Abstract
Sarcopenia of age is prevalent and costly and proven pharmacological interventions are currently lacking. The pathophysiology of sarcopenia is incompletely understood but appears to involve multiple pathways, including inflammation, hormonal dysregulation, impaired regeneration, mitochondrial dysfunction and denervation. There are several ways in which we might select potential pharmacological interventions for testing in clinical trials. These include a ‘bottom-up’ approach using basic science to elucidate the molecular processes involved and identify potential targets from this knowledge—a strategy that has led to the development of myostatin inhibitors. A ‘top-down’ approach might use observational data to examine the association between physical function and use of certain medications, such as the association between angiotensin-converting enzyme inhibitors with slower decline in physical function. Once a pharmacological intervention has been proposed, efficacy must be demonstrated in this complex multi-morbid population. Both muscle mass and muscle function need to be measured as outcomes, but these outcomes require large sample sizes and sufficient follow-up to detect change. Biomarkers that can predict the response of sarcopenia to intervention after a short time would greatly assist our ability to select candidate interventions in short proof-of-concept trials. Further development of trial methods is required to accelerate progress in this important area of medicine for older people.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sarcopenia is prevalent and costly but lacks effective pharmacological treatments. |
Pharmacological interventions could be selected by ‘bottom-up’, ‘top-down’ or combination approaches. |
A tiered approach to outcomes measurement is needed, with the development of biomarkers that predict longer-term outcomes. |
1 Introduction
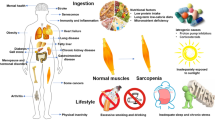
The term sarcopenia is derived from the Greek words for ‘poverty’ and ‘flesh’ and was first used in 1989 to describe age-related loss of muscle mass [1]. Sarcopenia is associated with poor current and future health [2], increased risk of physical disability, falls, poor quality of life and admission to hospital and care homes [3] as well as increased risk of fragility fracture [4]. It is also costly; the estimated direct healthcare cost attributable to sarcopenia in 2000 in the USA was $US18.5 billion [5], and this is only set to increase.
Sarcopenia remains largely undiagnosed and undertreated because of difficulties with a universally accepted definition, a need for equipment to measure muscle mass and dispute regarding which outcomes would best indicate treatment efficacy [6]. However, agreement has emerged that low muscle mass alone is of insufficient clinical relevance if not combined with muscle weakness and/or functional impairment [7]. The European Working Group on Sarcopenia in Older People (EWGSOP) consensus conference in 2010 proposed a diagnosis based on low muscle mass (appendicular lean mass/height2) with either low muscle strength (grip strength) or low physical performance (gait speed) [8]. Similar guidelines have emerged from other bodies, including in the USA [9]; however, the diagnostic criteria for sarcopenia continue to evolve as our understanding of the epidemiology and pathophysiology improve.
Sarcopenia is prevalent in older people; it occurs in at least 1 in 20 community-dwelling individuals and up to one in three frail older people living in nursing homes, and prevalence increases with age [10]. There is a clear need for intervention in sarcopenia; however, the best way to identify candidate interventions and choose which to progress into clinical trials and clinical application is less clear. This paper reviews two main aspects of pharmacological intervention in sarcopenia. First, we consider the ways in which candidate pharmacological interventions might be selected for testing in clinical trials. Second, we discuss what criteria might need to be met to deem a pharmacological intervention as successful and the various different outcome data that might therefore be required.
2 Pathophysiology of Sarcopenia
Multiple physiological pathways contribute to the maintenance of muscle mass and function. These affect cellular function, turnover, growth, repair and the net balance of protein synthesis and degradation [11]. Loss of muscle mass can therefore occur from alterations in multiple interacting pathways. It is therefore possible that several different subtypes of sarcopenia may be associated with different pathways. Key pathways thought to be involved in muscle tissue metabolism [12] are demonstrated in Fig. 1. A comprehensive account of the pathophysiology of sarcopenia is beyond the scope of this article, but Fig. 1 serves to illustrate the complex biology of the condition.
