Abstract
Lesinurad (Zurampic®) is an oral selective inhibitor of the URAT1 and OAT4 uric acid (UA) transporters of the kidney, via which it inhibits UA reabsorption and thus increases renal UA excretion and lowers serum UA (sUA) levels. Lesinurad 200 mg once daily is indicated for use in combination with a xanthine oxidase inhibitor (XOI) to treat hyperuricaemia in adults with gout who have not achieved target sUA levels with an XOI alone. Approval was based on three 12-month phase 3 trials that evaluated lesinurad in combination with allopurinol in adults with gout inadequately responsive to allopurinol (CLEAR 1 and 2) and in combination with febuxostat in adults with tophaceous gout (CRYSTAL). The target sUA level of <6 mg/dL at 6 months (primary endpoint) was achieved by significantly more lesinurad plus allopurinol than placebo plus allopurinol recipients in the CLEAR trials. In CRYSTAL (which enrolled patients regardless of prior XOI experience, and included 3 weeks of febuxostat before randomization), the proportion of patients who achieved an sUA target of <5 mg/dL did not reach statistical significance between lesinurad plus febuxostat and placebo plus febuxostat at 6 months (primary endpoint), although significantly favoured the lesinurad plus febuxostat group at 12 months. Notably, the sUA target of <5 mg/dL at 6 months was met with lesinurad plus febuxostat in the CRYSTAL subgroup that had uncontrolled hyperuricaemia at baseline, despite having received febuxostat pre-randomization. Lesinurad plus XOI regimens were generally not associated with improvements in flares and tophi in these trials, although clinical benefit became more apparent in 12-month extension studies; the regimens were also generally well tolerated. Thus, lesinurad, in combination with an XOI, is an emerging option for the treatment of hyperuricaemia in adults with gout who have not achieved target sUA levels with an XOI alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lowers serum UA concentrations via inhibition of URAT1 and OAT4 (UA transporters of the kidney) |
Administered orally once daily in combination with an XOI |
Added to an XOI regimen, enables many gout patients with hyperuricaemia to achieve target sUA levels |
Improves clinical parameters (e.g. tophus number/size and rate of gout flares needing treatment) in the long term (over up to 24 months) |
Generally well tolerated, with most adverse events being mild to moderate and transient |
1 Introduction
Gout is a common disabling inflammatory arthritis characterized by painful periods of severe joint inflammation [1,2,3]. A key pathogenic factor in gout development is hyperuricaemia [a level of serum uric acid (sUA) above the threshold of saturation, which is 6.8 mg/dL at 37 °C and physiological pH], resulting from renal/extra-renal under excretion of UA and/or its overproduction (e.g. via the liver) [1, 2]. The high levels of sUA promote the formation and accumulation of monosodium urate (MSU) crystals in the joints and tissues, that are capable of inducing acute self-limiting inflammatory responses (i.e. flares) [1, 2]; crystals can also accumulate in the kidneys, forming kidney stones that can be damaging [4]. If hyperuricaemia is not controlled, acute flares can begin to occur more frequently and take longer to resolve, with inflammatory responses to the MSU crystals eventually becoming chronic in some patients, resulting in joint and bone damage [1, 2]. Patients with such advanced gout often have subcutaneous deposits of MSU, known as tophi, which can be disfiguring and restrictive [1, 2] and increase overall disease burden [5]. In addition, patients with hyperuricaemia/gout can have comorbidities, including renal and cardiovascular disease [6], that require consideration.
Pharmacological management of gout includes the use of anti-inflammatory drugs to treat acute flare symptoms, UA-lowering therapy (ULT) to reduce sUA to levels that allow MSU crystal dissolution (i.e. <6 mg/dL, or <5 mg/dL if gout is severe [3]), and prophylactic use of anti-inflammatories early during ULT to manage the transient risk of flares that initially occurs upon MSU crystal dispersion [1, 3, 7]. ULT is a key element of gout management, with three classes of UA-lowering drugs being available for use: xanthine oxidase inhibitors (XOIs) [inhibit UA production], uricases (convert UA to readily excretable allantoin) and uricosurics (normalize/increase UA excretion by the kidneys) [1].
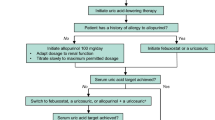
Lesinurad (Zurampic®) is the latest uricosuric agent to become commercially available. It is a selective uric acid reabsorption inhibitor approved in several countries, including the USA [8] and EU [9], for use in combination with an XOI to treat hyperuricaemia in adults with gout who have not achieved target sUA levels with an XOI alone. This article overviews key pharmacological, therapeutic efficacy and tolerability data relevant to the use of the drug in this indication, focusing on the recommended dosage of 200 mg once daily wherever possible.
2 Pharmacodynamic Properties of Lesinurad
Lesinurad inhibits UA reabsorption by selectively inhibiting two apical UA transporters in the kidney: UA transporter 1 (URAT1) [the key protein involved in reabsorbing UA from the tubular lumen] and OAT4 (a protein involved in diuretic-induced hyperuricaemia) [8,9,10]. These two transporters are inhibited by the drug dose-dependently [10] and with micromolar potency [8, 10] (e.g. mean lesinurad concentrations of 3.53 and 2.03 µmol/L were required to inhibit URAT1 and OAT4 by 50% in one in vitro study, making lesinurad more potent than some of the other uricosuric agents evaluated, including probenecid [10]). Lesinurad did not inhibit the in vitro activity of GLUT9, another reabsorptive UA transporter [10], but did inhibit that of some transporter proteins involved in UA secretion, namely OAT1 and OAT3 (IC50 values of ≈4 and ≈3.5 µmol/L) but not BCRP [10, 11]; however, the inhibition of OAT1 and 3 is not clinically relevant [11] (Sect. 3.1).
