (i.e. heterogeneity could be greater in people without DMD exposure).
2.4 Relapses and Disability
We allowed for an annual age-related decrease in the annualized relapse rate (ARR) (Table 1) [32]. The variation in relapse rates across subgroups 1–5 in the simulation model was based on the results of the BCMS cohort analysis. We selected the overall ARR (0.40 at symptom onset) by reviewing observational studies reporting relapses among older adults with MS [12, 13, 32] and the limited number of observational studies of DMD discontinuation among older adults [14, 16].
We assumed the risk of disability progression was based on the current EDSS level (Fig. 1, Table 1). Within subgroups, progression was independent of relapses. This assumption highlights the role of aging in progression [29, 30] and was similar to previous studies that projected long-term outcomes for people with relapsing-onset MS [33, 34]. To estimate the relative risk of disability progression among subgroups, we analyzed the BCMS cohort using Kaplan–Meier survival analyses, overall and by subgroup, with a primary endpoint of median time to EDSS 6 (sustained, with no subsequent EDSS scores below 6.0). We used these results to develop adjustments (multipliers) that accounted for the variation across subgroups in the overall rate of disability progression (Table 1). Across subgroups, we assumed that relapses and disability progression co-varied (e.g. those with a lower initial ARR progress slower in disability) based on the clinical view that residual disability may accrue following relapses, as shown by short-term data from clinical trials [35,36,37].
2.5 Disease-Modifying Drug (DMD) Treatment
Parameters and ranges for the relative risk of relapse or disability worsening while exposed to a DMD (interferon-β, fingolimod, and natalizumab) were obtained from a network meta-analysis that examined relapses and disability worsening over 24 months [20]. For disability worsening, we assumed that treatment effectiveness was proportional to a person’s relapse activity as represented by the ARR, which decreased with age (as shown by ‘a’ in Fig. 1 and Table 1). This assumption was based on evidence that DMDs are most effective in people who are younger and who have more frequent relapses, with limited efficacy, if any, for people with secondary progressive MS [8, 9, 38]. Although some studies have found no DMD benefit among adults ≥ 40 years of age [39], these results represent group averages. Because DMDs might offer benefits for older adults who continue to have disease activity, we linked effectiveness to disease activity as a conservative assumption (rather than assuming that all benefits stop at age 40 years, for example). We modeled the reduction in the risk of disability worsening while taking a DMD (RRDMD) as a function of the ARR at a given age (rt) compared with the ARR at the age of MS symptom onset (rt0): 1 − (1 − RRDMD)*(rt/rt0). For example, if the ARR had decreased by half since symptom onset, then the reduction in the risk for disability worsening with a DMD would also decrease by half. The treatment effectiveness for relapse was assumed to be constant (and thus DMD benefits for relapses decreased with age only, by decreased ARR).
2.6 Utilities
Utilities are a preference-based measure for health-related quality of life. QALYs weight years of life by utilities. Utilities for disability status and relapse events were derived from published assessments of people in the US with relapsing-onset MS [33, 40]. The disutility (i.e. utility decrement) for a relapse was assumed to last 3 months. We compared the annual DMD benefits (prior to consideration of treatment burden) with DMD disutilities derived from published literature based on patient preferences regarding treatment delivery [33, 41, 42]. To do this, we translated our 10-year results into annual threshold values that represent the magnitude of perceived treatment burden equal to the projected DMD benefit. For example, if an individual was willing to ‘trade’ 30 quality-adjusted days per year in order to be free of DMD burdens, then the benefit of a DMD outweighs the burden of treatment only if the projected benefit is > 30 quality-adjusted days annually.
2.7 Analysis
We used a 10-year time horizon (the duration of continuation or discontinuation) in order to allow sufficient time to capture disability outcomes, and because a longer time duration of assumed DMD continuation or discontinuation may be less relevant to older adults making treatment decisions. Analyses required two sets of simulations (age 30–55 years and 55–64 years, for age 55 years analysis) because people in subgroups with a lower ARR were more likely to be relapse-free, thus the prevalence of subgroups changed over time among cohort members who were in a relapse-free interval (electronic supplementary Table 1). Model simulations were based on the average results of 30,000 simulated individuals.
