Abstract
Background
There are tools and criteria in the literature aimed at distinguishing between appropriate and inappropriate medicines use. However, many have not been externally validated with regard to patient-related outcomes, potentially limiting their use in clinical practice.
Objectives
The aim of the study was to conduct a systematic review to summarise (1) available prescribing appropriateness assessment tools and criteria, and (2) their associations with patient-related outcomes (external validity).
Methods
A systematic review was conducted using MEDLINE, EMBASE and Informit (Health Collection) databases to screen for articles in English that examined (1) tools to assess the appropriateness of prescribing and (2) associations of tools with patient-related outcomes, published between 2000 and 2016, without any limits placed on the study design, participant age or setting.
Results
After screening 1710 articles, removing duplicates and shortlisting relevant articles, 42 prescribing assessment tools were identified. Out of the 42 tools, 78.6% (n = 33) provided guidance around stopping inappropriate medications, 28.6% (n = 12) around starting appropriate medications, 61.9% (n = 26) were explicit (criteria based) and 31.0% (n = 13) had been externally validated, with hospitalisation being the most commonly used patient-related outcome (n = 9, 21.4%).
Conclusion
The results of this systematic review highlight the need for evidence-based and externally validated tools, which combine the different aspects of medication management to optimise patient-related outcomes. PROSPERO registration number: CRD42017067233.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While there is a range of tools for the assessment of the appropriateness of prescribing in the literature, the results of this systematic review highlight the need for evidence-based tools that combine the various aspects of medication management in order to optimise health outcomes. |
Less than 50% of available tools have been externally validated, limiting their use in clinical practice. It is important to develop tools that are proven to improve common patient-related outcomes such as falls and hospitalisation. |
1 Background
Multimorbidity, defined as the co-existence of two or more chronic health conditions, is common in the older population [1]. The complexity of therapeutic management of multimorbidity for both health professionals and patients or carers is well recognised. Multimorbidity is associated with decreased quality of life, self-rated health, mobility and functional ability as well as increases in hospitalisations, physiological distress, use of healthcare resources, mortality and costs [2,3,4]. Clinical guidelines often suggest the use of multiple medications for the management of a single disease, with limited consideration of comorbidities and concurrent medications [5]. As a result, despite following best practice guidelines, patients are frequently prescribed multiple medications, commonly referred to as polypharmacy, which can interact with each other and their comorbid conditions [6].
Polypharmacy is associated with adverse health outcomes including mortality, falls, adverse drug reactions (ADRs), increased length of stay in hospital and readmission to hospital soon after discharge [7,8,9]. A systematic review from 2014 contained 50 studies in the community setting, of which the majority demonstrated relationships between polypharmacy and a range of outcomes including falls, ADRs, hospitalisation, mortality, functional status and cognition [10]. The risk of adverse effects and harm increases with increasing numbers of medications. Harm can result from a multitude of factors including drug–drug interactions and drug–disease interactions in older patients with multimorbidity. Older patients are at even greater risk of adverse effects due to decreased renal and hepatic function, lower lean body mass and reduced hearing, vision, cognition and mobility [11]. Whilst in many instances the use of multiple medicines or polypharmacy may be clinically appropriate, it is important to identify patients with inappropriate polypharmacy that may place patients at increased risk of adverse events and poor health outcomes.
While there is no consensus definition of polypharmacy, the most commonly encountered definition in the literature is the use of five or more medications [12,13,14,15,16]. This numerical definition of polypharmacy is unable to distinguish between appropriate and inappropriate medication use to provide a meaningful clinical evaluation of the risk of harm in everyday practice. It is important to consider the appropriateness of therapy using a holistic approach of considering concurrent medication classes and comorbidities present, to distinguish between appropriate and inappropriate polypharmacy to identify patients at risk of poor health outcomes.
There are prescribing tools and criteria in the literature aimed at facilitating the identification of appropriate and inappropriate medications, facilitating the deprescribing of potentially inappropriate medications and optimising the use of appropriate therapy. Important considerations in practice during medication review and rationalisation include stopping or minimising use of inappropriate medications, starting or optimising the use of appropriate medications, considering dosing of medications, accounting for the impact of renal function on drug clearance and reviewing any drug–drug or drug–disease interactions [17, 18]. There are tools available in the literature that present a scoring system where a rating or score is provided to indicate the degree of polypharmacy or potential for harm, such as the Drug Burden Index (DBI) [19] and the anticholinergic scales such as the Anticholinergic Risk Scale (ARS) [20] and Anticholinergic Drug Scale (ADS) [21]. On the other hand, there are tools that do not provide a score or rating but a list of criteria for appropriate or inappropriate prescribing such as the Beers criteria [22] and Screening Tool of Older Person’s Prescriptions and Screening Tool to Alert doctors to Right Treatment (STOPP START) criteria [23]. Given the myriad of tools and criteria available, a summary of available tools would be useful for clinicians in practice to understand which characteristics of the medication review and rationalisation processes are included in each of these tools and therefore which tools may be relevant to specific patient scenarios in everyday practice. Additionally, it is unclear whether each of these tools and criteria has been validated to be clinically relevant, in terms of their association with common patient-related outcomes such as falls, hospitalisation and mortality. Some tools and criteria have been studied in great detail, such as the DBI, which has been associated with decline in functional and cognition status, falls, hospitalisation and mortality across populations in the US, UK, Australia, Finland, Netherlands, Canada and New Zealand in the community, nursing home and hospital settings [24]. Other tools have been proposed theoretically, without external validation.
The aims of this study were to address these gaps in the existing literature by conducting a systematic review to summarise the range of tools and criteria to assess appropriateness of medication prescribing in the existing literature and for each of the identified tools and criteria, to determine their association with patient-related outcomes (external validation).
2 Methods
2.1 Data Sources and Search Strategy
2.1.1 Systematic Review 1—Tools and Criteria for Assessing Prescribing Appropriateness
MEDLINE, EMBASE and Informit (Health Collection) databases were searched between the years 2000 and 2016 as these databases are most likely to cover medicine-related topics including tools and criteria for assessment of the appropriateness of prescribing. Databases and search terms were selected after consultation with an academic librarian specialising in health-related database searches.
The following search terms (Medical Subject Headings [MeSH] and keywords) were used in EMBASE (relevant MeSH headings were used in MEDLINE):
‘polypharmacy/’ (MeSH) OR ‘multiple medication*’ (keyword) OR ‘multiple medicine*’ (keyword) OR ‘multiple drug*’ (keyword) OR ‘many medication*’ (keyword) OR ‘many medicine*’ (keyword) OR ‘many drug*’ (keyword) OR ‘potentially inappropriate prescribing’ (keyword) OR ‘potentially inappropriate medication’ (keyword) OR ‘potentially inappropriate medicine’ (keyword) OR ‘potentially inappropriate drug’ (keyword) (for all articles exploring polypharmacy and inappropriate prescribing)
AND
‘tool*’ (keyword) OR ‘criter*’ (keyword to include all words related to the word criteria) OR ‘index’ (keyword) OR ‘clinical assessment tool’ (MeSH) (for all articles exploring tools or criteria).