By inhibiting UA reabsorption, lesinurad increases renal excretion of UA and consequently lowers levels of sUA [8, 9], as demonstrated in single-[10, 12] and multiple-[12] dose studies in healthy volunteers. However, lesinurad must be used in combination with an XOI [8, 9], since combined use of these agents augments the sUA-lowering effect of lesinurad (because UA excretion is increased while UA production is reduced) and reduces the likelihood of adverse renal outcomes (Sect. 5.1) (as less UA is available for excretion) [9]. For instance, healthy volunteers had mean reductions in sUA of ≈46% at 6 h and 26% at 24 h after administration of lesinurad 200 mg alone; further reductions of 25 and 19% were seen when lesinurad was coadministered with febuxostat [9]. The sUA-lowering effect and benefits of adding lesinurad to an XOI regimen have been confirmed in patients with gout in various clinical studies [13,14,15,16,17,18,19], including three phase 3 trials and their extensions (Sect. 4) [15,16,17,18,19].
Lesinurad should be taken with food (Sect. 6) [8, 9], as doing so may improve the sUA-lowering effects of the drug [9, 12]. Lesinurad should not be coadministered with salicylates at dosages of >325 mg/day, as the UA-lowering benefits of lesinurad may be reduced [9].
Cardiac repolarization in healthy volunteers was not altered to any clinically relevant extent by single lesinurad doses of up to 1600 mg (i.e. supratherapeutic) in a thorough QT interval, crossover study [20]. Moreover, in vitro, lesinurad did not induce PPARγ activity at concentrations much greater than its maximal plasma concentration (PPARγ activation appears to elevate cardiovascular risk) and displayed no mitochondrial toxicity, properties that have been seen with some other uricosuric agents [10]. However, major cardiovascular events (MACE) have occurred with lesinurad, although infrequently (Sect. 5).
3 Pharmacokinetic Properties of Lesinurad
Oral lesinurad is absorbed rapidly, with the drug reaching maximum plasma concentrations 1–4 h after administration of a single lesinurad tablet in the fed or fasted state [8, 12] (200–600 mg in healthy volunteers, where specified [12]). The fat content of food does not appear to impact lesinurad exposure [8, 9], which increases dose-proportionally across single oral doses of 5–1200 mg [8, 9, 12]. Lesinurad has ≈100% absolute bioavailability [8, 9] and does not accumulate with repeated dosing [8, 9, 12].
Lesinurad is highly (>98%) bound to plasma proteins (particularly albumin), has a small mean volume of distribution at steady state (≈20 L after intravenous administration) [8, 9] and does not extensively penetrate/partition into erythrocytes [9]. Metabolism of lesinurad occurs predominantly via CYP2C9, producing metabolites that have no known role in the drug’s UA-lowering effects [8, 9] and have minimal plasma exposure (<10% of the parent drug) [8]. Much of a lesinurad dose is excreted via the urine within 7 days (63%; ≈30% as unchanged parent drug), with >60% being recovered via this route within 24 h of administration; 32% of the dose is excreted via the faeces [8, 9]. The high renal clearance of lesinurad (25.6 mL/min) relative to the typical rate of glomerular filtration suggests that active renal secretion is key in the drug’s excretion [9]. Lesinurad has a total body clearance of ≈6 L/h [8] and an elimination half-life of ≈5 h after administration of a single 200 mg tablet [12].
3.1 Special Patient Groups and Potential Drug Interactions
Lesinurad should be used with caution in patients who are known or suspected to be poor CYP2C9 metabolizers, as exposure to the drug [and thus the risk of renal adverse events (AEs); Sect. 5.1] may be increased [8, 9]. For instance, in a study that evaluated daily lesinurad 200–600 mg (with or without an XOI) in patients with gout and healthy volunteers (n = 67), overall exposure to lesinurad 400 mg/day was 111 and 22% higher in poor or intermediate versus extensive CYP2C9 metabolizers, although individual values remained within the extensive metabolizer range [9].
Exposure to lesinurad also increases with decreasing renal function [8, 9, 21]. For instance, in an open-label phase 1 trial, the area under the plasma concentration-time curve of lesinurad after a single 200 mg dose was 34 and 65% higher in adults with mild or moderate renal impairment than in those with normal renal function [21]. No dosage adjustments are required in patients with mild or moderate renal impairment [creatinine clearance (CLCR) ≥45 mL/min in USA [8]; 30–89 mL/min in EU, with caution advised if CLCR is 30 to <45 mL/min, as data are limited [9]]. Lesinurad is contraindicated in patients with severe renal impairment (CLCR <30 mL/min), end-stage renal disease and/or receiving dialysis, as well as in kidney transplant recipients, as it is not expected to be effective in these settings, given its mechanism of action (Sect. 2) [8, 9].
Lesinurad does not require dosage adjustment in patients with mild or moderate hepatic impairment (Child-Pugh class A or B) [8, 9]; however, as the drug has not been studied in patients with severe hepatic impairment, no dosage recommendations are given for these patients in the EU [9] and lesinurad is not recommended in this setting in the USA [8]. Lesinurad pharmacokinetics are not altered to any clinically meaningful extent by gender, race, ethnicity or age, according to population pharmacokinetic analysis [8, 9]; elderly patients (aged ≥65 years [9]) do not require dosage adjustment [8, 9], although caution is advised in the EU in those aged ≥75 years, as there is limited experience with the drug in these patients [9].
Coadministering lesinurad with inhibitors or inducers of CYP2C9 may increase or decrease lesinurad exposure, respectively; consequently, caution is advised when using lesinurad in combination with moderate CYP2C9 inhibitors [8, 9], and monitoring for reduced lesinurad efficacy may be necessary when lesinurad is used in conjunction with CYP2C9 inducers [9]. Coadministering lesinurad with inhibitors of (microsomal [9]) epoxide hydrolase is not recommended, as lesinurad metabolism may be affected [8, 9].