2.8 Sensitivity Analyses
We examined a range of utility weights for the quality-of-life adjustments for disability status [33]. We also evaluated QALYs assuming all relapses were severe (0.0755) or mild (0.01575), which increased or decreased the weight of relapses relative to disability status in QALY outcomes, respectively. This sensitivity analysis represents scenarios where an individual perceives relapses as more/less severe or places more/less value on relapse prevention. Relapse is an important outcome, but, implicitly, relapses are weighted less than disability status in QALYs because relapses are infrequent events.
We examined heterogeneity by ARR only (subgroups with no differences by disability status) to evaluate the impact of our assumption that relapses and disability co-varied. We investigated the impact of a lower or higher ARR (averaged across subgroups) at age 55 years, with a focus on relapse outcomes. Lastly, we assessed the impact of a longer time horizon on quality-of-life outcomes over the full remaining lifetime. This analysis evaluated lifetime QALYs for DMD continuation or discontinuation from 55 or 70 years of age, up to 100 years of age.
3 Results
3.1 Quality-Adjusted Life-Years
Among simulated adults with an EDSS score below 6 and a 5-year relapse-free interval, DMD treatment continuation yielded more QALYs than discontinuation in both age cohorts, prior to consideration of treatment-related disutility (Table 2). However, the QALY gains of continuation over discontinuation decreased with patient age. At age 55 years, the projected benefit of DMDs ranged from 0.007 QALYs for fingolimod to 0.017 QALYs for natalizumab, while at age 70 years, these QALY gains were only 0.002 and 0.006 QALYs for fingolimod and natalizumab, respectively. Continuation with interferon-β yielded QALY gains similar to those of fingolimod (0.008 QALYs at age 55 years; 0.002 QALYs at age 70 years) due to having similar effectiveness. Natalizumab had the largest benefit regardless of age, due to its greater effectiveness in preventing relapses and disability compared with interferon-β and fingolimod (Table 1) [20].
3.2 Relapses and Disability
Among those who discontinued DMDs, relapses were lower at age 70 years than age 55 years, and, accordingly, we found a larger benefit for DMD continuation at age 55 years compared with age 70 years for relapse outcomes (Table 2). For those who continued a DMD, we found a 3.3–5.8 percentage point reduction in the percentage of people with a relapse within 10 years for natalizumab, 2.3–3.5 for fingolimod, and 1.0–1.1 for interferon-β. DMD continuation resulted in a modest reduction in the percentage of people who reached EDSS 6 within 10 years (0.4–1.6 points at age 55 years and 0.0–0.5 at age 70 years) (Table 2).
3.3 Decision Threshold
3.3.1 DMD Benefit versus Burden
For older adults who perceive reduced health-related quality of life when taking a DMD, the quality-of-life losses from the drug burden may outweigh the benefits derived from the DMD effectiveness. We found that if a person was willing to give up less than one quality-adjusted life-day per year (0.3–0.6 at age 55 years and 0.1–0.2 at 70 years) to avoid the DMD burden and expense, then discontinuation of the DMD would be recommended (Table 3). These annual threshold values are smaller than the estimated disutility for DMDs (47–74 quality-adjusted life-days).
Estimates for the disutility of interferon-β incorporated minor adverse events such as soreness or infection around the injection site; disutilities for fingolimod and natalizumab were based on treatment administration only and did not include additional burdens related to adverse events such as serious infections [33, 41, 42].
3.4 Sensitivity Analyses
Examining higher or lower values for the relapse disutility produced few to no differences from the main analysis, particularly at age 70 years (electronic supplementary Table 2). Similarly, a range of different utility weights for disability status yielded small differences compared with the main analysis (electronic supplementary Table 3). Results were similar to the main analysis when examining heterogeneity by ARR only (subgroups with no differences by disability status) and a lower or higher ARR (electronic supplementary Table 4). Assuming a lower or higher ARR, the reduction in the percentage of people with a relapse (0.2–3.3 and 0.7–7.3 percentage points, respectively) was marginally lower or higher than the main analysis. Extending the duration of DMD continuation or discontinuation from 10 years to the full lifetime (45 years at age 55 years, and 30 years at age 70 years) produced annual QALY gains that were similar to the main analysis and very low compared with typical treatment burdens (0.3–0.7 quality-adjusted life-day per year at age 55 years, and 0.1–0.2 at age 70 years).