The following search terms were used in Informit (Health Collection) and applied to all fields including the title and abstract:
‘polypharmacy’ OR ‘multiple medication*’ OR ‘multiple medicine*’ OR ‘multiple drug*’ OR ‘many medication*’ OR ‘many medicine*’ OR ‘many drug*’ OR ‘potentially inappropriate prescribing’ OR ‘potentially inappropriate medication*’ OR ‘potentially inappropriate medicine’ OR ‘potentially inappropriate drug*’
AND
‘tool’ OR ‘criter*’ (to include all words related to the word criteria) OR ‘index’ OR ‘assess*’ (to include all words branching from the word assess such as assessment).
Prescribing of medications can be inappropriate regardless of the number of medications. The probability of inappropriate prescribing, however, increases with increasing number of medications, commonly defined as polypharmacy. We therefore included both ‘polypharmacy’ (and terms such as ‘multiple medication*’ and ‘many medication*’) as well as potentially inappropriate prescribing (and terms such as ‘potentially inappropriate medication*’) to capture the range of tools for the assessment of the appropriateness of prescribing in the literature. Given the various definitions of polypharmacy in the literature, the term ‘polypharmacy’ was not limited to a specific definition in order to include all studies referring to tools that assess appropriateness of polypharmacy.
If articles referred to tools or criteria but were not the original research article describing the formulation and development of the tool, the original article describing the tool was found and included in the review.
2.1.2 Systematic Review 2—Shortlisted Tools and Their Association with Patient-Related Outcomes (External Validation)
MEDLINE, EMBASE and Informit (Health Collection) databases were searched between the years 2000 and 2016 as these databases are most likely to cover medicine-related topics including associations of shortlisted prescribing appropriateness tools and patient-related outcomes. Databases and search terms were selected after consultation with an academic librarian specialising in health-related database searches.
The following search terms (MeSH and keywords) were used for each shortlisted tool in EMBASE (relevant MeSH headings were used in MEDLINE):
‘Name of tool or criteria’ (as a keyword, for example, ‘Beers criteria’)
AND
‘outcome*’ (keyword) OR ‘Treatment Outcome/’ (MeSH) OR ‘Outcome Assessment/’ (MeSH) OR ‘validat*’ (keyword to include all words related to the word validate) to find articles exploring each of the shortlisted tools and their associations with patient-related outcomes.
The following search terms were used in Informit (Health Collection) and applied to all fields including the title and abstract:
‘Name of tool’ (for example, ‘Beers criteria’)
AND
‘outcome’ OR ‘validat*’ (to include all words related to the word validate).
Articles were required to explore any association or probability of each of the shortlisted tools to predict outcomes such as hospitalisation, falls, mortality, ADRs, mobility and cognition. Specific outcomes were not used as search terms to prevent limiting to particular outcomes and to include the range of outcomes that have been studied in the literature. No limits were placed on the location (country), setting or age of participants to include studies conducted across various settings and populations. Exclusion criterion was articles that were duplicates.
2.2 Systematic Reviews 1 and 2
Results were limited to studies in English that were already published or in press. Reference lists of relevant articles and the grey literature were screened to identify other relevant articles. The search strategy was developed in consultation with a librarian specialising in health databases, with a pre-determined protocol developed collaboratively with the authors for search methods and selection of relevant articles. The reporting of this systematic review conforms to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist [25].
2.3 Critical Appraisal (Risk of Bias Assessment)
Once articles were shortlisted for prescribing assessment tools and associations with patient-related outcomes, the Critical Appraisal Skills Programme (CASP) tool was applied to each of those articles for critical appraisal and quality assessment, as shown in Electronic Supplementary Material Appendix S1 [26].
For quality assessment of shortlisted articles for tools and external validation, data items extracted depended on the type of study as guided by the CASP tool. For example, for cohort studies, data items extracted included whether the study addressed a clearly focused issue, if the cohort was recruited in an acceptable way, if the exposure and outcome were accurately measured to minimise bias, if the authors identified and accounted for all important confounders and if the follow up of subjects was complete and for an appropriate duration of time. While the CASP tool presents a list of questions for quality assurance, there are no clear guidelines regarding scoring or grading studies according to answers to those questions. Therefore, the four authors discussed the CASP and decided to score studies out of 8 for cohort and case control studies (a total of 12 questions for cohort studies and 11 questions for case control studies), as answers to questions 7, 8, 11 and 12 (results, precision of results, whether results fit with findings from other studies and implications for practice) in cohort studies and 7, 8 and 11 (results, precision of results and whether results fit with findings of other studies) in case control studies do not affect the quality of the study. It was decided by the authors that a score of 0–4 would be considered as low quality, 5–6 considered as medium quality and 7–8 as high quality for cohort and case control studies graded using the CASP tool. For randomised controlled trials, a score out of 9 was used (total 11 questions), as answers to questions 7 and 8 do not affect the quality of the study. A score of 0–5 would be considered as low quality, 6–7 as medium quality and 8–9 as high quality.
2.4 Study Selection and Data Extraction
Articles were shortlisted according to the inclusion and exclusion criteria. After the initial database search and primary screening of article titles and abstracts, articles were categorised as relevant, irrelevant or unsure. The appropriateness of inclusion of each article classed as relevant or unsure was discussed by all four authors. A pre-defined data extraction template was developed by all authors and then applied to ensure consistent data extraction from each of the identified studies.
Data items extracted for systematic review 1 included whether (1) the tool presented a scoring system for polypharmacy; (2) the Delphi method or expert panel was used in developing the tool; (3) information around stopping inappropriate medications and starting appropriate medications was provided; (4) alternative treatment options were suggested; (5) dosing of medications was considered (this was further divided into whether the tool simply mentioned dosing or whether it was predominantly based on dosing); (6) the impact of renal function on drug clearance was considered; (7) the tool focused on specific drug class(es); (8) the tool considered drug–drug or drug–disease interactions; and (9) the tool was implicit (requiring clinicians to apply the tool to a specific patient scenario) or explicit (criteria-based tool); as these are important considerations for prescribing appropriateness tools and criteria.
Data items extracted for systematic review 2 included the name of the tool or criteria; the outcomes investigated (such as hospitalisation, mortality and decline in cognition); the number of studies showing a positive, negative or no association between the specific tool and patient-related outcome; as well as study characteristics such as location (country), setting and age of participants. Data items extracted for critical appraisal of validation studies have been outlined in Sect. 2.3.
Once the primary data extraction was complete, all authors met and reviewed the content analysis for each of the extracted studies, with data further categorised and summarised in tables.
The PROSPERO registration number for this systematic review (systematic reviews 1 and 2) is CRD42017067233.
3 Results
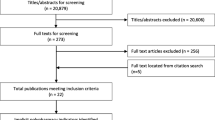
A total of 1710 articles were identified with 42 prescribing appropriateness tools and criteria meeting the inclusion criteria for systematic review 1 [11, 19,20,21,22,23, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. Figure 1 shows a flowchart of study selection according to the PRISMA checklist for systematic review 1.