Some key cellular pathways involved in muscle tissue signalling and metabolism. 4EBP1 4E binding protein 1, AMPK 5′AMP activated protein kinase, BAD Bcl-2 associated death promoter, eIF2B eukaryotic initiation factor 2B, FoxO forkhead box subgroup O, GSK3β glycogen synthase kinase 3 beta, IGF insulin-like growth factor, LC-3 microtubule-associated proteins 1A/1B light chain 3A, MAFbx muscle atrophy F-box, MAPK mitogen-activated protein kinase, MAP2K mitogen-activated protein kinase kinase, mTOR mammalian target of rapamycin, MuRF-1 muscle RING-finger protein-1, NFκβ nuclear factor kappa-light-chain-enhancer of activated B cells, PI3 K phosphoinositide 3 kinase, PKB protein kinase b, ROS reactive oxygen species, S6K1 ribosomal protein S6 kinase beta-1, TNF tumour necrosis factor
3 Selection of Pharmacological Intervention
3.1 What Does an Ideal Intervention Look Like?
An ideal pharmacological intervention would be effective in all cases of sarcopenia, no matter what the causative pathology, and would ideally be effective at treating other diseases of old age to avoid exacerbating the problem of polypharmacy. It should be inexpensive, orally administered with an acceptable frequency of administration to aid adherence, and have a low risk of causing side effects.
There are various means by which pharmacological interventions could be selected for testing in trials against sarcopenia. These include ‘top-down’ approaches through the use of observational data or reviewing the off-target effects of existing drugs. They also include a ‘bottom-up’ approach using insights from basic biology or ‘omics’ techniques to select pharmacological targets. These are summarised in Fig. 2.
3.2 Top-Down Approaches
Employing observational studies to examine the association between physical function and medication use in routine clinical practice is a fruitful approach to target identification. Such approaches have demonstrated that angiotensin-converting enzyme (ACE) inhibitors are associated with slower decline in muscle mass, muscle strength and walking speed [13, 14]. Knowledge of the interaction of angiotensin at a cellular level in muscle (inhibition of insulin-like growth factor [IGF]-1 action as well as stimulation of nuclear factor kappa-light-chain-enhancer of activated B cells [NF-κβ] and tumour necrosis factor [TNF]-α) has since provided supportive evidence for this interaction. Observational studies have also demonstrated that people with higher levels of antioxidants have better physical function [15]. This finding accords with insights from basic biology given that elevated levels of reactive oxygen species and pro-inflammatory cytokines are found in many conditions where low muscle mass is found and are thought to activate NF-κβ or the ubiquitin proteasome system via muscle RING-finger protein (MuRF)-1 resulting in protein degradation [12]. Allopurinol, an inhibitor of xanthine oxidase that is known to reduce oxidative stress across a range of diseases, including vascular disease [16], has been associated with improved functional outcomes in patients undergoing rehabilitation [17], again suggesting that agents that reduce oxidative stress are worth testing in patients with sarcopenia.
Other agents have been noted as having positive associations with muscle function, but biological plausibility is either lacking or the findings suggest our incomplete understanding of biological pathways. An example here is HMG-CoA reductase inhibitors (statins), which have been associated with greater proximal muscle strength in community-dwelling individuals [18, 19]. This is despite the known phenomenon of statin-induced myopathy. Observational studies have also revealed adverse associations with function, for example, with the use of furosemide and calcium channel blockers [20]. While such adverse findings might appear less useful at first glance, such data may help practitioners avoid making sarcopenia worse and might also suggest new avenues to explore in terms of underlying mechanisms that lead to sarcopenia.
3.3 Bottom-Up Approaches
A ‘bottom-up approach’ starts with the molecular processes involved in muscle atrophy and sarcopenia and identifies potential targets from this knowledge. Such an approach has revealed that myostatin (a member of the transforming growth factor [TGF]-β superfamily) plays a crucial role in muscle growth via interaction with the activin receptor [6]. Myostatin negatively regulates muscle growth via inhibition of the phosphoinositide 3 kinase/protein kinase B (PI3K/AKT) pathway and reduction of the levels of myogenic regulatory factors [12]. Myostatin is inhibited by a protein called follistatin.