4 Discussion
In this simulation study of three different DMDs commonly used to treat people with MS, we found that the benefit of continuing DMDs among older adults was 0.007–0.017 QALYs at age 55 years, and 0.002–0.006 QALYs at age 70 years (Table 2). Despite the low gain in QALYs for DMD continuation, our results generally showed a reduction in the percentage of individuals with a relapse or reaching EDSS 6 (‘requires a cane to walk’) over 10 years. Whether these potential benefits are worth the burden of continuing to take a DMD will depend on an individual’s preferences, clinical history, and previous MS disease activity, including relapses and disability progression. The treatment disutilities (depending on personal preferences) that would result in no benefit associated with DMD continuation were small compared with average patient preferences reported in the literature (Table 3) [33, 41, 42]. Our work highlights the importance of gaining a better understanding of aging with relapsing-onset MS.
For people with relapsing-onset MS, along with their clinicians, weighing the perceived treatment burden with uncertain benefits may require a challenging conversation or series of conversations. People may be unaware of age-related factors in the risks versus benefits of DMDs and some may have a strong preference for or against taking a specific DMD. Individuals have different preferences and perceptions of treatment burdens [33, 41, 42]. For example, for interferon-β, the 25th–75th percentile of estimated disutilities ranged from 0.230 (84 quality-adjusted life-days per year) to 0.001–0.003 (0.4–1.1 quality-adjusted life-days per year). Patient preferences are generally affected by convenience of drug delivery, perceptions of potential benefits, and risks of adverse events [42,43,44], as well as health insurance coverage and out-of-pocket cost. Even for people who are currently able to afford their treatment, uncertainty about changes in coverage, cost, and income may add to the burden of taking expensive drugs such as DMDs. In the US, drug costs are typically higher than in other countries, and DMDs can cost upwards of $US60,000 per person per year [7].
Broadly, our study contributes to the growing literature suggesting a lack of DMD benefits for older adults with MS. For example, the results of a regression model extending findings from a meta-analysis indicated no DMD benefit for the average person with MS over 53 years of age [45]. However, few people aged in their 50 s or older were included in the clinical studies that were included in the meta-analysis, and, clinically, DMDs are generally believed to offer some benefits for MS patients who continue to have disease activity such as relapses. We assumed DMD benefits persisted with increasing age in proportion to ARR decreases with age and still found very low benefits for continuing DMDs at 55 or 70 years of age. Previous studies in MS using Markov models to project long-term outcomes did not incorporate a decrease in relapses with age or examine stopping a DMD among older adults [33, 34, 46,47,48]. Thus, these previous models may have overestimated the benefits of DMDs for older adults by not accounting for the change in relapses with age. Observational studies have shown that people who discontinue DMDs when they are older are less likely to relapse compared with those who discontinue at a younger age [14, 16,17,18, 49]; however, these studies have been limited by sample size and duration of follow-up.
Our study has limitations, in part because it is inherently challenging to model aging with MS; the literature is sparse and we had to make several key assumptions. We could have overestimated the benefits of the DMDs if relapses eventually stop or ‘burn out’ [23, 50] rather than being a continuous linear decline as assumed in our model. Based on available data, we used relapses and disability status to calculate QALYs. It would be of value for future studies to include other outcomes that are important to people with MS, such as cognitive function or employment status. These outcomes are also lacking in, for example, phase III clinical trials.
Our study has several limitations in estimating treatment effectiveness. Age-related changes in the disease pathology of relapsing-onset MS or accrual of comorbidities may affect an older adult’s response to a DMD or the risks associated with DMDs. We linked DMD effectiveness for disability worsening to disease activity based on the ARR. In addition, our model does not include comorbidities, JC viral status, or MRI, which is a biomarker of disease activity. Disability may accrue with or without the presence of a clinical relapse, possibly via distinct pathological processes, which may occur simultaneously [51]. Current evidence did not allow us to separately quantify treatment effectiveness based on inflammatory versus degenerative pathology or other biomarkers.
We used estimates for DMD efficacy based on the results of a network meta-analysis that ranked DMDs [20], however such meta-analyses in MS have produced varying results and rankings [52, 53]. Thus, small differences in study outcomes may not be clinically meaningful. We modeled three different DMDs, which represent a subset of those currently available. The use of these DMDs will also differ across jurisdictions. Essentially, our study encompasses scenarios of first- and second-generation DMDs with varying degrees of effectiveness, different modes of treatment administration, and both biologic and small molecule agents. Future work could incorporate estimates, such as relapse rates or treatment effectiveness, based on newly completed studies of older adults with relapsing-onset MS. This would allow the projection of long-term outcomes with updated or refined disease- and treatment-related assumptions, or comparison of a different set of DMDs.