3.1 Systematic Review 1—Tools and Criteria for Assessing Prescribing Appropriateness
Table 1 presents a summary of various prescribing appropriateness tools in the existing literature and characteristics of each of these tools such as providing guidance around stopping inappropriate medications, starting appropriate medications and drug dosing. Table 2 shows a breakdown of each of the different tools and specific characteristics. Tools were divided into two broad categories: (1) tools with a scoring system where a rating or score is provided (n = 9, 21.4%) [19,20,21, 42, 58,59,60,61,62] and (2) tools that do not provide a score or rating but a list of criteria for appropriate or inappropriate prescribing (n = 33, 78.6%) [11, 22, 23, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Tools that provide a scoring or rating system, allowing users to evaluate the level of appropriateness or inappropriateness of prescribing, include the DBI, ADS, ARS and the Medication Appropriateness Index (MAI) [19,20,21, 58]. Out of all tools providing a score, the Anticholinergic Cognitive Burden Scale was the only tool to provide guidance around interpretation of scores [61]. It states the need for clinical intervention at a score ≥ 3. This type of guidance is not available for other tools with a scoring system. These tools with a scoring system do not provide direct guidance around stopping specific inappropriate medications and starting appropriate therapy but quantify the burden of polypharmacy. On the other hand, commonly used tools that do not provide a scoring system do provide criteria for appropriate and/or inappropriate prescribing. For example, while the Beers criteria 2015 does not provide a scoring system, it identifies potentially inappropriate medication (PIM) use in the elderly, interactions of drugs with other drugs, diseases/syndromes, drugs to be used with caution in the elderly, drugs requiring dose adjustment in renal impairment and drugs with strong anticholinergic properties [22]. Additionally, the Beers criteria 2015 state the quality of evidence and strength of recommendations that are important considerations in evidence-based practice.
Out of the 42 shortlisted tools, 61.9% (n = 26) were explicit or criteria based [22, 23, 27,28,29,30,31, 33,34,35,36,37,38,39,40,41, 44, 49,50,51,52,53,54,55,56,57], with the remaining being implicit tools that require input from clinicians depending on the given patient scenario (n = 16, 38.1%) [11, 19,20,21, 32, 42, 43, 45,46,47,48, 58,59,60,61,62]. All tools with a scoring system were implicit. Additionally, seven tools without a scoring system were implicit, namely the Assess, Review, Minimize, Optimize, Reassess (ARMOR) tool, Tool to Improve Medications in the Elderly via Review (TIMER), Individualized Medication Assessment and Planning (iMAP) tool, Prescribing Optimization Method (POM) tool, Hyperpharmacotherapy Assessment Tool (HAT), Need and indication, Open questions, Tests and monitoring, Evidence and guidelines, Adverse events, Risk reduction or prevention, Simplification and switches (NO TEARS) tool and Tool to Reduce Inappropriate Medications (TRIM).
While deprescribing inappropriate therapy and optimising appropriate therapy are both important aspects of medication management, 33 tools (78.6%) provided guidance around stopping inappropriate medications [11, 22, 23, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45, 47,48,49,50,51,52,53,54,55,56,57] and only 12 tools (28.6%) provided guidance around starting appropriate medications [23, 27, 32, 37, 40, 44,45,46, 48, 50, 53, 55]. Out of the 42 tools and criteria, 11 tools (26.2%) suggested safer alternative treatment options to replace inappropriate therapy [28, 31, 36, 37, 39,40,41, 49, 52, 56, 57]. Whilst the dose of a medication is an important consideration in clinical practice, only 64.3% (n = 27) of tools considered drug dosing. The degree to which each tool considered dosing varied significantly between the different tools. Out of the 27 tools that considered dosing, only the DBI was predominantly based on dosing. In the DBI, the exact doses of anticholinergic and sedative medications for a specific patient are considered in order to provide a score [19]. Other tools simply mentioned appropriate dose of one or more selected medications, such as the Beers criteria [22]. Similarly, the impact of renal function on drug clearance is a significant consideration in practice but less than half of the tools (n = 19, 45.2%) took this into consideration.
There are tools that focus on specific drug class(es) (n = 4, 9.5%) and these are predominantly limited to tools with a scoring system. These tools, namely the DBI, ADS, ARS and Anticholinergic Cognitive Burden Scale focus on anticholinergics only (the DBI additionally focuses on sedatives) [19,20,21, 61].
3.2 Systematic Review 2—Shortlisted Tools and Their Association with Patient-Related Outcomes (External Validation)
A summary of associations of shortlisted prescribing tools and criteria with patient-related outcomes (external validation) is shown in Table 3. There were nine patient-related outcomes that were investigated for the different tools: hospitalisation, mortality, falls, cognitive decline, functional decline, ADRs, decline in quality of life, discharging home after hospitalisation and renal failure. Measures used for hospitalisation included admission to hospital, readmission to hospital after 30 days or 12 months, length of stay in hospital and drug-related hospitalisations. Studies measured mortality as death (regardless of setting) or in-hospital mortality. Falls were measured as the occurrence of falls in hospital, occurrence of falls regardless of setting or recurrent falls regardless of setting (two or more falls in the previous year). Studies measured cognitive decline using the Mini Mental State Examination, Abbreviated Mental Test or Short Blessed Test. Functional decline included measurement of physical function using the Barthel Index, Instrumental Activities of Daily Living, the Short Performance Physical Battery, dynamic balance tests using coordinated stability tasks, Frailty Deficit Index, grip weakness and the Cardiovascular Health Study criteria. ADRs were defined as adverse effects of medications for a specified duration of time such as 30 days or no specified duration of therapy. Decline in quality of life was measured using the EuroQol Group 5 dimensions (EQ-5D) and EuroQol Visual Analog Scale (EQ VAS) instruments, which measure health effects on the quality of life of a person.
Less than half (n = 14, 33.3%) of the 42 shortlisted prescribing tools and criteria have been investigated for association with at least one patient-related outcome, in 53 separate studies conducted across different countries, age groups and settings as shown in Table 4 [20, 63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114]. The majority of studies investigating associations between tools and outcomes used cohort study designs (n = 46) [20, 63, 65, 66, 68,69,70,71,72, 74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89, 93, 94, 96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114], with the remaining studies being randomised controlled trials (n = 2) [67, 92] and case control studies (n = 5) [64, 73, 90, 91, 95].
Of the 14 tools that were investigated with regard to association with outcomes, 13 (92.9% of all tools explored external validation and 31.0% of all shortlisted tools) were positively associated with one or more patient-related outcome [19,20,21,22,23, 50, 55, 56, 58,59,60,61,62]. None of the studies showed a negative association between the tools and outcomes. Out of the 14 tools that were investigated, the majority were tools with a scoring system (n = 8, 57.1%), for example the DBI and ARS. Validation studies for prescribing assessment tools were most commonly conducted in Europe (n = 18, 34.0%), across Belgium, Finland, France, Germany, Italy, Netherlands, Norway, Austria and Sweden in the community, nursing home and hospital settings in various age groups, with the youngest patients being 60 years or older in a study conducted in Germany to validate the Fit fOR The Aged (FORTA) tool. The remaining validation studies were conducted in the US, Australia, UK and Israel.
Of the 14 tools that explored external validation, the DBI, ARS, Beers criteria (1991, 1997, 2003 and 2012), STOPP START and ADS were studied most extensively. The Beers criteria, STOPP START, ARS and DBI were associated with the highest number of patient-related outcomes (six outcomes for the Beers criteria, STOPP START and ARS and five outcomes for DBI), with all tools having been associated with hospitalisation, mortality, falls and functional decline.