Myostatin inhibition is required for muscle growth and development [6], and levels of myostatin have been observed to be increased in conditions with similarities to sarcopenia, such as heart failure and AIDS-related cachexia [21]. Pharmacological manipulation of myostatin has therefore become a key research target in sarcopenia [22]. Several different approaches have been taken to this, including myostatin-blocking antibodies, myostatin propeptide, follistatin, follistatin-related proteins, soluble myostatin receptors, small interfering RNA and small chemical inhibitors [22]. Several agents have now progressed to clinical trials, for example, the humanised monoclonal antibody to myostatin, LY2495655. A multicentre randomised controlled trial in 2015 of LY2495655 in older people with recent falls and low muscle strength met its primary endpoint of increased appendicular lean mass at 24 weeks. It also improved fast gait speed, stair climbing time and chair rise with arms but not other functional measures [23].
Ultimately, a long-term imbalance between rate of protein synthesis and breakdown is thought to have the potential to exacerbate age-related loss of muscle tissue [12] due to reduced total and essential amino acid intake as well as altered anabolic–catabolic balance. Branched chain amino acids, such as leucine, have been demonstrated to boost pathways leading to increased protein translation [2]. However, older skeletal muscle has been noted as having significant anabolic resistance to protein nutrition during immobilisation [24], and this has led to the suggestion that recommendations for protein intake should be increased for older people [2].
Approaches altering downstream effects in favour of anabolism are likely therefore to needed in addition to nutritional supplementation. For example, the manipulation of myostatin represents an approach where a conserved signalling pathway has been reviewed [11] and manipulated. Several generic pathway points have been considered with respect to sarcopenia such as caspase inhibitors or proteasome inhibitors [6]. However, many of these would be involved in the cellular transcription pathway more generally and may have widespread effects, including the potential for promotion of cancerous changes.
It is likely that new targets and treatment strategies will emerge as the pathways involved in sarcopenia are elucidated through reviews of gene expression patterns and more information is gained through metabolomics and proteomics [25, 26]. The FRAILomic initiative is collecting urine and blood biomarkers from 75,000 participants who will be stratified as frail or not frail according to Fried’s criteria [27]. Over 70% of the participants are aged >65 years [27]. The challenge of this approach will be the volume of information and the massive task of translating this information into the development of new pharmacological agents. The clinical trials involved will be in a complex population with multiple diseases that contribute to physical, cognitive and functional disability. This large variability will increase the difficulty of detecting both treatment effects and adverse effects [7]. The fact that currently used drugs have a better known side-effect profile provides further advantage to their reappropriation and may pave the way for further ‘new tricks for old drugs’ [2].
The number of potential biological targets is likely to be far higher than the number of existing drugs that could be repurposed. There is therefore a limit to what observational data can tell us about target selection, and some way of refining our ability to select from the large number of targets suggested by basic biology will be needed. If a specific target or biomarker can be developed, then it may be that action on these new targets could be screened for using high-throughput drug screening, using both marketed drugs and those from compound libraries. Developing robust validated preclinical models is therefore essential if we are to effectively screen large numbers of candidate molecules, but this task is made more complex by the large number of biological pathways involved in the genesis of sarcopenia.
4 Outcome Measures
The history of the development of osteoporosis diagnosis and treatment reveals interesting parallels with the field of sarcopenia. Little progress was made in developing effective treatments for osteoporosis until a set of diagnostic criteria were agreed; the development of effective treatments then helped to establish these diagnostic criteria in clinical practice. One of the main issues with sarcopenia research and treatment has been the lack of an established core outcome set [28]. In health, it appears there is a relatively strong correlation between muscle mass and strength; however, the assumption that an increase in muscle mass will be associated with an increase in function does not always hold [11]. In general there is a ‘hierarchy of response’ to any given intervention [11]. For example, in response to resistance training in older people, a large effect may be seen in quadriceps strength but this may not translate into improved physical function [29]. Similarly, interventions might increase muscle mass but not necessarily muscle strength; a situation seen in short-term trials of myostatin inhibitors [30]. The difficulty in selecting pharmacological interventions is therefore exacerbated by the difficulty in deciding how to declare an intervention successful.
