Abstract
Background
Older adults living with dementia may have a higher risk of medication toxicity than those without dementia. Optimising prescribing in this group of people is a critically important yet challenging process.
Objective
Our aim was to systematically review the evidence for the effectiveness of interventions for optimising prescribing in older people with dementia.
Methods
This systematic review searched the Pubmed, Embase, CINAHL, PsycINFO and Cochrane Library electronic databases for studies that evaluated relevant interventions. Experimental, quasi-experimental and observational studies published in English prior to August 2018 were included. Data were synthesised at a narrative level.
Results
The 18 studies accepted for review included seven randomised, two nonrandomised controlled, five quasi-experimental and four observational studies. Half the studies were conducted in nursing homes and the other half in hospital and community settings. There was great variability in the interventions and outcomes reported and a meta-analysis was not feasible. The three randomised and four nonrandomised studies examining medication appropriateness all reported improvements on at least one measure of the outcome. Six studies reported on interventions that identified and resolved drug-related problems. The results for other outcomes, including the number of medications (10 studies), healthcare utilisation (7 studies), mortality (7 studies), quality of life (3 studies) and falls (3 studies), were mixed and difficult to synthesise because of variability in the study design and measures used.
Conclusion
Emerging evidence suggests that interventions in older people with dementia may have positive effects on medication appropriateness and resolution of drug-related problems; however, whether optimisation of medication results in clinically meaningful outcomes remains uncertain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Studies evaluating interventions to optimise prescribing in older people with dementia have used variable study designs, interventions and outcomes. |
Interventions may have some effects on medication appropriateness but effects on clinical and patient-reported outcomes remain uncertain. |
Future well-designed studies reporting on outcomes relevant to patients are needed. |
1 Introduction
Dementia describes a clinical syndrome stemming from a number of underlying conditions that are characterised by progressive deterioration of behavioural and cognitive functioning [1]. Dementia often has a gradual onset and is a progressive, irreversible and life-limiting condition [2, 3]. Approximately 50 million people are living with dementia worldwide, and, every year, approximately 10 million people are newly diagnosed, therefore the number of cases is expected to reach 152 million in 2050 [4]. Dementia has enormous social, economic and health costs that will continue to rise with the ageing population and growing number of people living with dementia [4].
People with dementia commonly have comorbid medical conditions [5,6,7] and over half are taking five or more drugs [7,8,9]. Even when adjusting for sex, age and number of comorbidities, on average they are taking more medications than people without dementia [5]. Nevertheless, there is some evidence that they may be undertreated [6, 10, 11], which may be due to several reasons, such as reduced ability to notice or report symptoms of their disease and medication adverse effects [6]. Potentially inappropriate medication (PIM) use in persons with dementia is underresearched [12], but available studies from different settings and different countries show a high prevalence of PIM use in these patients (range 10.2–63.4%) [5, 9, 13,14,15,16,17,18].
Several reasons make older adults, in particular those living with dementia, more vulnerable to the adverse effects of medications compared with younger adults [19]. Ageing-induced alterations in pharmacokinetics and pharmacodynamics, as well as additional physiological changes in people with dementia, put older people with the disease at a higher risk of medication toxicity [20, 21]. Moreover, the evidence to guide prescribing in older adults is limited and the case is even worse in people with dementia as they have been reported to be excluded from 85% of published clinical studies [22]. Taking these factors into account, together with the complexity of medication regimens, the high prevalence of PIMs and changes in goals of care as the disease progresses in older people with dementia, makes optimising medication prescribing in this group of people a critically important yet challenging process. This process should involve prescribing of beneficial drugs, withdrawing inappropriate medications, and ongoing review of medication appropriateness [2, 21].
Different interventions can be undertaken for the purpose of optimising medication prescribing in older people, including those with dementia. These interventions can work through targeting over- or underprescribing or appropriate monitoring of medications [23, 24]. The evidence for interventions to optimise medication prescribing in older adults across settings has been evaluated through different systematic reviews [19, 25,26,27,28], but, to date, none of these reviews had patients with dementia as their main population of interest. A systematic review that looked at interventions conducted by pharmacists in an inpatient setting to improve appropriate prescribing did not find any studies in dementia patients [19]. A recent systematic review of interventions to improve medication management for dementia patients highlighted that very few randomised controlled trials (RCTs) of this purpose have been conducted that focused on dementia patients [24]. That review included randomised studies of dementia patients of all ages in community setting or care homes. Given the few studies that met these criteria, a broader scope including studies conducted with other designs and in other settings may give a better insight into the current evidence for the effectiveness of interventions aimed at improving prescribing practice in people living with dementia.
Through conducting this systematic review, we aimed to establish the evidence for effectiveness of available interventions for optimising prescribing in older people with dementia in any settings. Specifically, we aimed to describe the interventions used to optimise prescribing and summarise the evidence for these interventions in terms of medication and patient-related outcomes.