Out of the nine patient-related outcomes that were investigated, nine tools (21.4%) were associated with hospitalisation, making this the most commonly reported outcome. The next most commonly reported outcomes were mortality (n = 7, 16.7%) and functional decline (n = 7, 16.7%). The least commonly reported outcomes were discharge home after hospitalisation (n = 1, 2.4%, namely the Medication Regimen Complexity Index [MRCI]) and renal failure (n = 1, 2.4%, namely the FORTA).
4 Discussion
There are many tools to assess the appropriateness of prescribing in the existing literature, which cover different aspects of the medication review and rationalisation processes. This was the first systematic review to summarise the range of tools and criteria available in the literature and assess the associations of each of these tools with patient-related outcomes.
Out of the 42 shortlisted tools, nine provided a scoring system regarding the degree of polypharmacy and the potential for harm as a result. The remaining 33 tools provided a list of criteria for appropriate or inappropriate prescribing. None of the tools provided both a rating regarding the level of polypharmacy and guidance around stopping inappropriate medications and starting appropriate medications, limiting their clinical applicability for informing clinicians in practice and facilitating the quality use of medicines.
While deprescribing inappropriate therapy and optimising appropriate therapy are both important aspects of medication management, 33 tools (78.6%) provided guidance around stopping inappropriate medications and only 12 tools (28.6%) provided guidance around starting appropriate medications, limiting the clinical relevance of the remaining tools in terms of the medication optimisation process. Often in practice, part of this process involves choosing a safer alternative medication in order to minimise harm and optimise health outcomes. Only 26.2% (n = 11) of tools, however, suggested alternative treatment options. Out of the 42 shortlisted tools, 61.9% (n = 26) were explicit (criteria based), with the remaining being implicit tools that require input from clinicians depending on the given patient scenario (n = 16, 38.1%). Similarly, a systematic review of prescribing appropriateness criteria included 46 tools (excluding tools that focus on specific drug classes), 61% of which were explicit criteria [115]. The study concluded that none of the currently available tools combine the various aspects of inappropriate prescribing, with underprescribing being mentioned by only 13.0% of the 46 tools included, despite the fact that underprescribing is an important aspect of inappropriate prescribing.
Whilst the dose of a medication is an important consideration in clinical practice, only 64.3% (n = 27) of tools considered drug dosing. Only one tool (the DBI) takes into account specific doses for a given patient to provide a score and only considers the doses of anticholinergic and sedative medicines when calculating the score. The remaining tools either simply mentioned appropriate dose of one or more selected medications or did not consider dosing at all, making these tools less clinically relevant, given dosing is an important consideration in assessing safety, efficacy and side effects of medications. Similarly, accounting for the impact of renal function on drug clearance is a significant consideration in practice, especially in multimorbid geriatric patients with compromised kidney function who may be at increased risk of harm from the use of some medication classes and at specific doses. Less than 50% of tools, however, took this into consideration.
Out of the 42 shortlisted tools, 9.5% focused on specific drug classes (anticholinergic agents and/or sedatives only). These are medication classes associated with harm and it is important to consider these when assessing medication management in the geriatric setting. Tools that focus on specific drug classes only are likely to be less relevant in clinical practice, as patients are often prescribed a range of medication classes not limited to specific classes such as anticholinergics and sedatives, making these tools less generalisable across the wider patient population.
Examples of specific tools and their limitations include the MRCI, which is appropriate for evaluating the medication administration burden for patients but does not in any way account for the pharmacology of medications and cumulative risks and side effects of combinations of specific medications or classes of medications [59]. The DBI focuses on anticholinergics and sedatives only but anticholinergics and sedatives are not clearly defined in the original article. Additionally, the mathematical formula for calculating the DBI requires the minimum effective daily dose of the drug, which may not be clearly defined for certain medications and can vary according to recommendations from one country to another [116]. There are tools that were developed for specific populations only, such as the tool by Chang et al. that is limited to the Taiwanese population and the PRISCUS list that is limited to the German population, that have not yet been validated in other healthcare settings [56, 57]. Other criteria such as the Healthcare Effectiveness Data and Information Set (HEDIS) 2016 and the NO TEARS tool include a list of statements such as ‘Adherence to Antipsychotic Medications for Individuals With Schizophrenia’ in the HEDIS, with an attachment containing a list of antipsychotic medications but no guidance around how to assess this, making these criteria less useful for clinicians in practice [43, 50].
Out of the 42 shortlisted tools, external validation has been explored for only 33.3% (n = 14) of the tools, with 31.0% (n = 13) having been validated in terms of at least one patient-related outcome. Out of these 13 tools that have been externally validated, the majority were tools with a scoring system (8/13 = 61.5%), which helps clinicians quantify the risk of harm. A recent study argues that risk stratification is a key component of assessing appropriateness of therapy and therefore having a scoring system can help quantify the burden of polypharmacy [117]. The systematic review by Kaufmann and others in 2014 found only 17.9% of tools (n = 8) to be clinically validated with regard to outcomes in the literature [115]. The current systematic review provides an update to this study and includes various other studies exploring associations with clinical outcomes conducted after 2014 such as the validation of the FORTA in 2016, which was not included in the systematic review conducted in 2014.
The DBI, ARS, Beers criteria (1991, 1997, 2003 and 2012), STOPP START and ADS were studied most extensively. The Beers criteria, STOPP START, ARS and DBI were associated with the highest number of patient-related outcomes (six outcomes for the Beers criteria, STOPP START and ARS and five outcomes for DBI), with all tools having been associated with hospitalisation, mortality, falls and functional decline. This reiterates the importance of using a scoring system to provide clinicians with an assessment of the burden of medications and potential for harm (ARS and DBI) as well as a list of criteria for appropriate or inappropriate prescribing (Beers criteria and STOPP START) to guide medication review, rationalisation and deprescribing. Based on the validation studies regarding patient-related outcomes, the Beers criteria, STOPP START, ARS and DBI may be more clinically relevant compared with the range of other tools and criteria available.
Validation studies for prescribing assessment tools have most commonly been conducted in Europe in the community, nursing home and hospital settings in various age groups with other studies in the US, Australia, UK and Israel. While prescribing appropriateness tools have been validated in different parts of the world, researchers have stated the need for developing internationally validated criteria that account for differences in cost and patterns of medication use in different countries [118].
A recent commentary argued that while there are tools that have been studied and designed in the older population, there is a need for tools that are specifically designed and validated for complex older patients with a number of comorbidities [119]. The commentary states that older patients have compromised organ function such as impaired kidney and liver activity, where simple recommendations for stopping or starting medications is not as useful and more detailed and specific guidance is required, as clinical decision making can be very challenging [119]. Another commentary stated that the usefulness of different criteria is determined once they are validated in different subgroups of the older population and refined according to validation studies [120]. Additionally, a study argued that while there may be criteria around clinical decision making, there appears to be no guidance around prioritisation of these clinical decisions [121]. The challenge in developing new evidence-based prescribing assessment tools lies in ensuring comprehensiveness and clinical relevance while concurrently ensuring these criteria are easy to use and practical for everyday practice [119].