As lesinurad is a mild [8, 9] to moderate [9] CYP3A inducer, it may reduce exposure to drugs that are CYP3A substrates. Consequently, if coadministered with lesinurad, the efficacy of drugs that are sensitive CYP3A substrates should be monitored and hormonal contraceptives should be used with other forms of contraception [8, 9]. Lesinurad also induces CYP2B6 weakly in vitro [9]; thus, monitoring the efficacy of CYP2B6 substrates coadministered with lesinurad is recommended in the EU [9]. Further in vitro evaluation demonstrated inhibition of various transporter proteins with lesinurad, including OAT1 and OAT3 (key renal transporters) and OATP1B1, OATP1B3 and OCT1 (hepatic transporters), but minimal/no inhibition of OCT2, BCRP, P-gp, MATE1 or MATE2K; however, lesinurad displayed no clinically relevant inhibition of OAT1, OAT3, OATP1B1 or OCT1 in healthy volunteers [11].
4 Therapeutic Efficacy of Lesinurad
This section discusses the efficacy of oral lesinurad, in combination with the XOIs allopurinol [15, 16] or febuxostat [17, 22], in adults with gout, as evaluated in three 12-month, randomized, double-blind, phase 3 studies: CLEAR 1 [15], CLEAR 2 [16] and CRYSTAL [17]. These multicentre trials enrolled patients aged 18–85 years with gout (as per American Rheumatism Association criteria) who had a body mass index of <45 kg/m2 and an estimated CLCR of ≥30 mL/min [15, 16, 22]. Across the trials, patients had a mean age of 51–54 years and were predominantly male (94–96%) and Caucasian (76–80%). Some data are from abstracts [17,18,19, 23,24,25]. Although all trials assessed lesinurad dosages of 200 and 400 mg/day, wherever possible, only the recommended dosage of 200 mg/day is discussed.
4.1 In Combination with Allopurinol
In CLEAR 1 [15] and 2 [16], patients were required to have had two or more gout flares in the last year and an inadequate hypouricaemic response to allopurinol (as the sole ULT for their gout) at a stable, medically appropriate dosage (see Table 1 for details) in the ≥8 weeks prior to screening; an sUA of ≥6.5 mg/dL at screening and ≥6.0 mg/dL ≈1 week prior to study treatment was also required. Patients had been diagnosed with gout a mean of ≈12 years previously [15, 16] and the majority had no target tophi at baseline (84% [16]; 86% [15]). After ≈2 weeks of gout flare prophylaxis, patients were randomized to receive lesinurad (200 or 400 mg/day) or placebo, once daily in combination with allopurinol (at the pre-study dosage) for 12 months (Table 1); patients continued prophylaxis for gout flares through to the end of month 5.
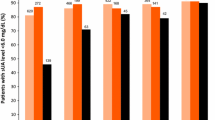
In adults with gout inadequately responsive to allopurinol, adding once-daily lesinurad 200 mg to the regimen was effective in reducing sUA levels, with significantly more lesinurad plus allopurinol than placebo plus allopurinol recipients achieving a target sUA of <6 mg/dL after 6 months of treatment in CLEAR 1 and 2 (primary endpoint; Table 1) [15, 16]. The benefits of lesinurad plus allopurinol in terms of this parameter were rapid and prolonged, with between-group differences significantly favouring lesinurad plus allopurinol (p < 0.0001 vs. placebo plus allopurinol) from 1 month of treatment onwards, through to month 12 (Table 1). The proportions of patients achieving the more stringent sUA targets of <5 or <4 mg/dL were likewise significantly (p < 0.01) greater in the lesinurad plus allopurinol than in the placebo plus allopurinol arm at each timepoint of the 12-month treatment period (see Table 1 for month 6 and 12 data). Consistent with these findings, lesinurad plus allopurinol recipients had significantly lower mean sUA levels than placebo plus allopurinol recipients at all timepoints, including month 12 (Table 1) [15, 16].
In terms of clinical outcomes, the mean rate of gout flares that required treatment was low and did not significantly differ between the lesinurad plus allopurinol and placebo plus allopurinol groups in the last 6 months of CLEAR 1 (0.57 vs. 0.58) [15] or CLEAR 2 (0.73 vs. 0.83) [16], i.e. after gout flare prophylaxis had ceased. Among the few patients who had target tophi at baseline, none of 18 lesinurad plus allopurinol recipients versus 29% of 17 placebo plus allopurinol recipients showed complete resolution of at least one tophi by month 12 in CLEAR 1 (p = 0.02 for between-group difference) [15] and 31% of 35 lesinurad plus allopurinol recipients versus 33% of 33 placebo plus allopurinol recipients achieved this outcome in CLEAR 2 [16].
Longer term, the benefits of lesinurad 200 mg/day plus allopurinol became more apparent during the 12-month open-label extension of CLEAR 1 and 2 [18, 19]. Of the 239 extension participants originally randomized to this regimen in one of the parent studies, 64% had an sUA level of <6 mg/dL after 12 months’ treatment in the extension (i.e. after 24 months total treatment) [data estimated from a graph] [18]. At this timepoint, 43.8% of the 32 patients with target tophi at baseline had complete resolution of at least one of the tophi (vs. 25.0% at parent study end), the total area of all target tophi was reduced from baseline by 41.8% (vs. 11.6% at parent study end) and 5.6% of all patients had gout flares requiring treatment (vs. 7.9% at parent study end) [19]. Of note, patients who switched from placebo plus allopurinol to lesinurad plus allopurinol at the start of the extension (n = 121) had similar benefits to those seen with the regimen in the parent trials [18].