4.1 Progression of Candidate Interventions Through Trial Stages
Different outcome measures may be of use at different trial phases. For example, early-phase outcomes need to be highly responsive (so as to minimise sample size) but should correlate with later trial outcomes. Such outcomes might reflect molecular and cellular changes in muscle without necessarily reflecting whole body function. Middle phase outcomes should reflect physical function and again need to correlate with later-phase outcomes; a failure of such outcomes to predict late-phase trial success renders any middle-phase results of little value. Late-phase outcomes need to reflect what is important to individual patients, health services and society as a whole. Again, parallels can be drawn with osteoporosis, where bone turnover markers, bone mineral density and fractures form key outcomes from early-, middle- and late-phase trials. Potential early-, middle- and late-phase outcomes for sarcopenia trials are suggested in Table 1.
4.2 Early-Phase Outcomes
4.2.1 Biomarkers
Biomarkers are objectively measurable indicators of biological or pathological processes and have the potential to be used as markers of response to a therapeutic intervention [31]. Measuring changes in physical function and muscle mass in sarcopenia requires sufficient time for measurable changes to occur (typically months), and because of the variability of these measures, sample sizes of typically 50–150 per arm are required. Reliable, responsive, easy-to-measure biomarkers for the detection and monitoring of sarcopenia could allow smaller, more rapid proof-of-concept trials of pharmacological interventions [26].
Numerous biomarkers have been proposed [26] but are often not specific to sarcopenia [25]. Type III collagen is a subtype of collagen found in skeletal muscles. During its synthesis from procollagen III the N-terminal propeptide (P3NP) is cleaved and released into the circulation in direct proportion to type III synthesis and is therefore a measure of tissue remodelling [32]. In a cross-sectional study, plasma concentrations of P3NP were found to be inversely related to total and appendicular lean mass in postmenopausal women but not in older men [33].
C-terminal agrin fragment (CAF) is another proposed biomarker. During neuromuscular remodelling, agrin is cleaved by neurotrypsin-releasing CAF, which is detectable in plasma. Increases in serum CAF have been associated with neuromuscular junction disruption, muscle fibre atrophy and dysfunction [26] and have been reported to be elevated in older adults with sarcopenia compared with age-matched controls [32]. In a trial of 23 older adults, women had a higher baseline circulating CAF than men, and the level increased by 10.4% after 6 weeks of resistance training. This increase was correlated with significant changes of cross-sectional area of vastus lateralis (p = 0.008) [32]. A study in 2013 investigated CAF levels in patients admitted acutely with hip fractures and found that serum levels were significantly higher (p < 0.001) in all patients with sarcopenia (diagnosed using the EWGSOP definition) at 172.2 pmol/L in sarcopenic patients and 93.0 pmol/L in non-sarcopenic patients [34]. This suggests that CAF levels may be of relevance in both community-dwelling and hospitalised patients with sarcopenia.
It may be that biomarkers such as P3NP and CAF have a role in helping to identify sarcopenia subtypes, but considerable work is needed to establish whether these biomarkers can predict response of muscle to interventions and hence whether they are valid measures for use in trials. It is probable that given the multiple pathways involved in the pathogenesis of sarcopenia, a panel of biomarkers will need to be measured so that responses in a particular pathway affected by a candidate intervention are not missed.
4.3 Middle-Phase Outcomes
4.3.1 Muscle Mass
Assessment of muscle mass can be made using dual energy X-ray absorptiometry (DXA), computed tomography (CT) or magnetic resonance imaging (MRI) [35]. In particular, CT and MRI scanning have been used widely in oncology studies assessing the impact of sarcopenia [36]. Bioelectrical impedance analysis can also be used to predict lean muscle mass; results depend on the instrument used and the conversion equation employed and are also subject to variation depending on peripheral oedema and hydration status [37]. However, bioimpedance is quick and easy to perform and undoubtedly has a key role in screening for sarcopenia, although it is not the preferred technique for measuring muscle mass change in trials. A non-radiological method to assess skeletal muscle mass has been validated. This is based on D3-creatine dilution from an oral dose and detection of urinary creatinine enrichment by isotope ratio mass spectrometry. This has been used in longitudinal assessment of changes in skeletal muscle mass [38] and reportedly has the potential to be superior to DXA [26]. However, it requires specialist techniques that are likely to have limited availability [26].