It would be ideal to develop a tool that combines the different characteristics to formulate a holistic tool that provides structured guidance around appropriate and inappropriate medication use in the older population, who are more susceptible to adverse medicine events, as well as providing a scoring system to indicate the burden of polypharmacy and the resultant risk of harm for clinicians in practice. While some tools to assess prescribing appropriateness have been studied extensively with regard to external validation, studies are alluding to the need for validation in interdisciplinary models of care such as pharmacist-led clinics where the clinical relevance of these tools is unclear [117]. Additionally, researchers have identified that patients visiting multiple prescribers can increase the risk of adverse medicine events and prescribing indicators need to consider the number of prescribers in assessing appropriate and inappropriate polypharmacy [117].
A strength of this systematic review is the novelty of summarising the range of prescribing appropriateness assessment tools available in the literature. While previous studies have attempted to explore external validation of a subgroup of polypharmacy assessment tools such as that of anticholinergic scales and the DBI, this systematic review assessed the external validation for the range of tools and criteria available, without limiting which types of tools and criteria were studied [116, 122]. Other studies have excluded subgroups of tools that focus on specific drug classes, whereas this systematic review included the range of tools and criteria in the literature [115]. Additionally, while previous studies have simply explored associations of outcomes with a selected range of prescribing criteria, this systematic review applied the CASP tool to assess and grade the quality of studies exploring each association [116].
Given the significant variability in study designs, methodologies, patient characteristics and settings (such as community dwelling, nursing home and hospital) and resultant heterogeneity, it was not possible to group and analyse outcomes based on study participants and settings, which could have provided clinicians further clarification regarding which outcomes are clinically significant for subgroups of patients (for example, community-dwelling patients compared with those admitted to hospital). Articles in English were included, meaning there may be tools and criteria in other languages that are clinically useful and validated but have not been included in this review. The CASP tool was used for quality assessment of studies. The CASP, however, has limitations, which means that the quality of studies may have potentially been over- or under-estimated by using this tool.
The results of this review highlight the need for evidence-based tools that are internationally recognised, externally validated, easy to use in everyday practice and account for both over and under prescribing [119, 123]. Researchers have suggested the need for tools containing accurate clinical information and that are practical to use [54, 119, 124]. There is a need to develop evidence-based resources and tools that cater specifically to drug use in multimorbid geriatric patients [125] and have been validated externally to elucidate their relevance in clinical practice.
5 Conclusion
There are many tools and criteria available in the existing literature to assess the appropriateness of prescribing, with each tool covering different aspects of medication review and management. There does not appear to be any one tool that combines all these different aspects and that has been validated against key patient-related outcomes such as hospitalisation, mortality and falls, which would be useful for clinicians in practice looking to optimise medication use. Such a tool is needed to aid clinicians who wish to tailor medication management for patients who may be at risk of medication-related harm.
References
Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013. https://doi.org/10.1093/epirev/mxs009.
Roughead EE, Vitry AI, Caughey GE, et al. Multimorbidity, care complexity and prescribing for the elderly. Aging Health. 2011;7(5):695–705. https://doi.org/10.2217/ahe.11.64.
Caughey GE, Ramsay EN, Vitry AI, et al. Comorbid chronic diseases, discordant impact on mortality in older people: a 14-year longitudinal population study. J Epidemiol Community Health. 2010;64(12):1036–42. https://doi.org/10.1136/jech.2009.088260.
Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Aging Res Rev. 2011;10(4):430–9. https://doi.org/10.1016/j.arr.2011.03.003.
Vitry AI, Zhang Y. Quality of Australian clinical guidelines and relevance to the care of older people with multiple comorbid conditions. Med J Aust. 2008;189(7):360–5.
Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. https://doi.org/10.1001/jama.294.6.716.
Milton JC, Hill-Smith I, Jackson SHD. Prescribing for older people. BMJ. 2008;336(7644):606–9. https://doi.org/10.1136/bmj.39503.424653.80.
Caughey GE, Roughead EE, Pratt N, et al. Increased risk of hip fracture in the elderly associated with prochlorperazine: is a prescribing cascade contributing? Pharmacoepidemiol Drug Saf. 2010;19(9):977–82. https://doi.org/10.1002/pds.2009.
Caughey GE, Roughead EE, Vitry AI, et al. Comorbidity in the elderly with diabetes: identification of areas of potential treatment conflicts. Diabetes Res Clin Pract. 2010;87(3):385–93. https://doi.org/10.1016/j.diabres.2009.10.019.
Fried TR, O’Leary J, Towle V, et al. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–72. https://doi.org/10.1111/jgs.13153.
Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3(2):383–9.
Shimizu K, Ishii S, Tanaka T, et al. Use of potentially inappropriate medication and polypharmacy in community-dwelling Japanese elderly population from the Kashiwa study. J Am Geriatr Soc. 2014;62:S249–50. https://doi.org/10.1111/jgs.12870.
Sato I, Akazawa M. Polypharmacy and adverse drug reactions in Japanese elderly taking antihypertensives: a retrospective database study. Drug Healthc Patient Saf. 2013;5(1):143–50. https://doi.org/10.2147/DHPS.S45347.
Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Epidemiol Community Health. 2012;65(9):989–95. https://doi.org/10.1016/j.jclinepi.2012.02.018.
Fulton MM, Riley Allen E. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract. 2005;17(4):123–32. https://doi.org/10.1111/j.1041-2972.2005.0020.x.
Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. https://doi.org/10.1186/s12877-017-0621-2.
Pollock M, Bazaldua OV, Dobbie AE. Appropriate prescribing of medications: an eight-step approach. Am Fam Physician. 2007;75(2):231–6.
NPS MEDICINEWISE. Identifying inappropriate prescribing. In: Older people & medicines. 2013. http://www.nps.org.au/topics/ages-life-stages/for-individuals/older-people-and-medicines/for-health-professionals/inappropriate-prescribing. Accessed 15 March 2017.
Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781–7. https://doi.org/10.1001/archinte.167.8.781.
Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–13. https://doi.org/10.1001/archinternmed.2007.106.
Carnahan RM, Lund BC, Perry PJ, et al. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–6. https://doi.org/10.1177/0091270006292126.
American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. https://doi.org/10.1111/jgs.13702.
Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
Kouladjian L, Gnjidic D, Chen TF, et al. Drug burden index in older adults: theoretical and practical issues. Clin Interv Aging. 2014;9:1503–15. https://doi.org/10.2147/CIA.S66660.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
CASP UK. CASP Checklists. In: Critical Appraisal Skills Programme (CASP) Making sense of evidence. CASP UK. 2017. https://www.casp-uk.net/casp-tools-checklists. Accessed 10 May 2017.
Holmes HM, Sachs GA, Shega JW, et al. Integrating palliative medicine into the care of persons with advanced dementia: identifying appropriate medication use. J Am Geriatr Soc. 2008;56(7):1306–11. https://doi.org/10.1111/j.1532-5415.2008.01741.x.
Tommelein E, Petrovic M, Somers A, et al. Older patients’ prescriptions screening in the community pharmacy: development of the Ghent Older People’s Prescriptions community Pharmacy Screening (GheOP3S) tool. J Public Health (Oxf). 2016;38(2):e158–70. https://doi.org/10.1093/pubmed/fdv090.
Berdot S, Bertrand M, Dartigues J-F, et al. Inappropriate medication use and risk of falls—a prospective study in a large community-dwelling elderly cohort. BMC Geriatr. 2009;9:30. https://doi.org/10.1186/1471-2318-9-30.