2 Methods
2.1 Protocol and Registration
This systematic review was undertaken and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. The protocol of the review was registered with the international prospective register of systematic reviews (PROSPERO; CRD42017073358) [30].
2.2 Eligibility Criteria
2.2.1 Types of Studies
Experimental (RCTs and nonrandomised controlled studies), quasi-experimental (pre-post design) and observational studies (either with or without concurrent controls) were included in the review. Although observational studies were stated to be excluded in the registered protocol [30], the decision was made to include them in the final review to have a better overview of the available interventions.
2.2.2 Participants
Studies of any settings were included if participants were aged 65 years and older (or if the mean age was 65 years or over if the age range was not reported) and the participants had dementia. The studies met the inclusion criteria for the presence of dementia if they fulfilled one or more of the following criteria: 1, at least 50% of their population had a clinical diagnosis of dementia of any type; 2, at least 50% of their population had scores indicating dementia measured by a validated assessment scale (for example Mini–Mental State Examination < 24 [31]); or 3, the mean score of the population measured by a validated assessment scale was suggestive of dementia.
2.2.3 Interventions
This review focuses on interventions that target optimising the whole medication regimen. In the registered protocol [30], we indicated including ‘any’ interventions. However, as a review of interventions that target a specific medication class (namely antipsychotics) has already been published [32], we changed our focus to include interventions that include the total medication regimen. The intervention could involve a single profession or a multidisciplinary team and could be led by physicians, pharmacists, nurses or any other healthcare professionals.
2.2.4 Comparator(s)/Control
For studies that included a control group, comparison could be between the intervention and the study-defined usual care or the same group in before-after studies.
2.2.5 Outcome Measures
The primary outcome of this systematic review was medication appropriateness, measured by validated tools or study-defined criteria. Secondary outcome measures were drug-related problems, number of medications, healthcare utilisation, all-cause mortality, quality of life (using any measure) and falls. Studies were included if they reported on any of these outcomes.
2.3 Information Sources and Search Strategy
The Pubmed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO and Cochrane Library databases were searched from inception to May 2017. The search strategy from a Cochrane systematic review titled “Interventions to optimise prescribing for older people in care homes” [25] was used and adapted to suit the search criteria of this review. A professional librarian assisted with designing the search strategy. Both text words and Medical Subject Heading (MeSH) terms were used in the search strategy to filter publications based on type of intervention (to optimise prescribing) and the population (older people with dementia). The detailed electronic search strategy can be found in Online Resource 1. Google Scholar was searched for grey literature to identify guidelines, reports or conference proceedings that may include relevant information. Reference lists and citations of the relevant articles and reviews were searched in order to identify any additional studies. Only full-length articles or reports of original studies published in English were included. The search was updated in August 2018 to include any relevant studies published since the previous search.
2.4 Study Selection
After removal of duplicates and screening titles, two reviewers (LSH and DL) independently screened the abstracts and then evaluated the eligibility of the full-text articles. Any disagreement was resolved by discussion and, if required, by seeking advice from a third reviewer (NP).
2.5 Data Extraction and Synthesis
Two authors (LSH and DL) independently extracted data from the included studies using a pre-piloted form. The extracted data included author, publication year and country, study design, setting, population (number at baseline and number completed, description, mean age, proportion of female participants, proportion of dementia patients and how dementia was measured), intervention (type, by whom it was delivered, duration, frequency and follow-up period, number in this group), comparison group, if any (description and number in this group), and outcomes measured (measurement, results). Meta-analysis was considered, but if not feasible due to the heterogeneity of the interventions and outcomes reported, the data were synthesised on a narrative level.
2.6 Assessment of Risk of Bias
The risk of bias of the studies was independently assessed by two reviewers (LSH and DL). The following quality assessment tools were used: for the RCTs, the Cochrane Collaboration’s Tool for assessing risk of bias in randomised trials [33]; for nonrandomised controlled studies, the ROBINS-I (Risk Of Bias In Non-randomised Studies - of Interventions) [34]; for quasi-experimental before and after studies, the National Institutes of Health’s Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group [35]; and for the observational studies, the Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [36]. Any disagreement was resolved by discussion and, if required, by seeking advice from a third reviewer (NP).
3 Results
3.1 Study Selection
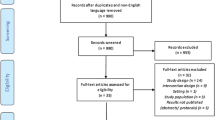
The PRISMA flow diagram showing the process of selecting eligible studies is shown in Fig. 1. A total of 1342 records were identified after removing duplicates, of which 66 studies were found to be suitable for full-text review. Of these, 18 eligible papers were included in the final review [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Updating the search in August 2018 resulted in two additional papers that were reports of a study by Gustafsson et al. [37] already included in the review [55, 56]. The results from these studies were combined with those of the original study. Characteristics of the included studies are summarised in Table 1.