4.3.2 Functional Measures Including Muscle Strength
Because the definition of sarcopenia encompasses both muscle mass and muscle function; it is essential to include measurement of muscle function in any suite of sarcopenia outcomes. Isometric hand grip has been widely used as a general indicator of functional status [39], as have measures of lower limb extremity strength such as leg press strength and isokinetic leg extension strength [39]. These measures evaluate muscle function directly and thus provide insight into physiology. However, they do not always reflect the way in which muscle is used in daily life, and some measures (e.g. grip strength) may not be responsive to interventions. Other measures examine muscle function at a whole body level, for example, the time to walk 400 m [39], 6-min walking distance [40], gait speed (usually over a 3- or 4-m course) and timed up and go test [41]. These measures better reflect how muscles are used in activities of daily living, but many are composites of muscle, nerve and cardiorespiratory function, which may dilute the impact of muscle-specific intervention effects.
4.3.3 Composite Measures of Function
The Short Physical Performance Battery (SPPB) is a widely used composite measure of lower extremity function [42] that has been validated and shown to be responsive to intervention [43] and associated with a clinically meaningful change [35]. It consists of measures of walking, balance and sit to stand [43] and has been shown to correlate with quality of life [44]. There are perhaps more data linking the SPPB with relevant outcomes such as death, hospitalisation, future dependency and falls than for any other functional measure; the SPPB is also easy to perform. It is therefore emerging as one of the functional measures of choice in sarcopenia trials and is under consideration as a surrogate marker of efficacy for future sarcopenia drug licensing.
4.3.4 Activities of Daily Living and Quality of Life Measures
Questionnaires that assess quality of life, functional status and psychological state are also potential outcome measures [42]. These can either be generic questionnaires or specific to sarcopenia, for example, SarQOL (sarcopenia and quality of life) is a quality-of-life measure that aims to be more specific to sarcopenia with seven main domains [45] and has recently been validated in English [46].
4.4 Late Outcomes
These outcomes are much less specific in terms of alteration in muscular function but represent outcomes that will be of greatest importance to patients and health and social care funders. Late outcomes might include measures such as number of falls, hospital admissions, need for care assistance or risk of institutionalisation [35].
Given the likely heterogeneity of both individuals and pathophysiological subtypes of sarcopenia, the inspection of single variables will inevitably result in a partial and incorrect picture. Multiplex analyses are gaining increasing plausibility with the development of ‘omics’ techniques, and this approach may well be of increasing importance in an approach to biomarkers of sarcopenia. These analyses involve complex statistics but might potentially allow the early detection of subclinical syndromes as well as aid clinical diagnosis and monitor response to treatment [26]. The downside of such methods is that the multivariate nature of such analyses will likely require relatively large numbers of patients. Any use of biomarkers to predict response to interventions in sarcopenia is limited by the lack of data on efficacious interventions—it is necessary to demonstrate that an intervention improves sarcopenia before one can tell whether changes in a biomarker for sarcopenia predict improvements in sarcopenia itself.
5 Conclusion
Sarcopenia is not currently routinely diagnosed in clinical practice. This is unlikely to become the case until a proven benefit of intervention can be established. Studies have taken place for many years evaluating exercise interventions as well as nutritional supplementation in sarcopenia. Resistance training remains the key intervention proven to improve muscle function and physical performance in patients with sarcopenia. Nutritional and some pharmacological interventions may only be of benefit when combined with exercise training; however, exercise interventions may not necessarily add benefit to all pharmacological interventions.
Exercise interventions in sarcopenia are likely only to be applicable to a limited proportion of the population given their baseline functional status. Ideal agents might mimic exercise as an intervention, perhaps by acting on the same physiological pathways and thus would not need to be combined with exercise and be of benefit in patients who cannot or will not exercise. For example, a trial that assessed 130 older patients with functional impairment found that the group supplemented with perindopril had an improvement in exercise capacity equivalent to that reported after 6 months of exercise training [47]. A further trial demonstrated that perindopril did not enhance the effect of exercise training on physical function, providing further support that the effects of exercise and pharmacological intervention in this case are not additive [48].