Zhan C, Sangl J, Bierman AS, et al. Potentially inappropriate medication use in the community-dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey. JAMA. 2001;286(22):2823–9.
Winit-Watjana W, Sakulrat P, Kespichayawattana J. Criteria for high-risk medication use in Thai older patients. Arch Gerontol Geriatr. 2008;47(1):35–51. https://doi.org/10.1016/j.archger.2007.06.006.
Niehoff KM, Rajeevan N, Charpentier PA, et al. Development of the tool to reduce inappropriate medications (TRIM): a clinical decision support system to improve medication prescribing for older adults. Pharmacotherapy. 2016;36(6):694–701. https://doi.org/10.1002/phar.1751.
Rancourt C, Moisan J, Baillargeon L, et al. Potentially inappropriate prescriptions for older patients in long-term care. BMC Geriatr. 2004;4:9. https://doi.org/10.1186/1471-2318-4-9.
PrescQIPP. Optimising safe and appropriate medicines use. 2017. https://www.prescqipp.info/projects/optimising-safe-and-appropriate-medicines-use. Accessed 2 Nov 2017.
Nyborg G, Straand J, Klovning A, et al. The Norwegian General Practice-Nursing Home criteria (NORGEP-NH) for potentially inappropriate medication use: a web-based Delphi study. Scand J Primary Health Care. 2015;33(2):134–41. https://doi.org/10.3109/02813432.2015.1041833.
Mimica Matanovic S, Vlahovic-Palcevski V. Potentially inappropriate medications in the elderly: a comprehensive protocol. Eur J Clin Pharmacol. 2012;68(8):1123–38. https://doi.org/10.1007/s00228-012-1238-1.
Kojima T, Mizukami K, Tomita N, et al. Screening tool for older persons’ appropriate prescriptions for Japanese: Report of the Japan Geriatrics Society Working Group on “Guidelines for Medical Treatment and its Safety in the Elderly”. Geriatr Gerontol Int. 2016;16(9):983–1001. https://doi.org/10.1111/ggi.12890.
Maio V, Del Canale S, Abouzaid S. Using explicit criteria to evaluate the quality of prescribing in elderly Italian outpatients: a cohort study. J Clin Pharm Ther. 2010;35(2):219–29. https://doi.org/10.1111/j.1365-537(2710).2009.01094.x.
Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM List: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71(7):861–75. https://doi.org/10.1007/s00228-015-1860-9.
Ruths S, Straand J, Nygaard HA. Multidisciplinary medication review in nursing home residents: what are the most significant drug-related problems? The Bergen District Nursing Home (BEDNURS) study. Qual Saf Health Care. 2003;12(3):176–80.
Mann E, Bohmdorfer B, Fruhwald T, et al. Potentially inappropriate medication in geriatric patients: the Austrian Consensus Panel List. Wien Klin Wochenschr. 2012;124(5–6):160–9. https://doi.org/10.1007/s00508-011-0061-5.
Hassan NB, Ismail HC, Naing L, et al. Development and validation of a new prescription quality index. Br J Clin Pharmacol. 2010;70(4):500–13. https://doi.org/10.1111/j.1365-2125.2009.03597.x.
Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329(7463):434. https://doi.org/10.1136/bmj.329.7463.434.
Basger BJ, Chen TF, Moles RJ. Validation of prescribing appropriateness criteria for older Australians using the RAND/UCLA appropriateness method. BMJ Open. 2012. https://doi.org/10.1136/bmjopen-2012-001431.
Drenth-van Maanen AC, van Marum RJ, Knol W, et al. Prescribing optimization method for improving prescribing in elderly patients receiving polypharmacy: results of application to case histories by general practitioners. Drugs Aging. 2009;26(8):687–701. https://doi.org/10.2165/11316400-000000000-00000.
Roth MT, Ivey JL, Esserman DA, et al. Individualized medication assessment and planning: optimizing medication use in older adults in the primary care setting. Pharmacotherapy. 2013;33(8):787–97. https://doi.org/10.1002/phar.1274.
Haque RM. ARMOR: a tool to evaluate polypharmacy in elderly persons. Ann Long-Term Care. 2009;17(6):26–30.
Lee SS, Schwemm AK, Reist J, et al. Pharmacists’ and pharmacy students’ ability to identify drug-related problems using TIMER (Tool to Improve Medications in the Elderly via Review). Am J Pharmaceut Educ. 2009;73(3):52.
McLeod PJ, Huang AR, Tamblyn RM, et al. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ. 1997;156(3):385–91.
National Committee for Quality Assurance. HEDIS 2016. 2016.
Van der Linden L, Decoutere L, Flamaing J, et al. Development and validation of the RASP list (Rationalization of Home Medication by an Adjusted STOPP list in Older Patients): a novel tool in the management of geriatric polypharmacy. Eur Geriatr Med. 2014;5(3):175–80. https://doi.org/10.1016/j.eurger.2013.12.005.
Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63(8):725–31. https://doi.org/10.1007/s00228-007-0324-2.
Wenger NS, Roth CP, Shekelle P. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(Suppl 2):S247–52. https://doi.org/10.1111/j.1532-5415.2007.01328.x.
Naugler CT, Brymer C, Stolee P, et al. Development and validation of an improving prescribing in the elderly tool. Can J Clin Pharmacol. 2000;7(2):103–7.
Kuhn-Thiel AM, Weiss C, Wehling M. Consensus validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging. 2014;31(2):131–40. https://doi.org/10.1007/s40266-013-0146-0.
Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Deutsches Ärzteblatt Int. 2010;107(31–32):543–51. https://doi.org/10.3238/arztebl.2010.0543.
Chang CB, Yang SY, Lai HY, et al. Using published criteria to develop a list of potentially inappropriate medications for elderly patients in Taiwan. Pharmacoepidemiol Drug Saf. 2012;21(12):1269–79. https://doi.org/10.1002/pds.3274.
Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045–51.
George J, Phun YT, Bailey MJ, et al. Development and validation of the Medication Regimen Complexity Index. Ann Pharmacother. 2004;38(9):1369–76. https://doi.org/10.1345/aph.1D479.
Linjakumpu T, Hartikainen S, Klaukka T, et al. A model to classify the sedative load of drugs. Int J Geriatr Psychiatry. 2003;18(6):542–4. https://doi.org/10.1002/gps.846.
Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–20. https://doi.org/10.2217/1745509X.4.3.311.
Evans DC, Gerlach AT, Christy JM, et al. Pre-injury polypharmacy as a predictor of outcomes in trauma patients. Int J Crit Illn Inj Sci. 2011;1(2):104–9. https://doi.org/10.4103/2229-5151.84793.
Gnjidic D, Le Couteur DG, Abernethy DR, et al. Drug burden index and Beers criteria: impact on functional outcomes in older people living in self-care retirement villages. J Clin Pharmacol. 2012;52(2):258–65. https://doi.org/10.1177/0091270010395591.
Wehling M, Burkhardt H, Kuhn-Thiel A, et al. VALFORTA: a randomised trial to validate the FORTA (Fit fOR The Aged) classification. Age Ageing. 2016;45(2):262–7. https://doi.org/10.1093/ageing/afv200.