4.2 In Combination with Febuxostat
In CRYSTAL, patients were required to have tophaceous gout (i.e. one or more tophi measuring ≥5 and ≤20 mm in diameter on the hands/wrists and/or feet/ankles) and an sUA of either ≥8 mg/dL (if not currently receiving an approved ULT) or ≥6.0 mg/dL (if receiving allopurinol or febuxostat at an appropriate dosage) [22]; patients had been diagnosed with gout a mean of 14.7 years earlier [17]. After receiving febuxostat 80 mg/day for 3 weeks, patients were randomized to receive lesinurad (200 or 400 mg) or placebo once daily in combination with the febuxostat regimen for 12 months [17]. At baseline, 50% of patients had not attained an sUA level of <5 mg/dL after the pre-randomization febuxostat treatment period [8].
The proportion of patients who achieved an sUA level of <5 mg/dL did not significantly differ between once-daily lesinurad 200 mg plus febuxostat and placebo plus febuxostat at 6 months (primary endpoint), although significantly favoured lesinurad plus febuxostat at 12 months (Table 1) [17]. There was also significant benefit in the lesinurad plus febuxostat versus the placebo plus febuxostat group in the proportion of patients achieving sUA targets of <3 and <4 mg/dL, but not <6 mg/dL, at each of these timepoints (Table 1) [17]. Notably, among the patients (n = 59 and 51) who had an sUA level of ≥5 mg/dL at baseline (i.e. had inadequately-controlled hyperuricaemia despite 3 weeks of febuxostat prior to randomization), more lesinurad plus febuxostat than placebo plus febuxostat recipients achieved a target sUA level of <5 mg/dL at month 6 (44 vs. 24%; 95% CI 0.03–0.38) and at all other timepoints [9].
With regard to clinical outcomes, no significant difference was evident between the lesinurad plus febuxostat and placebo plus febuxostat groups in the proportion of patients who had complete resolution of at least one tophus by month 12 (26 vs. 21%) [9, 17]; however, tophus area was reduced with the lesinurad plus febuxostat regimen at this timepoint (by 55.8 vs. 31.3% with placebo plus febuxostat; p < 0.05) [17].
The benefit of lesinurad plus febuxostat became more evident during the CRYSTAL extension [19]. Among the 64 extension participants originally randomized to lesinurad 200 mg/day plus febuxostat who continued the regimen for a further 12 months, after 24 months’ total therapy, 53.1% had achieved complete resolution of at least one tophus (vs. 26.6% at parent study end), the sum area of all target tophi was reduced from baseline by 68.3% (vs. 54.8% at parent study end) and 6.3% of patients had gout flares requiring treatment (vs. 10.9% at parent study end) [19]. Data from patients who switched from placebo plus febuxostat to lesinurad plus febuxostat at the start of the extension (n = 33) generally supported those with the regimen in the parent study [23].
4.3 Additional Analyses
When primary endpoint data from CLEAR 1 [15] and 2 [16] were assessed by patient age, race, sex, baseline sUA, thiazide diuretic use and renal function, adding lesinurad 200 mg/day to ongoing allopurinol therapy provided benefit (vs. adding placebo) consistent with that seen in the overall trial population, although some subgroups for which quantitative data were provided were small and had wide confidence intervals (namely patients on thiazide diuretics or with CLCR <60 mL/min; n = 52–85). However, in a pooled CLEAR analysis [24], significantly (p < 0.05) more lesinurad 200 mg/day plus allopurinol than placebo plus allopurinol recipients achieved an sUA <6.0 mg/dL at 6 or 12 months regardless of whether their baseline CLCR was ≥90, <90 or <60 mL/min (total n = 154–497 per subgroup). Similarly, in each of the same renal subgroups of CRYSTAL [25], the proportion of patients who achieved an sUA <5 mg/dL was numerically higher with lesinurad 200 mg/day plus febuxostat than with placebo plus febuxostat at 6 and 12 months, with the between-regimen difference reaching significance (p < 0.05) at the latter timepoint in the <90 and ≥90 mL/min subgroups (the two largest; total n = 143 and 66).
In a pooled analysis of CLEAR 1 and 2 and CRYSTAL [26], significantly (p < 0.001) more lesinurad (200 or 400 mg/day) plus XOI regimen recipients achieved sUA target levels and had no gout flares (post hoc composite endpoint) than placebo plus XOI recipients (no further details reported).
5 Tolerability of Lesinurad
Oral lesinurad 200 mg/day, in combination with allopurinol or febuxostat, was generally well tolerated when used for up to 24 months to treat hyperuricaemia in adults with gout in phase 3 trials [15,16,17] and their extensions [18, 23] (Sect. 4). AEs with these regimens in clinical trials were generally mild or moderate [9, 15, 16] and resolved without discontinuing treatment [9]. Further discussion in this section focuses on pooled data from these studies and on the recommended lesinurad dosage of 200 mg/day. Note, some data are from abstracts [17, 27, 28], and lesinurad must not be used as monotherapy (Sect. 5.1).
In a pooled analysis of the three pivotal 12-month phase 3 trials (Sect. 4), the tolerability profile of lesinurad 200 mg/day was generally similar to that of placebo, when each was used in combination with an XOI [27]. In the respective groups, the exposure-adjusted incidence rate (EAIR) per 100 patient-years (PY) was 25 and 20 for AEs possibly related to study drug, 6 and 7 for serious treatment-emergent AEs, 8 and 7 for treatment-emergent AEs leading to study drug discontinuation and 0.5 and 0 for fatal treatment-emergent AEs [27]. The AEs that occurred most commonly in the lesinurad plus XOI group and with ≥1% greater incidence than in the placebo plus XOI group in this analysis included headache, influenza, blood creatinine increased (see also Sect. 5.1) and gastroesophageal reflux disease (Fig. 1) [8]. In the individual phase 3 trials, lesinurad plus XOI recipients had similar clinical laboratory test results (excluding renal tests) and urinalysis outcomes to those of placebo plus XOI recipients, and vital signs did not change notably in either treatment group, where specified [15, 16].