3.2 Study Characteristics
3.2.1 Study Design
Study designs were categorised using the algorithm proposed by Grimes and Schulz [57] and included randomised trials (n = 7, including three RCTs [37, 38, 41], three cluster RCTs [40, 42, 43], and a stepped-wedge, cluster-randomised study [39]), nonrandomised controlled studies (n = 2) [44, 45], quasi-experimental before and after studies (n = 5) [46,47,48,49,50] and observational before and after studies with no control group (n = 4) [51,52,53,54].
3.2.2 Country and Setting
The included studies were from the UK (n = 4) [39, 41, 43, 46], Australia (n = 3) [38, 42, 51], the US (n = 2) [53, 54], Israel (n = 2) [45, 49], Sweden (n = 1) [37], Spain (n = 1) [52], Italy (n = 1) [47], Switzerland (n = 1) [48], Finland (n = 1) [40], Japan (n = 1) [44] and Norway (n = 1) [50]. Half of the studies (9/18) [38,39,40,41,42,43, 46, 50, 51] were conducted in residential care settings and the other half were performed in hospital [37, 45, 47, 48, 52,53,54] and community settings [44, 49] (seven and two, respectively).
3.2.3 Participants
The included studies involved 3047 participants with a mean age ranging from 78.7 [39] to 86.9 years [50]. For the majority of the studies (16/18) [37,38,39,40,41,42,43, 45, 47,48,49,50,51,52,53,54], female participants accounted for more than half of the population. Dementia was defined by reporting clinical diagnosis of dementia in 13 studies [37, 39, 40, 42, 44, 46, 48,49,50,51,52,53,54] and by reporting scores indicating dementia in a validated assessment scale in five studies [38, 41, 43, 45, 47]. Based on 16 studies that reported the number or percentage of people with dementia [37,38,39,40, 42,43,44,45,46, 48,49,50,51,52,53,54], the studies included at least 1925 participants living with dementia. The two remaining studies only reported the mean score on a cognitive test [41, 47]. Eleven studies reported that over 70% of their participants had dementia [37,38,39,40, 43,44,45,46, 52,53,54], with five reporting all their participants had dementia [37, 44, 46, 52, 54].
3.2.4 Interventions
A variety of interventions were evaluated in the studies. Four studies evaluated deprescribing interventions that aimed to manage polypharmacy [38, 44, 45, 49]. Of these four studies, doctors led the intervention in three studies [38, 45, 49] and the fourth study also involved a pharmacist [44]. Clinical medication review by pharmacists was the main part of the intervention in three studies [37, 41, 43]. Three studies involved multidisciplinary teams [42, 50, 52], two looked at nurse-led medication monitoring interventions aimed at minimising adverse drug reactions [39, 46], two evaluated geriatric assessment and consultation conducted by geriatricians [51, 53], two evaluated interdisciplinary geriatric and psychiatric care in hospital [48, 54], one evaluated an educational intervention, i.e. training of nursing staff in a residential care setting [40], and one study evaluated the use of a computerised prescription support system [47].
3.2.5 Primary Outcome (Medication Appropriateness)
Seven studies evaluated medication appropriateness [37, 40, 42, 47, 48, 51, 54]. In these seven studies, the interventions varied significantly and included training of nursing staff [40], multidisciplinary case conferencing [42] and comprehensive geriatric assessment [51] in the residential care setting; and interdisciplinary geriatric and psychiatric care [48, 54] and medication review, either by clinical pharmacists [37] or using a computerised prescription support system [47], in the hospital setting. We found no relevant studies conducted in the community setting. Assessments of appropriateness reported in four of these studies were measured independently by pharmacists, physicians, nurses or the research team [40, 42, 48, 51]. In one study, the assessment was performed by a computerised prescription support system [47], and, in two studies, the person responsible for the assessment was not clearly stated [37, 54]. The time between the intervention delivery and follow-up outcome measurement varied from immediately after intervention (i.e. post-hospital discharge [37, 47, 48, 54] or after the videoconference by geriatricians [51]) to 3 months [42] and 6 and 12 months [40]. Medication appropriateness was assessed using the Medication Appropriateness Index (MAI) [42], Beers criteria [47, 54], and the French version of the Screening Tool of Older Persons’ Prescriptions (STOPP) and Screening Tool to Alert doctors to Right Treatment (START) criteria [48]. Moreover, two studies used a composite of different criteria (Beers criteria, Anticholinergic Risk Scale, more than two psychotropic medications, nonsteroidal anti-inflammatory drugs and proton pump inhibitors [40]; and Beers, McLeod, Laroche, PRISCUS and the Norwegian General Practice criteria [51]), and one study used a selection of quality indicators defined by the Swedish National Board of Health and Welfare [37].