Pugh MJ, Marcum ZA, Copeland LA, et al. The quality of quality measures: HEDIS® quality measures for medication management in the elderly and outcomes associated with new exposure. Drugs Aging. 2013;30(8):645–54. https://doi.org/10.1007/s40266-013-0086-8.
Henschel F, Redaelli M, Siegel M, et al. Correlation of incident potentially inappropriate medication prescriptions and hospitalization: an analysis based on the PRISCUS list. Drugs Real World Outcomes. 2015;2(3):249–59. https://doi.org/10.1007/s40801-015-0035-4.
Frankenthal D, Lerman Y, Kalendaryev E, et al. Intervention with the screening tool of older persons potentially inappropriate Prescriptions/Screening Tool to Alert doctors to Right Treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014;62(9):1658–65. https://doi.org/10.1111/jgs.12993.
Moriarty F, Bennett K, Cahir C, et al. Potentially inappropriate prescribing according to STOPP and START and adverse outcomes in community-dwelling older people: a prospective cohort study. Br J Clin Pharmacol. 2016;82(3):849–57. https://doi.org/10.1111/bcp.12995.
Gosch M, Wortz M, Nicholas JA, et al. Inappropriate prescribing as a predictor for long-term mortality after hip fracture. Gerontology. 2014;60(2):114–22. https://doi.org/10.1159/000355327.
Tosato M, Landi F, Martone AM, et al. Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing. 2014;43(6):767–73. https://doi.org/10.1093/ageing/afu029.
Cahir C, Bennett K, Teljeur C, et al. Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br J Clin Pharmacol. 2013;77(1):201–10. https://doi.org/10.1111/bcp.12161.
Maggiore RJ, Dale W, Gross CP, et al. Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: effect on chemotherapy-related toxicity and hospitalization during treatment. J Am Geriatr Soc. 2014;62(8):1505–12. https://doi.org/10.1111/jgs.12942.
Price SD, Holman CD, Sanfilippo FM, et al. Impact of specific Beers criteria medications on associations between drug exposure and unplanned hospitalisation in elderly patients taking high-risk drugs: a case-time-control study in Western Australia. Drugs Aging. 2014;31(4):311–25. https://doi.org/10.1007/s40266-014-0164-6.
Onder G, Landi F, Liperoti R, et al. Impact of inappropriate drug use among hospitalized older adults. Eur J Clin Pharmacol. 2005;61(5–6):453–9. https://doi.org/10.1007/s00228-005-0928-3.
Fick DM, Mion LC, Beers MH, et al. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31(1):42–51. https://doi.org/10.1002/nur.20232.
Stockl KM, Le L, Zhang S, et al. Clinical and economic outcomes associated with potentially inappropriate prescribing in the elderly. Am J Manag Care. 2010;16(1):e1–10.
Dedhiya SD, Hancock E, Craig BA, et al. Incident use and outcomes associated with potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother. 2010;8(6):562–70. https://doi.org/10.1016/s1543-5946(10)80005-4.
Pasina L, Djade CD, Tettamanti M, et al. Prevalence of potentially inappropriate medications and risk of adverse clinical outcome in a cohort of hospitalized elderly patients: results from the REPOSI study. J Clin Pharm Ther. 2014;39(5):511–5. https://doi.org/10.1111/jcpt.12178.
Somers A, Mallet L, van der Cammen T, et al. Applicability of an adapted medication appropriateness index for detection of drug-related problems in geriatric inpatients. Am J Geriatr Pharmacother. 2012;10(2):101–9. https://doi.org/10.1016/j.amjopharm.2012.01.003.
Olsson IN, Runnamo R, Engfeldt P. Medication quality and quality of life in the elderly, a cohort study. Health Qual Life Outcomes. 2011;9:95. https://doi.org/10.1186/1477-7525-9-95.
Hanlon JT, Sloane RJ, Pieper CF, et al. Association of adverse drug reactions with drug-drug and drug-disease interactions in frail older outpatients. Age Ageing. 2011;40(2):274–7. https://doi.org/10.1093/ageing/afq158.
Lund BC, Carnahan RM, Egge JA, et al. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother. 2010;44(6):957–63. https://doi.org/10.1345/aph.1M65710.1345/aph.1M657A.
Wimmer BC, Dent E, Visvanathan R, et al. Polypharmacy and medication regimen complexity as factors associated with hospital discharge destination among older people: a prospective cohort study. Drugs Aging. 2014;31(8):623–30. https://doi.org/10.1007/s40266-014-0185-1.
Wimmer BC, Dent E, Bell JS, et al. Medication regimen complexity and unplanned hospital readmissions in older people. Ann Pharmacother. 2014;48(9):1120–8. https://doi.org/10.1177/1060028014537469.
Peklar J, O’Halloran AM, Maidment ID, et al. Sedative load and frailty among community-dwelling population aged ≥ 65 years. J Am Med Dir Assoc. 2015;16(4):282–9. https://doi.org/10.1016/j.jamda.2014.10.010.
Gnjidic D, Le Couteur DG, Hilmer SN, et al. Sedative load and functional outcomes in community-dwelling older Australian men: the CHAMP study. Fundam Clin Pharmacol. 2014;28(1):10–9. https://doi.org/10.1111/j.1472-8206.2012.01063.x.
Taipale HT, Bell JS, Soini H, et al. Sedative load and mortality among residents of long-term care facilities: a prospective cohort study. Drugs Aging. 2009;26(10):871–81. https://doi.org/10.2165/11317080-000000000-00000.
Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–83. https://doi.org/10.1111/j.1532-5415.2011.03491.x.
Pasina L, Djade CD, Lucca U, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs Aging. 2013;30(2):103–12. https://doi.org/10.1007/s40266-012-0044-x.
Lampela P, Lavikainen P, Garcia-Horsman JA, et al. Anticholinergic drug use, serum anticholinergic activity, and adverse drug events among older people: a population-based study. Drugs Aging. 2013;30(5):321–30. https://doi.org/10.1007/s40266-013-0063-2.
Zimmerman KM, Salow M, Skarf LM, et al. Increasing anticholinergic burden and delirium in palliative care inpatients. Palliat Med. 2014;28(4):335–41. https://doi.org/10.1177/0269216314522105.
Kersten H, Molden E, Tolo IK, et al. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68(3):271–8. https://doi.org/10.1093/gerona/gls176.
Lowry E, Woodman RJ, Soiza RL, et al. Associations between the anticholinergic risk scale score and physical function: potential implications for adverse outcomes in older hospitalized patients. J Am Med Dir Assoc. 2011;12(8):565–72. https://doi.org/10.1016/j.jamda.2011.03.006.
Koshoedo S, Soiza RL, Purkayastha R, et al. Anticholinergic drugs and functional outcomes in older patients undergoing orthopaedic rehabilitation. Am J Geriatr Pharmacother. 2012;10(4):251–7. https://doi.org/10.1016/j.amjopharm.2012.06.003.
Kersten H, Molden E, Willumsen T, et al. Higher anticholinergic drug scale (ADS) scores are associated with peripheral but not cognitive markers of cholinergic blockade. Cross sectional data from Norwegian nursing homes. Br J Clin Pharmacol. 2013;75(3):842–9. https://doi.org/10.1111/j.1365-2125.2012.04411.x.