Pharmacological intervention in sarcopenia is therefore an extremely topical and relevant avenue in sarcopenia treatment. The best way to select pharmacological interventions for trials is likely to be a combination of the top-down and bottom-up approaches, acting in a complementary fashion. Further work is required to define the optimum set of outcome measures used in early-, middle- and late-phase trials; outcomes will need to be multidimensional to ensure success is defined not just by improvements in pathology and physiology but also in terms of patient-centred outcomes—improvement in function and increased independence. Future trials need to place these outcomes centre stage if patients and clinicians are to be convinced of the benefits of pharmacological interventions for sarcopenia.
References
Rosenberg I. Summary comments. Am J Clin Nutr. 1989;50:1231–3.
Sayer A, Robinson S, Patel H, et al. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing. 2013;42:145–50.
Roberts C, Dodds R, Sayer A. Current clinical care of older adults with sarcopenia. J Clin Densitom. 2015;18(4):193–8.
Grossmann M. Myostatin inhibition: a new treatment for androgen deprivation-induced sarcopenia? J Clin Endocrinol Metab. 2014;99:3625–8.
Janssen I, Shepard D, Katzmarzyk P, et al. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5.
Brotto M, Abreu E. Sarcopenia: pharmacology of today and tomorrow. J Pharmacol Exp Ther. 2012;343(3):540–6.
Cesari M, Fielding R, Benichou O, et al. Pharmacological interventions in frailty and sarcopenia: report by the international conference on frailty and sarcopenia research task force. J Frailty Aging. 2015;4(3):114–20.
Cruz-Jentoft A, Baeyens J, Bauer J, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–23.
Fielding R, Vellas B, Evans W, et al. Sarcopenia: an undiagnosed condition in older adults. current consensus definition: prevalence, etiology, and consequences. international working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56.
Cruz-Jentoft A, Landi F, Schneider S, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Intitiative (EWGSOP and IWGS). Age Ageing. 2014;43:748–59.
Compston J, Fearon K. Emerging therapeutic concepts for muscle and bone preservation/formation. Bone. 2015;80:157–61.
Lecker S, Jagoe R, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51.
Onder G, Penninx B, Balkrishnan R, et al. Relation between use of angiotensin converting enzyme inhibitors and muscle strenght and physical function in older women: an observational study. Lancet. 2002;359:926–30.
Carter C, Groban L. Role of the renin-angiotensin system in age-related sarcopenia and diastolic dysfunction. Aging Health. 2008;4(1):37–46.
Dodds R, Sayer A. Sarcopenia. Arq Bras Endocrinol Metabol. 2014;58(5):464–9.
George J, Carr E, Davies J, et al. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–16.
Beveridge L, Ramage L, McMurdo M, et al. Allopurinol use is associated with greater functional gains in older rehabilitation patients. Age Ageing. 2013;42(3):400–4.
Woo J, Leung J, Sham A, et al. Defining sarcopenia in terms of risk of physical limitations: a 5-year follow-up study of 3,153 Chinese men and women. J Am Geriatr Soc. 2009;57(12):2224–31.
Onder G, Della Vedova C, Landi F. Validated treatments and therapeutics prospectives regarding pharmacological products for sarcopenia. J Nutr Health Aging. 2009;13(8):746–56.
Ashfield T, Syddall H, Martin H, et al. Grip strength and cardiovascular drug use in older people: findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39:185–91.
Guasconi V, Puri P. Epigentic drugs in the treatment of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care. 2008;11(3):233–41.
Tsuchida K. Targeting myostatin for therapies against muscle-wasting disorders. Curr Opin Drug Discov Devel. 2008;11(4):487–94.
Becker C, Lord S, Studenski S, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet. 2015;3(12):948–57.
Budui S, Rossi A, Zamboni M. The pathogenetic basis of sarcopenia. Clin Cases Miner Bone Metab. 2015;12(1):22–6.