Compared with first-generation DMDs (e.g. interferon-β and glatiramer acetate), the newer, second-generation drugs (e.g. fingolimod and natalizumab) have a higher risk of severe adverse events [3,4,5, 54]. For example, natalizumab increases the risk of progressive multifocal leukoencephalopathy (PML), and cases of this potentially fatal viral disease have also been reported with the use of fingolimod [55, 56]. Among people with MS who were taking natalizumab and who contracted PML, approximately one in four died [55]. Screening for anti-JC virus antibodies reduces the risk of PML [4], however the risk of fatal infections may be perceived as a significant treatment burden.
Allowing for switching between DMDs was beyond the scope of our model, as was examining the treatment history (e.g. early treatment strategies) prior to the decision point at age 55 or 70 years. We assumed a person with MS who continued a DMD remained on the drug for 10 years, which may not always be applicable. For a treatment period of < 10 years, benefits may be proportionally reduced. Although switching between DMDs could be approximated by weighting the proportion of time on each drug, a detailed analysis of switching would need to account for possible patient burdens and harms related to medication changes.
Data were limited for quantifying the potential adverse events of DMD continuation or discontinuation and we did not examine the potential for ‘rebound’ disease activity after discontinuation (i.e. where the person can experience an increase in relapse rates and MRI lesions immediately after stopping a drug), which has been reported for natalizumab and fingolimod [57, 58]. Our study focused on the choice faced by clinically stable older adults with relapsing-onset MS who could potentially choose to either continue or discontinue DMDs. We did not specifically address reasons for discontinuation such as perceived lack of effectiveness, drug intolerance, or a change in JC virus status. The complexity of discontinuation decisions implies that an understanding of how the trajectory of relapsing-onset MS changes with age is important for people of all ages with this disease, so that they can make informed decisions.
Additional support for people with MS to enable their personal preferences to guide DMD discontinuation decisions would be valuable. These could include clinical decision aids as well as allowing for patient preferences in clinical guidelines and drug reimbursement policies. Policies and tools that assist older adults in weighing the treatment burden and risks they experience against the benefits of DMD continuation may improve the quality of life for people with relapsing-onset MS.
5 Conclusion
The MS literature has emphasized the importance of early initiation of DMDs among younger adults and treatment adherence, but less attention has been given to examining how age-related changes in DMD efficacy and effectiveness pertain to the treatment of older adults [6]. Although further direct clinical evidence is needed [20, 54], our study illustrates that the benefits of DMDs for older adults with relapsing-onset MS may be limited and outweighed by the burden of taking DMDs. Deprescribing medication can be a controversial topic [54, 59, 60]. Our work emphasizes that the decision regarding whether to discontinue a DMD should factor in patient preferences. Further research with a specific focus on older adults with MS will be invaluable for optimizing MS care across the lifespan.
References
Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991–2010). J Neurol. 2015;262:2352–63. https://doi.org/10.1007/s00415-015-7842-0.
Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–40. https://doi.org/10.1212/WNL.0000000000007035.
Biogen. Avonex (interferon beta-1a) prescribing information. Cambridge: Biogen; 2005.
Biogen. Tysabri (natalizumab) injection for intravenous use prescribing information. Cambridge: Biogen; 2018.
Novartis Pharmaceuticals Corporation. Gilenya (fingolimod) capsules prescribing information. East Hanover: Novartis; 2015.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90:777–88. https://doi.org/10.1212/WNL.0000000000005347.
Hartung DM, Bourdette DN, Ahmed S, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology. 2015;84:2185–92. https://doi.org/10.1212/WNL.0000000000002095.
Signori A, Schiavetti I, Gallo F, Sormani MP. Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol. 2015;22:960–6. https://doi.org/10.1111/ene.12690.
Palace J, Duddy M, Lawton M, et al. Assessing the long-term effectiveness of interferon-beta and glatiramer acetate in multiple sclerosis: final 10-year results from the UK multiple sclerosis risk-sharing scheme. J Neurol Neurosurg Psychiatry. 2019;90:251–60. https://doi.org/10.1136/jnnp-2018-318360.