Marcum ZA, Wirtz HS, Pettinger M, et al. Anticholinergic medication use and falls in postmenopausal women: Findings from the Women’s Health Initiative cohort study. BMC Geriatr. 2016;16:76. https://doi.org/10.1186/s12877-016-0251-0.
Kumpula EK, Bell JS, Soini H, et al. Anticholinergic drug use and mortality among residents of long-term care facilities: a prospective cohort study. J Clin Pharmacol. 2011;51(2):256–63. https://doi.org/10.1177/0091270010368410.
Mubang RN, Stoltzfus JC, Cohen MS, et al. Comorbidity-polypharmacy score as predictor of outcomes in older trauma patients: a retrospective validation study. World J Surg. 2015;39(8):2068–75. https://doi.org/10.1007/s00268-015-3041-5.
Evans DC, Cook CH, Christy JM, et al. Comorbidity-polypharmacy scoring facilitates outcome prediction in older trauma patients. J Am Geriatr Soc. 2012;60(8):1465–70. https://doi.org/10.1111/j.1532-5415.2012.04075.x.
Justiniano CF, Coffey RA, Evans DC, et al. Comorbidity-polypharmacy score predicts in-hospital complications and the need for discharge to extended care facility in older burn patients. J Surg Res. 2014;36(1):193–6. https://doi.org/10.1097/bcr.0000000000000094.
Mangoni AA, van Munster BC, Woodman RJ, et al. Measures of anticholinergic drug exposure, serum anticholinergic activity, and all-cause postdischarge mortality in older hospitalized patients with hip fractures. Am J Geriatr Psychiatry. 2013;21(8):785–93. https://doi.org/10.1016/j.jagp.2013.01.012.
Bostock CV, Soiza RL, Mangoni AA. Associations between different measures of anticholinergic drug exposure and Barthel Index in older hospitalized patients. Ther Adv Drug Saf. 2013;4(6):235–45. https://doi.org/10.1177/2042098613500689.
Dispennette R, Elliott D, Nguyen L, et al. Drug burden index score and anticholinergic risk scale as predictors of readmission to the hospital. Consul Pharm. 2014;29(3):158–68. https://doi.org/10.4140/TCP.n.2014.158.
Hilmer SN, Mager DE, Simonsick EM, et al. Drug burden index score and functional decline in older people. Am J Med. 2009;122(12):1142–9. https://doi.org/10.1016/j.amjmed.2009.02.021.
Gnjidic D, Cumming RG, Le Couteur DG, et al. Drug burden index and physical function in older Australian men. Br J Clin Pharmacol. 2009;68(1):97–105. https://doi.org/10.1111/j.1365-2125.2009.03411.x.
Gnjidic D, Bell JS, Hilmer SN, et al. Drug burden index associated with function in community-dwelling older people in Finland: a cross-sectional study. Ann Med. 2012;44(5):458–67. https://doi.org/10.3109/07853890.2011.573499.
Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and mortality in older people in residential aged care facilities. Drugs Aging. 2012;29(2):157–65. https://doi.org/10.2165/11598570-000000000-00000.
Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and physical function in older people in residential aged care facilities. Age Ageing. 2010;39(4):503–7. https://doi.org/10.1093/ageing/afq053.
Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc. 2011;59(5):875–80. https://doi.org/10.1111/j.1532-5415.2011.03386.x.
Best O, Gnjidic D, Hilmer SN, et al. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43(8):912–8. https://doi.org/10.1111/imj.12203.
Dauphinot V, Faure R, Omrani S, et al. Exposure to anticholinergic and sedative drugs, risk of falls, and mortality: an elderly inpatient, multicenter cohort. J Clin Psychopharmacol. 2014;34(5):565–70. https://doi.org/10.1097/jcp.0000000000000195.
Lonnroos E, Gnjidic D, Hilmer SN, et al. Drug burden index and hospitalization among community-dwelling older people. Drugs Aging. 2012;29(5):395–404. https://doi.org/10.2165/11631420-000000000-00000.
Lowry E, Woodman RJ, Soiza RL, et al. Drug burden index, physical function, and adverse outcomes in older hospitalized patients. J Clin Pharmacol. 2012;52(10):1584–91. https://doi.org/10.1177/0091270011421489.
Gnjidic D, Hilmer SN, Blyth FM, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther. 2012;91(3):521–8. https://doi.org/10.1038/clpt.2011.258.
Kaufmann CP, Tremp R, Hersberger KE, et al. Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol. 2014;70(1):1–11. https://doi.org/10.1007/s00228-013-1575-8.
Villalba-Moreno AM, Alfaro-Lara ER, Perez-Guerrero MC, et al. Systematic review on the use of anticholinergic scales in poly pathological patients. Arch Gerontol Geriatr. 2016;62:1–8. https://doi.org/10.1016/j.archger.2015.10.002.
Wang KCM, Veluswamy R. Evidence-based strategies to reduce polypharmacy: a review. OA Elderly Med. 2013;1:6.
Levy HB, Marcus EL, Christen C. Beyond the Beers criteria: a comparative overview of explicit criteria. Ann Pharmacother. 2010;44(12):1968–75. https://doi.org/10.1345/aph.1P426.
O’Mahony D, Gallagher PF. Inappropriate prescribing in the older population: need for new criteria. Age Ageing. 2008;37(2):138–41. https://doi.org/10.1093/ageing/afm189.
Taipale HT, Hartikainen S, Bell JS. A comparison of four methods to quantify the cumulative effect of taking multiple drugs with sedative properties. Am J Geriatr Pharmacother. 2010;8(5):460–71. https://doi.org/10.1016/j.amjopharm.2010.10.004.
Cadogan CA, Ryan C, Hughes CM. Appropriate polypharmacy and medicine safety: when many is not too many. Drug Saf. 2016;39(2):109–16. https://doi.org/10.1007/s40264-015-0378-5.
Hill-Taylor B, Walsh KA, Stewart S, et al. Effectiveness of the STOPP/START (Screening Tool of Older persons’ Potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: systematic review and meta-analysis of randomized controlled studies. J Clin Pharm Ther. 2016;41(2):158–69. https://doi.org/10.1111/jcpt.12372.
Lund BC, Steinman MA, Chrischilles EA, et al. Beers criteria as a proxy for inappropriate prescribing of other medications among older adults. Ann Pharmacother. 2011;45(11):1363–70. https://doi.org/10.1345/aph.1Q361.
Australian Medicines Handbook Pty Ltd. Prescribing for the elderly. In: The Australian Medicines Handbook. Australian Medicines Handbook Pty Ltd. 2017. https://amhonline-amh-net-au.access.library.unisa.edu.au/guides/guide-elderly. Accessed 10 May 2017.
Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–34. https://doi.org/10.1001/jamainternmed.2015.0324.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Nashwa Masnoon is supported by an Australian Government Research Training Program (RTP). No other sources of funding were used to assist in the preparation of this article.
Conflicts of interest
Nashwa Masnoon, Sepehr Shakib, Lisa Kalisch-Ellett and Gillian Caughey declare that they have no conflicts of interest relevant to the content of this review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Masnoon, N., Shakib, S., Kalisch-Ellett, L. et al. Tools for Assessment of the Appropriateness of Prescribing and Association with Patient-Related Outcomes: A Systematic Review. Drugs Aging 35, 43–60 (2018). https://doi.org/10.1007/s40266-018-0516-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-018-0516-8