Adverse events that occurred most commonly with lesinurad plus XOI and with ≥1% greater incidence than with placebo plus XOI in a pooled analysis of three pivotal 12-month phase 3 trials in patients with gout [8]. GERD gastroesophageal reflux disease, LES lesinurad, PL placebo, XOI xanthine oxidase inhibitor
Longer term, no new safety concerns were evident when data from the three 12-month trials and their 12-month extensions were pooled [27], with recipients of lesinurad 200 mg/day plus XOI therapy (n = 666) having an EAIR per 100 PY of 17 for AEs considered possibly related to study drug, 7 for both treatment-emergent AEs that were serious and those that caused study drug discontinuation and 0.8 for fatal treatment-emergent AEs.
Some patients with gout receiving lesinurad plus XOI therapy have experienced MACE, including non-fatal stroke, non-fatal myocardial infarction or cardiovascular death [8, 9]. For instance, the EAIR of adjudicated MACE events (per 100 PYs) in the pooled analysis of the phase 3 trials was 0.96 with lesinurad 200 mg/day plus XOI and 0.71 with placebo plus XOI (incidence rate ratio 1.36; 95% CI 0.23, 9.25) [8]. Longer term, the EAIR of MACE did not appear to notably increase over up to 24 months’ therapy in the pooled analysis of these trials and their extensions, being 1.05 per 100 PY with lesinurad 200 mg/day in combination with an XOI [27]. A causal relationship with lesinurad has not been established; however, because of the paucity of data, lesinurad is not recommended for patients with certain cardiovascular conditions in the EU (Sect. 6), although if these conditions are stable, the benefits/risks of using lesinurad may be considered [9].
5.1 Renal Profile
As a consequence of increased UA excretion by the kidneys (Sect. 2), recipients of lesinurad therapy can experience transient serum creatinine level increases, kidney stones and various other renal-related AEs [8, 9]. For instance, in the pooled analysis [28] of the three pivotal 12-month phase 3 trials, recipients of lesinurad 200 mg/day plus XOI or placebo plus XOI had EAIRs per 100 PY of 7.3 and 5.6 for any renal-related AE, 0 and 0.5 for serious renal-related AEs and 7.3 and 2.9 for serum creatinine elevations ≥1.5 × baseline. Elevated serum creatinine was the most common renal-related AE [28] and increased in incidence with increasing renal impairment (e.g. 3.0% of lesinurad plus XOI vs. 0.6% of placebo plus XOI recipients with CLCR ≥90 mL/min and 6.9 vs. 5.9% of recipients with CLCR ≥30 to <60 mL/min) [8]; however, the elevations usually resolved (84% of cases in the lesinurad plus XOI group and 75% of cases in the placebo plus XOI group), often without interrupting treatment (66 and 75% of cases) [28].
Indeed, few lesinurad plus XOI or placebo plus XOI recipients discontinued treatment because of renal-related adverse reactions (≈1% in each group) in this analysis [8], and renal function stayed constant across the treatment arms of the individual trials, where specified [15, 16]. Lesinurad 200 mg/day plus XOI did not increase the EAIR of kidney stone AEs relative to placebo plus XOI in the pooled analysis (0.8 vs. 2.2 per 100 PY) and these AEs were not often serious (0 vs. 0.2 per 100 PY) [28]. The incidence of renal failure in the lesinurad plus XOI group was low and approximately half that seen in the placebo plus XOI group (1.2 and 2.1%) and was not markedly impacted by baseline renal function [8].
Compared with lesinurad 200 mg/day, the higher lesinurad dosage of 400 mg/day was more commonly associated with renal AEs, including acute renal failure, especially when used as monotherapy [8, 9], and was thus recognized (prior to regulatory submission) as an inappropriate dosage. The US prescribing information (PI) features a boxed warning regarding the occurrence of acute renal failure with lesinurad, particularly in the monotherapy setting, and states that lesinurad should only be used in combination with an XOI [8]; similar warnings feature in the EU PI [9].
Longer-term exposure to lesinurad 200 mg/day plus XOI therapy for up to 24 months was not associated with clinically relevant increases in renal-related or kidney stone AEs when data from the three 12-month trials and their 12-month extensions were pooled [28]. For instance, for patients in the extension who had received lesinurad plus XOI in one of the parent trials, the EAIR per 100 PY was 8.4 for any renal-related treatment-emergent AE, 0.5 for any serious renal-related treatment-emergent AE, 7.7 for serum creatinine elevations ≥1.5 × baseline and 0.9 for kidney stones.
6 Dosage and Administration of Lesinurad
Lesinurad, used in combination with an XOI, is approved in the USA [8] and EU [9] for the treatment of hyperuricaemia in adults with gout (with or without tophi [9]) who have not achieved target sUA levels with an adequate dosage of an XOI alone. The recommended (and maximum) lesinurad dosage is 200 mg once daily, taken with food and water at the same time as the morning XOI dose [8, 9]; XOIs include febuxostat or allopurinol, with the recommended daily dose of the latter being ≥300 mg/day (or ≥200 mg/day in patients with CLCR <60 mL/min) [8, 9]. Treatment with lesinurad should be interrupted if XOI therapy is interrupted, as lesinurad should not be used as monotherapy. In addition, as UA may mobilize from tissue deposits during lesinurad therapy, as with any ULT, gout flare prophylaxis is recommended (for ≥5 months [9]) when initiating lesinurad; any flares during treatment should be managed concurrently [8, 9].
Renal function should be assessed before lesinurad is initiated and then monitored periodically during therapy (with close monitoring advised if baseline CLCR is <60 mL/min [8] or serum creatinine increases >1.5-fold vs. pre-treatment [8, 9]). Interruption of lesinurad is necessary if serum creatinine increases more than twofold versus pre-treatment [8, 9] or to >4.0 mg/dL [9], or if symptoms of acute UA nephropathy occur [8, 9]. Contraindications for lesinurad use include various renal impairment settings (CLCR <30 mL/min, end-stage renal disease, dialysis, kidney transplant; Sect. 3) as well as tumour lysis syndrome and Lesch–Nyhan syndrome [8, 9]. Lesinurad is not recommended for patients with unstable angina, uncontrolled hypertension, NYHA class III/IV heart failure, or recent stroke, deep vein thrombosis or myocardial infarction in the EU [9] and should not be used to treat asymptomatic hyperuricaemia in the USA [8]. Local prescribing information should be consulted for detailed information regarding drug interactions, use in special populations, contraindications and other warnings and precautions.