Awad A, Sütve O. Multiple sclerosis in the elderly patient. Drugs Aging. 2010;27:283–94. https://doi.org/10.2165/11532120-000000000-00000.
Filippi M, Wolinsky JS, Sormani MP, Comi G. Enhancement frequency decreases with increasing age in relapsing-remitting multiple sclerosis. Neurology. 2001;56:422–3. https://doi.org/10.1212/WNL.56.3.422.
Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79:1368–74. https://doi.org/10.1136/jnnp.2008.145805.
Soldán MMP, Novotna M, Zeid NA, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. 2015;84:81–8. https://doi.org/10.1212/WNL.0000000000001094.
Hua LH, Fan TH, Conway D, et al. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler J. 2019;25(5):699–708. https://doi.org/10.1177/1352458518765656.
Shirani A, Zhao Y, Karim ME, et al. Investigation of heterogeneity in the association between interferon beta and disability progression in multiple sclerosis: an observational study. Eur J Neurol. 2014;21:835–844. https://doi.org/10.1111/ene.12324.
Birnbaum G. Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: experience from a clinical practice. Int J MS Care. 2017;19:11–4. https://doi.org/10.7224/1537-2073.2015-032.
Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis—Clinical outcome and prognostic factors. Mult Scler J. 2017;23(9):1241–8. https://doi.org/10.1177/1352458516675751.
Kister I, Spelman T, Alroughani R, et al. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J Neurol Neurosurg Psychiatry. 2016;87:1133–7. https://doi.org/10.1136/jnnp-2016-313760.
US National Library of Medicine. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). ClinicalTrials.gov Identifier: NCT03073603. 2017. https://clinicaltrials.gov/ct2/show/NCT03073603. Accessed 26 Oct 2018.
Tramacere I, Del Giovane C, Salanti G, et al. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD011381.pub2.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444. https://doi.org/10.1212/WNL.33.11.1444.
Ebers GC, Heigenhauser L, Daumer M, et al. Disability as an outcome in MS clinical trials. Neurology. 2008;71:624–31. https://doi.org/10.1212/01.wnl.0000313034.46883.16.
Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–89. https://doi.org/10.1093/brain/awp070.
Statistics Canada. Life tables, Canada Provinces and Territories: 2000 to 2002 (updated July 2006). 2006. https://www150.statcan.gc.ca/n1/en/pub/84-537-x/2006001/4227757-eng.pdf?st=rJtWeNUj.
Ragonese P, Aridon P, Salemi G, et al. Mortality in multiple sclerosis: a review. Eur J Neurol. 2008;15:123–7. https://doi.org/10.1111/j.1468-1331.2007.02019.x.
Scalfari A, Knappertz V, Cutter G, et al. Mortality in patients with multiple sclerosis. Neurology. 2013;81:184–92. https://doi.org/10.1212/WNL.0b013e31829a3388.
Kingwell E, Leray E, Zhu F, et al. Multiple sclerosis: effect of beta interferon treatment on survival. Brain. 2019;142:1324–33. https://doi.org/10.1093/brain/awz055.
Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology. 2010;74:2004–15. https://doi.org/10.1212/WNL.0b013e3181e3973f.
Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disability. Brain. 2010;133:1914–29. https://doi.org/10.1093/brain/awq118.
Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006;66:172–7. https://doi.org/10.1212/01.wnl.0000194259.90286.fe.
Shirani A, Zhao Y, Karim ME, et al. Association between use of beta-interferon and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2012;308:247–56.
Schwehr N, Kuntz K, Butler M, et al. Age-related decreases in relapses among adults with relapsing-onset multiple sclerosis. Mult Scler J. 2019. https://doi.org/10.1177/1352458519866613(epub 29 Jul 2019).
Prosser L, Kuntz K. Cost effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health. 2004;7:554–68.
Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm. 2007;13:245–61. https://doi.org/10.18553/jmcp.2007.13.3.245.
Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528–32. https://doi.org/10.1212/01.WNL.0000096175.39831.21.
Hirst C, Ingram G, Pearson O, et al. Contribution of relapses to disability in multiple sclerosis. J Neurol. 2008;255:280–7. https://doi.org/10.1007/s00415-008-0743-8.
Vercellino M, Romagnolo A, Mattioda A, et al. Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand. 2009;119:126–30.