3.2.6 Other Outcomes
Other outcomes reported in the studies included the number of medications (10 studies) [38, 39, 41,42,43,44, 48, 52,53,54], healthcare utilisation (seven studies) [37, 38, 40, 41, 43, 45, 49], mortality (seven studies) [37, 38, 40, 41, 43, 45, 49], drug-related problems (six studies) [37, 39, 41, 43, 46, 50], quality of life (three studies) [38, 40, 44] and falls (three studies) [38, 41, 43].
3.3 Risk of Bias Within Studies
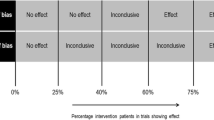
Figure 2 summarises the details of the risk of bias for the seven randomised studies included in the review. The studies were generally rated as having low risk of selection bias, except for two studies that had unclear risk of sequence generation [39] and allocation concealment [39, 43]. Blinding of participants and personnel did not appear to be conducted in any of the studies, however, in our judgement, the outcomes were not affected by this in four studies [37, 38, 41, 42]. The bias due to blinding of outcomes assessment was low for subjective outcomes in four studies [37, 38, 41, 42] and for objective outcomes in all the studies. Attrition bias risk was generally low, but was unclear for one or both types of outcomes in two studies [37, 43]. Reporting bias was adequate in three studies [38,39,40]. Four studies were judged to be at high risk of other bias due to different reasons, such as baseline differences, contamination and small sample sizes [38, 39, 41, 43]. The details of risk of bias assessment for the nonrandomised studies can be found in Tables 2, 3 and 4. The two nonrandomised controlled studies [44, 45] were judged to have serious risk of bias. Four of the five quasi-experimental before and after studies [46, 47, 49, 50] had only fair quality, and, as the observational before and after studies had no control group, they were not rated to have high quality [51,52,53,54].
3.4 Intervention Effects
The effects of interventions on outcomes reported in this paper are described in detail in Table 1. A summary of the findings by intervention type and outcome is presented in Table 5.
3.4.1 Primary Outcome (Medication Appropriateness)
Three randomised studies [37, 40, 42] and four nonrandomised studies [47, 48, 51, 54] reported on medication appropriateness. Change in the mean number of PIMs per participant was reported in four studies, of which one reported a significant reduction over the follow-up period of 12 months in the intervention group (− 0.43, 95% confidence interval [CI] − 0.71 to − 0.15) and no significant change in the control group [40]. The remaining three studies had no control group, but two showed a significant reduction (from 0.5 to 0.1 [47] and from 0.8 to 0.4 [54]) and one showed no significant change in the mean number of PIMs from admission to discharge [48]. Three studies reported a significant reduction in the prevalence of patients taking one or more PIMs, either over the 12-month follow-up period in the intervention group (− 11.7, 95% CI − 20.5 to − 2.9), with no significant change in the control group [40], or from admission to discharge (41.7–11.6% [47] and 77–19% [48]), with no control group. One study showed a significant decrease from admission to discharge in both the intervention (20.3–14.2%) and control (20.7–18.4%) groups [37]; however, this study reported a significantly greater reduction in the total number of PIMs in the intervention group when compared with the control group (numbers not reported) [37]. Only one study measured prevalence of patients with prescribing omissions and reported a significant decrease from admission (65%) to discharge (11%) [48]. In another study, a significantly greater improvement in the MAI was seen in the intervention group (mean change in MAI + 4.1, 95% CI 2.1–6.1) when compared with the control group [42], and one study reported that the intervention led to a change, i.e. stopping or altering, in 19.8% of high-risk medications [51].
3.4.2 Other Outcomes
Number of medications Ten studies, including five randomised [38, 39, 41,42,43] and five nonrandomised studies [44, 48, 52,53,54], reported outcomes related to the number of medications. Of these, three reported a significant reduction in the mean number of medications per patient, either over the 3-month follow-up period in the intervention group (7.1 ± 2.3 to 4.5 ± 2.1), with an increase in the control group (6.0 ± 2.7 to 6.7 ± 2.4) [44], or from admission to discharge (from 7.6 ± 4.1 to 5.9 ± 2.5 [48] and from 7.3 to 4.8 [52]). Another study reported a reduction in the mean number of medications per patient in the intervention group (− 1.9 ± 4.1) over the 12-month follow-up period, which was significantly different from the change in the control group [38]. Other studies reported no effect on the total number of medications [39], no significant difference between the intervention and control groups in their changes in the mean number of medications [43] and no change [41, 42, 54] or an increase in the mean number of medications [53].