Abellan van Kan G, Cameron Chulmea W, Gillette-Guyonet S, et al. Clinical trials on sarcopenia: methodological issues regarding phase 3 trials. Clin Geriatr Med. 2011;27(3):471–82.
Calvani R, Marini F, Cesari M, et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle. 2015;6:278–86.
Erusalimsky J, Grillari J, Grune T, et al. In search of ‘omics’-based biomarkers to predict risk of frailty and its consequences in older individuals: the FRAILOMIC initiative. Gerontology. 2016;62(2):182–90.
Reginster J, Cooper C, Rozzili R et al. Recommendations for the registration of drugs to treat sarcopenia. Aging Clin Exp Res. 2016;28;47–58.
Liu C, Latham N. Progressive resistance strength training for improving physical function in older adults: systematic review. Cochrane Database Syst Rev. 2009;3:CD002759.
Padhi D, Higano C, Shore N, et al. Pharmacologic inhibition of myostatin and changes in lean body mass and lower extremity size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2014;99:E1967–75.
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95.
Fragala M, Jajtner A, Beyer K, et al. Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle. 2014;5(2):139–48.
Berry S, Ramachandran V, Cawthon P, et al. Procollagen type II N-terminal peptide (P3NP) and lean mass: a cross-sectional study. J Frailty Aging. 2013;2:129–34.
Marzetti E, Calvani R, Lorenzi M, et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fracture patients. Exp Gerontol. 2014;60:79–82.
Abellan Van Kan G, Andre E, Bischoff-Ferrari H, et al. Carla task force on sarcopenia: propositions for clinical trials. J Nutr Health Aging. 2009;13(8):700–7.
Yip C, Dinkel C, Mahajan A, et al. Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging. 2015;6(4):489–97.
Baracos V, Kazemi-Bajestani S. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol. 2013;45(10):2302–8.
Loncar G, Omersa D, Cvetinovic N, et al. Emerging biomarkers in heart failure and cardiac cachexia. Int J Mol Sci. 2014;15(12):23878–96.
Pahor M. Mobility and functional outcomes for sarcopenia trials. J Frailty Aging. 2015;4(3):123–4.
Burton L, Sumukadas D, Witham M et al. Does spironolactone improve exercise capactiy in functionally impaired older people without heart failure? [abstract]. In: British Geriatrics Society Autumn Meeting, 12–14 Oct 2011. Brighton Centre. Brighton: British Geriatrics Society; 2011. Abstr no. 2.
Dennis R, Ponnappan U, Kodell R, et al. Immune function and muscle adaptations to resistance exercise in older adults: study protocol for a randomized control trial of a nutritional supplement. Trials. 2015;16:121.
LeBrasseur N, Lajevardi N, Miciek R, et al. Effects of testosterone therapy on muscle performance and physical function in older men with mobility limitations (The TOM trial): design and methods. Contemp Clin Trials. 2009;30(2):133–40.
LIFE Study investigators, Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165.
Oh B, Cho B, Choi H-C, et al. The influence of lower-extremity function in elderly individuals’ quality of life (QOL): an analysis of the correlation between SPPB and EQ-5D. Arch Gerontol Geriatr. 2014;58(2):278–82.
Beaudart C, Biver E, Reginster J, et al. Development of a self-administrated quality of life questionnaire for sarcopenia in elderly subjects: the SarQoL. Age Ageing. 2015;44(6):960–6.
Beaudart C, Edwards M, Moss C, et al. English translation and validation of the SarQoL®, a quality of life questionnaire specific for sarcopenia. Age Ageing 2016. doi:10.1093/ageing/afw192
Witham MW, Sumukadas D, McMurdo ME. ACE inhibitors for sarcopenia—as good as exercise training? Age Ageing. 2008;37:363–5.
Sumukadas D, Band M, Miller S, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69(6):736–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Amanda Kilsby, Avan Sayer and Miles Witham have no conflicts of interest relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Kilsby, A.J., Sayer, A.A. & Witham, M.D. Selecting Potential Pharmacological Interventions in Sarcopenia. Drugs Aging 34, 233–240 (2017). https://doi.org/10.1007/s40266-017-0444-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-017-0444-z