7 Current Status of Lesinurad in Hyperuricaemia of Gout
Gout can be debilitating if poorly controlled, impacting quality of life, social participation and the ability to work [1, 29]. For years, the focus of gout management was acute flare control, although growing knowledge of the condition highlighted the importance of also addressing the underlying pathophysiology of gout, namely hyperuricaemia [1, 30]. To this end, ULT is now a key management approach, with recent US [7] and EU [3] guidelines recommending its use in any patient with frequent [7]/recurrent [3] flares, tophus/tophi [3, 7], renal stones [3]/prior uroliathis [7] and/or chronic kidney disease (CKD) stage 2 or worse [7]. In addition, the EU guideline recommends considering ULT from the first presentation of gout, and advises that ULT is initiated close to when gout is first diagnosed in patients who are aged <40 years, have very high sUA levels and/or comorbidities (e.g. hypertension, renal impairment, heart failure or ischaemic heart disease), as gout symptoms are often worse in these groups [3].
XOIs (e.g. allopurinol, febuxostat) are the recommended first-line ULT agents for most patients [3, 7], with patients who do not achieve their sUA target then advised to switch to a uricosuric agent [3] or combination therapy with an XOI plus a uricosuric agent [3, 7]. A variety of uricosurics have historically been used in the treatment of gout, of which probenecid is the most widely available. However, probenecid is not without limitations [including various concomitant drug restrictions (e.g. due to OAT1 and 3 mediated drug-drug interactions) and regular administration, owing to a short half-life] [10, 29, 31, 32] and use of the drug has declined considerably over the years [33]. Thus, newer uricosuric options have been investigated.
Lesinurad is the first uricosuric agent available in the EU and USA that selectively inhibits URAT1 and OAT4 (Sect. 2) and is the only uricosuric for which efficacy and safety have been confirmed in robust, well-designed, clinical trials. The drug has the convenience of once-daily administration and is indicated in combination with an XOI to treat hyperuricaemia in adults with gout who have not achieved target sUA with an XOI alone (Sect. 6).
Its approval was based predominantly on the findings of three 12-month phase 3 trials, which showed sUA-lowering benefits with lesinurad when in combination with allopurinol in gout patients with an inadequate response to allopurinol (CLEAR 1 and 2; Sect. 4.1) and in combination with febuxostat in tophaceous gout (CRYSTAL; Sect. 4.2). In contrast to the CLEAR trials, not all sUA targets (including <5 mg/dL at 6 months; primary endpoint) were achieved by significantly more lesinurad plus febuxostat than placebo plus febuxostat recipients in CRYSTAL (Sect. 4.2). However, both XOI-naïve and -treated patients were eligible for this trial, and among those whose hyperuricaemia was still uncontrolled despite the pre-randomization febuxostat period (i.e. a patient group consistent with the approved indication), significantly more achieved the primary endpoint sUA target with lesinurad plus XOI than with placebo plus XOI (Sect. 4.2).
The extent by which sUA levels are reduced in gout patients correlates with the degree of clinical benefit achieved (in terms of tophus area and gout flares needing treatment), according to an exploratory pooled analysis of all of the treatment groups (including placebo) of CLEAR 1 and 2 and CRYSTAL [34]. However, in the individual studies, the sUA-lowering benefits of lesinurad plus XOI regimens were generally not associated with clinical improvements, perhaps due to the limited duration of the trials (understandable, given flares can initially increase during ULT), or (in CLEAR 1 and 2) the small number of patients with tophi. Clinical benefit of the lesinurad plus XOI regimens became more apparent during longer-term treatment (up to 24 months) in the study extensions (Sects. 4.1 and 4.2); thus, further longer-term robust data would be of interest, particularly given the potential benefits that disease control may have on measures such as quality of life, pain and/or disability [35, 36]. Although gout guidelines generally recommend that certain sUA targets are achieved with ULT [3, 7], a recent US guideline challenges the value of the ‘treat-to-target’ strategy versus approaches that use symptom minimization to guide treatment [37].
The CLEAR trials allowed allopurinol to be uptitrated to 800 or 900 mg/day at the investigator’s discretion, yet most patients received 300 mg/day (the most common dosage in clinical practice), as this was regarded as medically appropriate by the treating physicians prior to screening. Given this widely used dosage is likely to enable sUA target achievement in only half of patients [38], robust studies assessing lesinurad plus allopurinol in patients whose gout is inadequately responsive to the higher recommended dosages of allopurinol would be of interest. However, such studies may be of little use, given that in a recent gout trial that encouraged physicians to treat-to-target with allopurinol, 300 mg/day was still the most common dosage titrated to (perhaps due to perceptions of higher dosage intolerance), even though most of the recipients of this dosage failed to achieve sUA targets [39]. Pharmacoeconomic data and head-to-head trials versus other uricosurics would also be beneficial, although the likelihood of comparative trials being conducted in Europe may be limited by the extensive availability of lesinurad relative to other drugs of the class.