Kapoor R, Ho PR, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17:405–15. https://doi.org/10.1016/S1474-4422(18)30069-3.
Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol. 2009;256:405–15. https://doi.org/10.1007/s00415-009-0093-1.
Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006;66:1696–702. https://doi.org/10.1212/01.wnl.0000218309.01322.5c.
Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Patient and community preferences for treatments and health states in multiple sclerosis. Mult Scler. 2003;9:311–9. https://doi.org/10.1191/1352458503ms903oa.
Matza LS, Deger KA, Vo P, et al. Health state utilities associated with attributes of migraine preventive treatments based on patient and general population preferences. Qual Life Res. 2019;28(9):2359–72. https://doi.org/10.1007/s11136-019-02163-3.
Brennan VK, Dixon S. Incorporating process utility into quality adjusted life years: a systematic review of empirical studies. Pharmacoeconomics. 2013;31:677–91. https://doi.org/10.1007/s40273-013-0066-1.
Kremer IEH, Evers SMAA, Jongen PJ, Hiligsmann M. Comparison of preferences of healthcare professionals and MS patients for attributes of disease-modifying drugs: a best-worst scaling. Health Expect. 2018;21(1):171–80. https://doi.org/10.1111/hex.12599.
Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577. https://doi.org/10.3389/fneur.2017.00577.
Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Br Med J. 2003;326:522–5. https://doi.org/10.1136/bmj.326.7388.522.
Sanchez-De La Rosa R, Sabater E, Casado M, Arroyo R. Cost-effectiveness analysis of disease modifiying drugs (interferons and glatiramer acetate) as first line treatments in remitting-relapsing multiple sclerosis patients. J Med Econ. 2012;15:424–433. https://doi.org/10.3111/13696998.2012.654868.
Bin Sawad A, Seoane-Vazquez E, Rodriguez-Monguio R, Turkistani F. Cost-effectiveness of different strategies for treatment relapsing-remitting multiple sclerosis. J Comp Eff Res. 2017;6:97–108.
Bonenfant J, Bajeux E, Deburghgraeve V, et al. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol. 2017;24(2):237–44. https://doi.org/10.1111/ene.13181.
Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–93. https://doi.org/10.1016/S1474-4422(14)70256-X.
Cree BAC, Hollenbach JA, Bove R, et al. Silent progression in disease activity–free relapsing multiple sclerosis. Ann Neurol. 2019;85:653–66. https://doi.org/10.1002/ana.25463.
Claflin SB, Broadley S, Taylor BV. The effect of disease modifying therapies on disability progression in multiple sclerosis: a systematic overview of meta-analyses. Front Neurol. 2019;9:1150. https://doi.org/10.3389/fneur.2018.01150.
Fogarty E, Schmitz S, Tubridy N, et al. Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: systematic review and network meta-analysis. Mult Scler Relat Disord. 2016;9:23–30. https://doi.org/10.1016/j.msard.2016.06.001.
Butler M, Forte ML, Schwehr N, et al Decisional dilemmas in discontinuing prolonged disease-modifying treatment for multiple sclerosis. Report no.: 15-EHC012-EF. Rockville: Agency for Healthcare Research and Quality; 2015.
Tan I, McArthur J, Clifford D, et al. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology. 2011;77(11):1061–7.
Khatri B. Fingolimod in the treatment of relapsing–remitting multiple sclerosis: long-term experience and an update on the clinical evidence. Ther Adv Neurol Disord. 2016;9:130–47.
Hatcher SE, Waubant E, Nourbakhsh B, et al. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73:790–4. https://doi.org/10.1001/jamaneurol.2016.0826.
Clerico M, Artusi CA, Di Liberto A, et al. Natalizumab in multiple sclerosis: long-term management. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18050940.
Kister I. Disease-modifying therapies can be safely discontinued in an individual with stable relapsing-remitting MS—Yes. Mult Scler J. 2017;23:1188–90.
Tobin WO, Weinshenker BG. Disease-modifying therapies can be safely discontinued in an individual with stable relapsing-remitting MS—NO. Mult Scler J. 2017;23:1190–2. https://doi.org/10.1177/1352458517709957.
Kingwell E, van der Kop M, Zhao Y, et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia. Canada. J Neurol Neurosurg Psychiatry. 2012;83:61–6. https://doi.org/10.1136/jnnp-2011-300616.