Healthcare utilisation Seven studies, including five randomised [37, 38, 40, 41, 43] and two nonrandomised studies [45, 49], reported the effects on healthcare utilisation. Of these seven studies, two reported fewer days in hospital for the intervention group compared with the control group (1.4 days/person/year in the intervention group vs. 2.3 days/person/year in the control group [40], and 0.5 days/patient in the intervention group vs. 1.3 days/patient in the control group [43]), of which only one showed a statistically significant difference in hospital days [40]. One study reported lower 6-month drug-related readmission rates in the intervention group when compared with the control group (19% vs. 23%) [37]. In another study, the intervention group had a significantly lower referral rate to hospitals over the 12-month follow-up period when compared with the control group (12% vs. 30%) [45]. Other studies that reported hospitalisation outcomes either showed no significant effect [38, 41] or reported the numbers with no comparison [49]. No significant differences between the intervention and control groups were reported for general practitioner visits or ambulatory services use in three of four randomised studies that measured these outcomes [38, 40, 41]. The fourth study [43] showed mixed results for different primary care services, but numbers were too small for statistical comparison between the groups.
Mortality Mortality was reported in five randomised [37, 38, 40, 41, 43] and two nonrandomised studies [45, 49]. Four studies [37, 38, 40, 41] did not report significant effects on mortality over their follow-up periods of 6–12 months. Two studies showed a significantly lower number of deaths or mortality rates in the intervention group compared with the control group (4 vs. 14 [43] and 21% vs. 45% [45]). However, in one of these studies, this was only observed during the intervention phase and not over the whole study period [43]. One of the studies reported a 14% mortality rate during the follow-up after the deprescribing intervention, with the causes of death being unrelated to the intervention [49].
Drug-related problems Four randomised [37, 39, 41, 43] and two nonrandomised [46, 50] studies reported on drug-related problems. Four studies reported the total number of drug-related problems identified and the proportion of recommendations being acted upon as a result of interventions with pharmacist-led clinical medication review components (310 recommendations in 212 patients, of which 82% were acted upon [37]; 747 recommendations in 313 residents, of which 58% were acted upon [41]; 261 recommendations in 136 residents, of which 55% were acted upon [43]; and 719 recommendations in 142 residents, of which 65.6% were acted upon [50]). Two studies evaluated a nurse-administered adverse drug reaction profile for mental health medications [39, 46]. One study found that there were significantly more problems being detected and addressed per resident when the profile was applied (problems detected 15.8 ± 5.9; problems addressed 9.9 ± 4.5) compared with not applying the profile (problems detected 7.3 ± 3.2; problems addressed 6.0 ± 2.9) [39]. The other study reported that a total of 17.4 problems per resident were detected and 4.9 problems per resident were addressed, but there was no comparator group [46].
Quality of life Two randomised studies [38, 40] and one nonrandomised [44] study measured changes in quality of life, of which one showed no significant difference between the intervention and control groups [38]. The other study showed significantly slower decline in health-related quality of life in the intervention group compared with the control group (changes in quality of life − 0.038 [95% CI − 0.054 to − 0.022] in the intervention group vs. − 0.072 [95% CI − 0.089 to − 0.055] in the control group) [40]. Quality of life had a slower decline in the intervention group (− 0.03 ± 0.29) of another study reporting it as its primary outcome as well when compared with the control group (− 0.13 ± 0.29) over the 6-month follow-up period (statistical significance not reported) [44].
Falls Three randomised studies reported falls as an outcome [38, 41, 43], of which only one reported a significantly lower number of falls in the intervention group compared with the control group (0.8 ± 1.7 vs. 1.3 ± 3.1) [41].
4 Discussion
This systematic review identified 18 studies, including 7 randomised and 11 nonrandomised studies, evaluating interventions to optimise prescribing in older people with dementia. The majority of the studies were conducted in the residential care setting. All seven studies reporting on medication appropriateness showed some improvements, regardless of the type of intervention. The studies also reported drug-related problems being detected and addressed as a result of interventions. The evidence for the effect of the interventions on other clinical or medication-related outcomes, including the number of medications, quality of life, falls, mortality and healthcare utilisation, was uncertain and difficult to synthesise due to heterogeneity in study designs, outcome definitions and analyses, and inconsistency in reporting of the results.
Other published systematic reviews have explored the effects of interventions to optimise prescribing on medication appropriateness. These reviews concluded that optimising prescribing can result in some improvements in medication appropriateness in the general older population, including all settings [26], in nursing homes [25] and in the community [27]. The current review shows that there is emerging evidence that optimising prescribing can also improve medication appropriateness, specifically in older people living with dementia. The results for clinical outcomes are consistent with other systematic reviews of interventions for optimising prescribing in older adults, which reported conflicting or no evidence of effect of these interventions on patient outcomes [25, 26].