Lesinurad plus XOI regimens are generally well tolerated in patients with gout (Sect. 5). Although kidney stones can develop with uricosuric agents [40], no clinically relevant increase in renal-related or kidney stone AEs was evident with long-term lesinurad plus XOI therapy (Sect. 5.1), perhaps due to administration of lesinurad in the morning (when UA precipitation risk is lowest, due to high volume and pH of the urine) and/or the concomitant use of allopurinol (which inhibits UA production) [15, 16]. Indeed, using a uricosuric in combination with an XOI may reduce kidney stone risk [41]; whether standard urolithiasis prevention measures (e.g. urine alkalinization and high fluid intake) may minimize the likelihood of the acute renal failure that has been seen with some of the newer potent uricosurics under investigation, including lesinurad (Sect. 5.1) is not yet clear [31]. Lesinurad must always be taken with an XOI to minimize the risk of renal-related AEs (Sect. 5.1) and renal monitoring is required (Sect. 6).
CKD is a common comorbidity of gout [31], with such patients appearing to have an increased risk of CKD progression [42]. However, the UA-lowering efficacy of uricosuric agents likely declines with declining renal function [1, 29, 31, 40, 41], with lesinurad not expected to be effective (and is thus contraindicated) in patients with CLCR <30 mL/min, end-stage renal disease, a kidney transplant or requiring dialysis (Sect. 3). Another comorbidity of gout is cardiovascular disease (CVD), with elevated sUA levels being associated with increased cardiovascular risk [43,44,45]. Lesinurad is not recommended for gout patients with certain cardiovascular conditions in the EU (Sect. 6), as MACE have occurred with lesinurad regimens (Sect. 5), although a causal relationship has not been determined.
CKD, CVD and other comorbidities increase the likelihood of patient polypharmacy and thus drug interactions, with the potential for drug interactions with uricosuric agents being well recognized [29, 40]. However, lesinurad does not inhibit OAT 1 or 3 (which are key transporters in the renal secretion of numerous drugs) to any clinically relevant extent in vivo (Sect. 3.1), limiting the likelihood of some of the drug interactions that complicate probenecid use [32].
In conclusion, lesinurad, used in combination with an XOI, is an effective and generally well tolerated option for the treatment of hyperuricaemia in adults with gout who have not achieved target sUA levels with an adequate dosage of an XOI alone.
Data Selection Lesinurad: 115 records identified
Duplicates removed | 23 |
Excluded at initial screening (e.g. press releases; news reports; not relevant drug/indication) | 8 |
Excluded during initial selection (e.g. preclinical study; review; case report; not randomized trial) | 2 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 37 |
Cited efficacy/tolerability articles | 12 |
Cited articles not efficacy/tolerability | 33 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Lesinurad, Zurampic, gout, hyperuricemia, uric acid. Records were limited to those in English language. Searches last updated 17 March 2017 | |
References
Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388(10055):2039–52.
Perez-Ruiz F, Dalbeth N, Bardin T. A review of uric acid, crystal deposition disease, and gout. Adv Ther. 2015;32(1):31–41.
Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42.
National Kidney Foundation. Gout, hyperuricemia & chronic kidney disease. 2016. https://www.kidney.org/atoz/content/gout. Accessed 24 Mar 2017.
Khanna P, Tafesse E, Baumgartner S, et al. Comparing the burden of illness of patients with tophaceous and non-tophaceous gout in France, Germany, Italy, Spain, UK, and USA [abstract no. 226]. Arthritis Rheumatol. 2016;68(Suppl 10).
Nyberg F, Horne L, Morlock R, et al. Comorbidity burden in trial-aligned patients with established gout in Germany, UK, US, and France: a retrospective analysis. Adv Ther. 2016;33(7):1180–98.
Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–46.
Zurampic® (lesinurad) tablets, for oral use: US prescribing information. 2016. http://irwdpi.com/zurampic/zurampic.pdf. Accessed 3 Apr 2017.
Grünenthal GmbH. Zurampic 200 mg film-coated tablets: EU summary of product characteristics. 2016. http://www.ema.europa.eu/. Accessed 24 Mar 2017.
Miner J, Tan PK, Hyndman D, et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther. 2016;18(1):214.
Shen Z, Yeh LT, Wallach K, et al. In vitro and in vivo interaction studies between lesinurad, a selective urate reabsorption inhibitor, and major liver or kidney transporters. Clin Drug Investig. 2016;36(6):443–52.
Shen Z, Rowlings C, Kerr B, et al. Pharmacokinetics, pharmacodynamics, and safety of lesinurad, a selective uric acid reabsorption inhibitor, in healthy adult males. Drug Des Devel Ther. 2015;9:3423–34.
Perez-Ruiz F, Sundy JS, Miner JN, et al. Lesinurad in combination with allopurinol: results of a phase 2, randomised, double-blind study in patients with gout with an inadequate response to allopurinol. Ann Rheum Dis. 2016;75(6):1074–80.
Fleischmann R, Kerr B, Yeh LT, et al. Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatology. 2014;53(12):2167–74.
Saag KG, Fitz-Patrick D, Kopicko J, et al. Lesinurad combined with allopurinol. A randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis. Rheumatol. 2017;69(1):203–12.
Bardin T, Keenan RT, Khanna PP, et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis. 2016. doi:10.1136/annrheumdis-2016-209213.
Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad, a novel selective uric acid reabsorption inhibitor, in combination with febuxostat, in patients with tophaceous gout: the CRYSTAL phase III clinical trial [abstract no. SAT0329 plus poster]. Ann Rheum Dis. 2015;74(Suppl 2):778.
Saag KG, Becker MA, Storgard C, et al. Examination of serum uric acid (SUA) lowering and safety with extended treatment with lesinurad and allopurinol in subjects with gout [abstract no. THU0495]. Ann Rheum Dis. 2016;75(Suppl 2):371.
Bardin T, Dalbeth N, Terkeltaub R, et al. Clinical response of tophus and flares to extended use of lesinurad in combination with a xanthine oxidase inhibitor in patients with gout [abstract no. 209]. Arthritis Rheumatol. 2016;68(Suppl 10).
Shen Z, Gillen M, Tieu K, et al. Supratherapeutic dose evaluation and effect of lesinurad on cardiac repolarization: a thorough QT/QTc study. Drug Des Devel Ther. 2016;10:3509–17.