Acknowledgements
The authors gratefully acknowledge the BCMS clinic neurologists who contributed to the study through patient examination and data collection (current members at the time of data extraction are listed here, by primary clinic): University of British Columbia (UBC) MS Clinic: A. Traboulsee, MD, FRCPC (UBC Hospital MS Clinic Director and Head of the UBC MS Programs); A-L. Sayao, MD, FRCPC; V. Devonshire, MD, FRCPC; S. Hashimoto, MD, FRCPC (UBC and Victoria MS Clinics); J. Hooge, MD, FRCPC (UBC and Prince George MS Clinic); L. Kastrukoff, MD, FRCPC (UBC and Prince George MS Clinic); J. Oger, MD, FRCPC. Kelowna MS Clinic: D. Adams, MD, FRCPC; D. Craig, MD, FRCPC; S. Meckling, MD, FRCPC. Prince George MS Clinic: L. Daly, MD, FRCPC. Victoria MS Clinic: O. Hrebicek, MD, FRCPC; D. Parton, MD, FRCPC; K. Atwell-Pope, MD, FRCPC. The authors also thank Tom Duggan, Feng Zhu, and the Pharmacoepidemiology in MS Research Group (https://epims.med.ubc.ca/) for research support at UBC. We are indebted to all of the people with MS who contributed the data used in this study. The views expressed in this study do not necessarily reflect the views of each individual acknowledged.
The BeAMS Study Group (the long-term Benefits and Adverse effects of beta-interferon for Multiple Sclerosis): A. Shirani, Y. Zhao, C. Evans, M.L. van der Kop, G. Gustafson, J. Petkau, J. Oger. Role: Facilitated gaining funding applications and creation of the study cohort used in the simulation-related analyses in the current manuscript. Funding: Canadian Institutes of Health Research (CIHR) [MOP-93646] and the US National MS Society [#RG 4202-A-2]; 2009-12; Principal Investigator: Helen Tremlett.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Data analyses and modeling were performed by Natalie Schwehr. The first draft of the manuscript was written by Natalie Schwehr and all authors commented on later versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Natalie Schwehr, Karen Kuntz, Eva Enns, Nathan Shippee, Elaine Kingwell, Helen Tremlett, Adam Carpenter, and Mary Butler have no conflicts of interest that are directly relevant to the contents of this article. Eva Enns has received consulting fees from ViiV Healthcare for work unrelated to this manuscript. Helen Tremlett is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. Current research support was received from the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, and the Multiple Sclerosis Scientific Research Foundation. In addition, in the last 5 years, she has received research support from the Multiple Sclerosis Society of Canada (Don Paty Career Development Award); the Michael Smith Foundation for Health Research (Scholar Award), and the UK MS Trust; speaker honoraria and/or travel expenses to attend continuing medical education (CME) conferences from the Consortium of MS Centers (2013, 2018), the National MS Society (2014, 2016, 2018), European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) (2013, 2014, 2015, 2016, 2017, 2018, 2019), Biogen Idec (2014), and American Academy of Neurology (2013, 2014, 2015, 2016, 2019). All speaker honoraria are either declined or donated to an MS charity or to an unrestricted grant for use by Helen Tremlett’s research group. Adam Carpenter has received research funding for participation in clinical trials involving medications (interferon-β, natalizumab) discussed in this manuscript.
Funding
Natalie Schwehr received funding that partially supported this project, i.e. a Thesis Research Travel Grant from the University of Minnesota and 3 months of support as an Advanced Research Program Scholar under the University of Minnesota’s NIH Clinical and Translational Science Award (UL1TR002494). The funding sources played no role in the design, methods, data, or interpretation of the results of the study.
Ethical approval
Ethical approval was gained from the University of Minnesota Institutional Review Board and the UBC Clinical Research Ethics Board.
Data availability
Simulation model parameters were derived from analyses of a patient cohort (access to these data are restricted to on-site in Vancouver, BC, Canada) and published literature. The resulting parameters are presented in the Methods section and in the accompanying tables.
Additional information
Members of “The BeAMS Study group” are listed in the Acknowledgement section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schwehr, N.A., Kuntz, K.M., Enns, E.A. et al. Informing Medication Discontinuation Decisions among Older Adults with Relapsing-Onset Multiple Sclerosis. Drugs Aging 37, 225–235 (2020). https://doi.org/10.1007/s40266-019-00741-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-019-00741-1