Patients living with dementia have unique needs in relation to medication management. Carers have greater involvement in the management of patients, the progressive nature of dementia results in changes in the goals of a patient’s care during the course of the disease, and patients often have multiple comorbid conditions and tend to be prescribed multiple medications. Interventions to optimise medication prescribing in older people with dementia should specifically target these needs and should also consider the potential barriers to the process [2]. Multidisciplinary interventions that allow for the consideration of patients’ values and preferences, as well as the involvement of carers and general practitioners, may produce the best results [2]. Overall, the findings of this systematic review suggest that the current literature lacks studies of rigorous methodology evaluating interventions to optimise prescribing in older people with dementia. Although the results from the studies identified in this review suggest that these interventions might be effective in reducing inappropriate prescribing, different measures and tools were used to measure this outcome, and four of the seven studies reporting this outcome were nonrandomised trials of generally poor quality. For these reasons, no robust conclusion could be drawn. For some of the other medication and patient-related outcomes, there were very few or underpowered randomised studies, and the overall evidence for these outcomes was weak. With increased efforts in the development of resources to assist in the process of optimising prescribing in older dementia patients in recent years [20, 58], the development of suitable interventions should be the focus of future research. Moreover, these interventions should be evaluated in high-quality, well-designed studies that report on the outcomes relevant to dementia patients and their carers. Development of core outcome sets, similar to those recently developed for optimising prescribing in older residential care residents [59], but specific to older people with dementia, should also be considered in future research.
To the best of our knowledge, this review is the first to collate the evidence for the effectiveness of the interventions to optimise prescribing specifically in older people with dementia. To identify all potentially relevant studies, a comprehensive search strategy and broad inclusion criteria were used. As we aimed to collect the evidence for the effectiveness of those interventions that aim to optimise the whole medication regimen, we did not expect to detect many randomised studies. Therefore, all study designs were included in the review and no study was excluded because of the design or risk of bias.
This review also has limitations. The evaluated interventions varied significantly in type, frequency and duration. Settings, outcomes measured and follow-up duration of the studies were also variable. These differences preclude comparison of the studies, and generalisability of the results remains uncertain. While all studies included people with dementia in their study sample, only five were comprised solely of people with dementia, and none conducted a subgroup analysis on the intervention effect on people with dementia. Therefore, results of intervention effects for people with dementia should be interpreted with caution. We only included English-language studies, therefore language bias may have been introduced. This review included seven randomised studies with variable quality and the nonrandomised studies included in the review were generally judged to be of low quality. These limitations hamper a robust conclusion on the effectiveness of the interventions to optimise prescribing in older people with dementia from the available evidence.
5 Conclusion
This systematic review collates the evidence for the effectiveness of interventions to optimise prescribing in older people living with dementia in any setting. Eighteen studies evaluating eight different types of interventions were included and the effects of interventions on seven different outcomes were reviewed. Variability in the evaluated interventions, the design and quality of the studies, and outcomes reported made it difficult to draw robust conclusions from the available evidence. There is emerging evidence supporting improvement of medication appropriateness, however more research using well-designed trials is required to evaluate the impact of these interventions on outcomes relevant to dementia patients.
References
Guideline Adaptation Committee. Clinical practice guidelines and principles of care for people with dementia. Sydney: Guideline Adaptation Committee; 2016.
Reeve E, Bell SJ, Hilmer SN. Barriers to optimising prescribing and deprescribing in older adults with dementia: a narrative review. Curr Clin Pharmacol. 2015;10(3):168–77.
Page A, Etherton-Beer C, Seubert LJ, Clark V, King S, Clifford RM. Medication use to manage comorbidities for people with dementia: a systematic review. J Pharm Pract Res. 2018;48:356–67.
World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Accessed 3 Jan 2018.
Andersen F, Viitanen M, Halvorsen DS, Straume B, Engstad TA. Co-morbidity and drug treatment in Alzheimer’s disease. A cross sectional study of participants in the Dementia Study in Northern Norway. BMC Geriatr. 2011;11(1):58.
Bunn F, Burn A-M, Goodman C, Rait G, Norton S, Robinson L, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med. 2014;12:192–206.
Clague F, Mercer SW, McLean G, Reynish E, Guthrie B. Comorbidity and polypharmacy in people with dementia: insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing. 2017;46(1):33–9.
Kristensen RU, Norgaard A, Jensen-Dahm C, Gasse C, Wimberley T, Waldemar G. Polypharmacy and potentially inappropriate medication in people with dementia: a nationwide study. J Alzheimers Dis. 2018;63(1):383–94.
Somers M, Rose E, Simmonds D, Whitelaw C, Calver J, Beer C. Quality use of medicines in residential aged care. Aust Fam Phys. 2010;39(6):413.
Kuijpers MA, Van Marum RJ, Egberts AC, Jansen PA. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol. 2008;65(1):130–3.