Gillen M, Valdez S, Zhou D, et al. Effects of renal function on pharmacokinetics and pharmacodynamics of lesinurad in adult volunteers. Drug Des Devel Ther. 2016;10:3555–62.
US National Institutes of Health. ClinicalTrials.gov identifier NCT01510769. 2016. https://clinicaltrials.gov. Accessed 24 Mar 2017.
Dalbeth N, Jones G, Terkeltaub R, et al. Efficacy and safety in patients with tophaceous gout receiving lesinurad and febuxostat combination therapy: interim analysis of an extension study [abstract no. 2352]. Arthritis Rheumatol. 2015;67(Suppl 10).
Saag KG, Bardin T, So A, et al. Analysis of gout subjects receiving lesinurad and allopurinol combination therapy by baseline renal function [abstract no. 2112]. Arthritis Rheumatol. 2015;67(Suppl 10).
Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad and febuxostat combination therapy: analysis of treatment based on patient baseline renal function [abstract no. 2353]. Arthritis Rheumatol. 2015;67(Suppl 10).
Khanna PP, Baumgartner S, Robinson J. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with xanthane oxidase inhibitors: SUA and flares composite endpoint from phase III studies in gout patients (CLEAR 1, CLEAR 2 and CRYSTAL) [abstract no. THU0527]. Ann Rheum Dis. 2016;75(Suppl 2):382–3.
Becker MA, Keenan RT, Khanna P, et al. Integrated safety of lesinurad, a novel uric acid reabsorption inhibitor for the treatment of gout [abstract no. 207]. Arthritis Rheumatol. 2016;68(Suppl 10).
Terkeltaub R, Malamet R, Bos K, et al. Renal safety of lesinurad: a pooled analysis of phase III and extension studies [abstract no. 206]. Arthritis Rheumatol. 2016;68(Suppl 10).
Sattui SE, Gaffo AL. Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther Adv Musculoskelet Dis. 2016;8(4):145–59.
Keenan RT, Schlesinger N. New and pipeline drugs for gout. Curr Rheumatol Rep. 2016;18(6):32.
Terkeltaub R. Emerging uricosurics for gout. Expert Rev Clin Pharmacol. 2016:1–3. doi:10.1080/17512433.2017.1271709.
Lepist EI, Ray AS. Renal drug-drug interactions: what we have learned and where we are going. Expert Opin Drug Metab Toxicol. 2012;8(4):433–48.
Robbins N, Koch SE, Tranter M, et al. The history and future of probenecid. Cardiovasc Toxicol. 2012;12(1):1–9.
Terkeltaub R, Perez-Ruiz F, Storgard C, et al. Relationship between sustained lowering of serum urate levels and improvements in gout flares and tophus area: pooled exploratory analysis of gout subjects receiving lesinurad and xanthine oxidase inhibitor combination therapy [abstract no. SAT0313]. Ann Rheum Dis. 2015;74(Suppl 2):771–2.
Wood R, Fermer S, Ramachandran S, et al. Patients with gout treated with conventional urate-lowering therapy: association with disease control, health-related quality of life, and work productivity. J Rheumatol. 2016;43(10):1897–903.
Khanna P, Baumgartner S, Robinson J. The impact of gout flares on patient-reported assessments of pain and disability [abstract no. FRI0589 plus poster]. Ann Rheum Dis. 2016;75(Suppl 2):655–6.
Qaseem A, Harris RP, Forciea MA. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(1):58–68.
Singh JA, Storgard C, Baumgartner S, et al. Use of high-dose allopurinol to reach serum uric acid targets in patients with gout across multiple countries [abstract no. 1186]. In: ACR/ARHP Annual Meeting; 2013.
Becker MA, Fitz-Patrick D, Choi HK, et al. An open-label, 6-month study of allopurinol safety in gout: The LASSO study. Semin Arthritis Rheum. 2015;45(2):174–83.
Stamp LK, Chapman PT. Urate-lowering therapy: current options and future prospects for elderly patients with gout. Drugs Aging. 2014;31(11):777–86.
Bach MH, Simkin PA. Uricosuric drugs: the once and future therapy for hyperuricemia? Curr Opin Rheumatol. 2014;26(2):169–75.
Stack A, Blak B, Johnson M, et al. Association of gout with risk of advanced chronic kidney disease [abstract no. 3188]. Arthritis Rheumatol. 2016;68(Suppl 10).
Borghi C, Nuevo J, Medina J, et al. Association between serum uric acid levels and cardiovascular risk. A post hoc analysis of the European Cardiovascular Risk Patients: Disease Prevention and Management in Usual Daily Practice (EURIKA) Study [poster no. FRI0337]. In: 16th EULAR Annual European Congress of Rheumatology; 2015.
Baumgartner S, Morlock R. Hyperuricemia and the risk for cardiovascular disease: individual patient meta-analysis of 77,045 individuals enrolled in 15 epidemiologic studies [poster no. FRI0591]. In: EULAR Annual European Congress of Rheumatology; 2016.
Cheetham TC, Nichols GA, Harrold LR, et al. The association between serum uric acid levels and acute myocardial infarction in incident gout patients [abstract no. PMS18]. In: ISPOR 21st Annual International Meeting; 2016.
Acknowledgements
During the peer review process, the manufacturer of lesinurad was offered the opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Emma Deeks is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: S. Chohan, Arizona Arthritis and Rheumatology Associates, Phoenix, AZ, USA; A. L. Gaffo, University of Alabama at Birmingham and Rheumatology Section, Birmingham VA Medical Center, Birmingham, AL, USA; P. Khanna, Medicine-Rheumatology, University of Michigan, Ann Arbor, MI, USA.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Lesinurad: A Review in Hyperuricaemia of Gout. Drugs Aging 34, 401–410 (2017). https://doi.org/10.1007/s40266-017-0461-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-017-0461-y