Tan ECK, Jokanovic N, Koponen MPH, Thomas D, Hilmer SN, Bell SJ. Prevalence of analgesic use and pain in people with and without dementia or cognitive impairment in aged care facilities: a systematic review and meta-analysis. Curr Clin Pharmacol. 2015;10(3):194–203.
Parsons C. Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8(1):31–46.
Lau DT, Mercaldo ND, Harris AT, Trittschuh E, Shega J, Weintraub S. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis Assoc Disord. 2010;24(1):56.
Johnell K. Inappropriate drug use in people with cognitive impairment and dementia: a systematic review. Curr Clin Pharmacol. 2015;10(3):178–84.
Redston MR, Hilmer SN, McLachlan AJ, Clough AJ, Gnjidic D. Prevalence of potentially inappropriate medication use in older inpatients with and without cognitive impairment: a systematic review. J Alzheimers Dis. 2018;61(4):1639–52.
Ramsey CM, Gnjidic D, Agogo GO, Allore H, Moga D. Longitudinal patterns of potentially inappropriate medication use following incident dementia diagnosis. Alzheimers Dement Transl Res Clin Interv. 2018;4:1–10.
Bosboom PR, Alfonso H, Almeida OP, Beer C. Use of potentially harmful medications and health-related quality of life among people with dementia living in residential aged care facilities. Dement Geriatr Cogn Disord Extra. 2012;2(1):361–71.
Renom-Guiteras A, Thurmann PA, Miralles R, Klaassen-Mielke R, Thiem U, Stephan A, et al. Potentially inappropriate medication among people with dementia in eight European countries. Age Ageing. 2018;47(1):68–74.
Walsh KA, O’Riordan D, Kearney PM, Timmons S, Byrne S. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists’ interventions in secondary care. Age Ageing. 2016;45(2):201–9.
Page A, Potter K, Clifford R, McLachlan AJ, Etherton-Beer C. Medication appropriateness tool for co-morbid health conditions in dementia: consensus recommendations from a multidisciplinary expert panel. Intern Med J. 2016;46(10):1189–97.
Reeve E, Trenaman SC, Rockwood K, Hilmer SN. Pharmacokinetic and pharmacodynamic alterations in older people with dementia. Expert Opin Drug Metab Toxicol. 2017;13(6):651–68.
Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–40.
Alldred DP, Raynor DK, Hughes C, Barber N, Chen T, Spoor P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD009095.pub2.
McGrattan M, Ryan C, Barry HE, Hughes CM. Interventions to improve medicines management for people with dementia: a systematic review. Drugs Aging. 2017;34(12):907–16.
Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.CD009095.pub3.
Cooper JA, Cadogan CA, Patterson SM, Kerse N, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5(12):e009235.
Clyne B, Fitzgerald C, Quinlan A, Hardy C, Galvin R, Fahey T, et al. Interventions to address potentially inappropriate prescribing in community-dwelling older adults: a systematic review of randomized controlled trials. J Am Geriatr Soc. 2016;64(6):1210–22.
Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD008165.pub3.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Shafiee Hanjani L, Long D, Peel N, Peeters G, Freeman C, Hubbard R. Interventions to optimise prescribing in older people with dementia. PROSPERO 2017 (CRD42017073358). http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017073358. Accessed 1 Nov 2018.
Hartmaier SL, Sloane PD, Guess HA, Koch GG, Mitchell CM, Phillips CD. Validation of the minimum data set cognitive performance scale: agreement with the mini-mental state examination. J Gerontol A Biol Sci Med Sci. 1995;50(2):M128–33.
Coon JT, Abbott R, Rogers M, Whear R, Pearson S, Lang I, et al. Interventions to reduce inappropriate prescribing of antipsychotic medications in people with dementia resident in care homes: a systematic review. J Am Med Dir Assoc. 2014;15(10):706–18.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
National Institutes of Health. Quality assessment tool for before-after (pre-post) studies with no control group. Systematic evidence reviews and clinical practice guidelines. Washington, DC: National Institutes of Health; 2014.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: University of Ottawa; 2001. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 17 Jan 2018.
Gustafsson M, Sjölander M, Pfister B, Jonsson J, Schneede J, Lövheim H. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: a randomized controlled trial. Eur J Clin Pharmacol. 2017;73:827–35.
Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS One. 2016;11(3):e0149984.
Jordan S, Gabe-Walters ME, Watkins A, Humphreys I, Newson L, Snelgrove S, et al. Nurse-led medicines’ monitoring for patients with dementia in care homes: a pragmatic cohort stepped wedge cluster randomised trial. PLoS ONE. 2015;10(10):e0140203.
Pitkälä KH, Juola A-L, Kautiainen H, Soini H, Finne-Soveri UH, Bell JS, et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc. 2014;15(12):892–8.
Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes—randomised controlled trial. Age Ageing. 2006;35(6):586–91.
Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential aged care: a randomised controlled trial of case conferencing. Age Ageing. 2004;33(6):612–7.
Furniss L, Burns A, Craig SKL, Scobie S, Cooke J, Faragher B. Effects of a pharmacist’s medication review in nursing homes. Randomised controlled trial. Br J Psychiatry. 2000;176:563–7.
Sakakibara M, Igarashi A, Takase Y, Kamei H, Nabeshima T. Effects of prescription drug reduction on quality of life in community-dwelling patients with dementia. J Pharm Pharm Sci. 2015;18(5):705–12.
Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-positive approach for improving drug therapy in disabled elderly people. Isr Med Assoc J. 2007;9(6):430–4.
Jordan S, Gabe M, Newson L, Snelgrove S, Panes G, Picek A, et al. Medication monitoring for people with dementia in care homes: the feasibility and clinical impact of nurse-led monitoring. Sci World J. 2014;2014:843621.
Ghibelli S, Marengoni A, Djade CD, Nobili A, Tettamanti M, Franchi C, et al. Prevention of inappropriate prescribing in hospitalized older patients using a computerized prescription support system (INTERcheck®). Drugs Aging. 2013;30(10):821–8.
Lang PO, Vogt-Ferrier N, Hasso Y, Le Saint L, Dramé M, Zekry D, et al. Interdisciplinary geriatric and psychiatric care reduces potentially inappropriate prescribing in the hospital: interventional study in 150 acutely ill elderly patients with mental and somatic comorbid conditions. J Am Med Dir Assoc. 2012;13(4):406.e1–7.
Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–54.
Halvorsen KH, Ruths S, Granas AG, Viktil KK. Multidisciplinary intervention to identify and resolve drug-related problems in Norwegian nursing homes. Scand J Prim Health Care. 2010;28(2):82–8.
Poudel A, Peel NM, Mitchell CA, Gray LC, Nissen LM, Hubbard RE. Geriatrician interventions on medication prescribing for frail older people in residential aged care facilities. Clin Interv Aging. 2015;10:1043.
Brunet NM, Sevilla-Sánchez D, Novellas JA, Jané CC, Gómez-Batiste X, McIntosh J, et al. Optimizing drug therapy in patients with advanced dementia: a patient-centered approach. Eur Geriatr Med. 2014;5(1):66–71.
Saad M, Harisingani R, Katinas L. Impact of geriatric consultation on the number of medications in hospitalized older patients. Consult Pharm. 2012;27(1):42–8.
Chan VT, Woo BK, Sewell DD, Allen EC, Golshan S, Rice V, et al. Reduction of suboptimal prescribing and clinical outcome for dementia patients in a senior behavioral health inpatient unit. Int Psychogeriatr. 2009;21(1):195–9.
Gustafsson M, Sjolander M, Pfister B, Schneede J, Lovheim H. Effects of pharmacists’ interventions on inappropriate drug use and drug-related readmissions in people with dementia: a secondary analysis of a randomized controlled trial. Pharmacy (Basel). 2018;6(1):E7.
Pfister B, Jonsson J, Gustafsson M. Drug-related problems and medication reviews among old people with dementia. BMC Pharmacol Toxicol. 2017;18(1):52–62.
Grimes DA, Schulz KF. An overview of clinical research: the lay of the land. Lancet. 2002;359(9300):57–61.
Reeve E, Farrell B, Thompson W, Herrmann N, Sketris I, Magin P, et al. Evidence-based clinical practice guideline for deprescribing cholinesterase inhibitors and memantine. Sydney: The University of Sydney; 2018.
Millar AN, Daffu-O’Reilly A, Hughes CM, Alldred DP, Barton G, Bond CM, et al. Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes. Trials. 2017;18(1):175.
Acknowledgements
The authors would like to thank Ms. Christine Dalais (The University of Queensland Library) for her help with the development of the search strategy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Leila Shafiee Hanjani, Duncan Long, Nancye M. Peel, Geeske Peeters, Christopher R. Freeman and Ruth E. Hubbard declare that they have no conflicts of interest.
Funding
Leila Shafiee Hanjani is supported by an Australian Government Research Training Program (RTP), and Geeske Peeters is supported by a fellowship from the Global Brain Health Institute. This work was supported by the National Health and Medical Research Council (NHMRC) Partnership Centre for Dealing with Cognitive and Related Functional Decline in Older People (Grant no. GNT9100000). No other sources of funding were used to assist in the preparation of this article.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shafiee Hanjani, L., Long, D., Peel, N.M. et al. Interventions to Optimise Prescribing in Older People with Dementia: A Systematic Review. Drugs Aging 36, 247–267 (2019). https://doi.org/10.1007/s40266-018-0620-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-018-0620-9