Abstract
Gout is increasingly seen in the elderly population, in large part due to physiological decline in renal function with age, and as a result of therapy for comorbidities, in particular the use of diuretic therapies for hypertension and congestive heart failure. Urate-lowering therapy (ULT) is the cornerstone of successful long-term gout management with the aim of achieving a sustained reduction in urate (<0.36 mmol/L, or lower [<0.30 mmol/L] in those with tophi). After decades during which there has been relatively little interest in developing new agents to treat gout, the last 5–10 years has seen a plethora of new agents with several now used in routine clinical practice. There has also been a renewed focus on the optimal use of established ULT, specifically allopurinol, which remains the first-line therapy for most patients. There is emerging data on its use in patients with renal impairment and better recognition of risk factors of the rare but potentially lethal allopurinol hypersensitivity syndrome (AHS). Febuxostat, a new xanthine oxidase inhibitor, is now established in everyday practice. Uricosuric agents may be indicated in certain patient groups, whilst a new class of recombinant uricases (pegloticase) given by intravenous infusion may achieve dramatic and rapid urate-lowering effects. Cost and other factors have thus far limited its use to the very severe cases. Furthermore, increased understanding of urate metabolism has led to the development of a number of drugs currently under clinical evaluation. Common therapeutic targets are the urate transporters in the kidney and alternative xanthine oxidase inhibition pathways. These advances bode well for the better management of gout and hyperuricaemia in our elderly patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gout is one of the most common forms of inflammatory arthritis in those aged over 65 years. The highest prevalence (7.3 %) for men is within the 75–84 year age group while in women disease prevalence rises after the age of 85 years to 2.8 % [1]. Although gout frequently affects the elderly, there are few studies of urate-lowering therapies specifically in this population. The lack of clinical studies in this population provides challenges for clinicians treating elderly gout patients. The elderly frequently have multiple co-morbidities including renal impairment and heart disease, poly-pharmacy, and age-related physiological changes, all of which contribute to increased complexity in gout management. Clinicians caring for elderly patients are thus hampered by this lack of data.
Urate-lowering therapy (ULT) is central to successful long-term gout management. There are a number of potential mechanisms for lowering serum urate. These include inhibition of uric acid production through use of xanthine oxidase inhibitors, normalisation of renal uric acid excretion with the use of selective uric acid resorption inhibitors and dissolution of urate though use of recombinant uricases (Fig. 1). There are currently drugs available with each of these groups and with the resurgence of interest in gout there are a number of new agents under development. Herein we focus on the use of urate-lowering drugs in the elderly. Given the lack of specific data in this population, we have focused on the use of ULT in co-morbid conditions frequently encountered in the elderly.
2 General Principles
ULT is recommended when there is an established diagnosis of gout with two or more attacks of acute gout per year, presence of tophi, chronic kidney disease of stage 2 or more, or renal stones [2]. Sustained reduction of serum urate <0.36 mmol/L, or <0.30 mmol/L in the presence of tophi, leads to cessation of gouty attacks and resorption of tophi over time. It is important to recognise that patients may experience gout flares as serum urate reduces and these may continue for many months after target serum urate has been achieved. For this reason, patients should receive adequate prophylaxis against gout flares during the introduction of ULT and have an appropriate management plan for gout flares when they occur. Agents used include non-steroidal anti-inflammatory drugs (NSAIDs), colchicine and prednisone, all at low dose. Because of co-morbidities and other therapies, NSAIDs are often contraindicated in the elderly and low-dose colchicine (0.5 mg daily) or prednisone 5–7.5 mg daily may be considered for the first 6 months post-introduction of allopurinol. Patients should also have an ‘action plan’ for dealing with acute gout; our usual practice is for patients to have an emergency supply of prednisone to self-treat an acute attack. Patients should also be informed that the gout flares will abate over time if they remain adherent with ULT.
3 Current Urate-Lowering Therapies
Gout is increasingly seen in the elderly population due to a variety of factors; in particular, renal impairment and chronic diuretic use. ULT should be considered in most patients with established gout. There has been increasing recognition of the morbidity associated with poorly controlled gout and recent advances in new ULTs have been very welcome. There has also been a focus on optimal use of established therapies with a key goal being achieving a sustained target serum urate (<0.36 mmol/L, with a lower target of <0.30 mmol/L in those with tophaceous gout). ULT is generally lifelong, as withdrawal of therapy is usually associated with a recurrence of gout [3, 4]. A more recent study reported that patients who achieved a serum urate of <0.30 mmol/L while receiving ULT and < 0.52 mmol/L after ULT withdrawal could remain gout free for >4 years. This suggests that, for well controlled patients, intermittent treatment (5-yearly) might be an option [5]. The cost effectiveness of ULTs has not been specifically examined in the elderly where work absence is less relevant. However, poorly treated gout is associated with recurrent hospital admission [6], disability [7] and poor quality of life [8].
3.1 Xanthine Oxidase Inhibitors
The American College of Rheumatology and 3E guidelines recommend xanthine oxidase inhibition as the first-line treatment for gout [2, 9]. Of the two agents available (allopurinol and febuxostat), allopurinol is the more established agent and is commonly used as first-line therapy.
3.1.1 Allopurinol
Allopurinol is established as first-line ULT and is likely to remain so despite the introduction of newer ULTs. It is cheap, readily available and effective irrespective of the cause of hyperuricaemia. Allopurinol is converted to the active metabolite oxypurinol which is largely excreted through the kidneys. There is limited data on the use of allopurinol specifically in the elderly with some data from the post-hoc analysis of the CONFIRMS study [10] (see Sect. 3.1.2).
3.1.1.1 Use in Renal Impairment
Use of allopurinol in renal impairment remains controversial. Recommendations for dose reduction in renal impairment are based on the observation that allopurinol hypersensitivity syndrome (AHS) was more common in patients with renal impairment and recognition that oxypurinol excretion reduces as renal function declines [11]. However, clinical studies have not shown a relationship between higher allopurinol doses and AHS [12–14]. The relationship between oxypurinol concentrations and AHS remains unproven. Nonetheless, a recent study has shown that a high concentration of oxypurinol in the presence of HLA-B*5801, which is strongly associated with AHS, results in the activation of drug-specific T cells [15]. This provides a potential mechanism, at least for those who are HLA-B*5801, and suggests that there is a dose-dependent effect.
It is important to distinguish between allopurinol starting and maintenance doses when considering the relationship between allopurinol, renal function and AHS. Recent evidence suggests that allopurinol starting dose is an important risk factor for AHS. In a large case-controlled study, those who developed AHS were more likely to have started on higher than creatinine clearance (CLCR)-based allopurinol doses compared with controls (OR = 16.7, 95 % CI 5.7–47.6; p < 0.001). Furthermore, there was a significant increase in the percentage of patients developing AHS as the allopurinol dose corrected for estimated glomerular filtration rate (GFR) increased [16]. On the basis of this, it is recommended that allopurinol be commenced at a low dose and gradually increased.
The relationship between allopurinol maintenance dose and AHS in those with renal impairment is less clear. What is evident is that many patients fail to achieve the target serum urate when confined to CLCR-based allopurinol doses [14]. We have shown that in patients established on allopurinol, the dose can be gradually increased above the CLCR-based doses even in those with renal impairment [17]. A larger study addressing the occurrence of less severe allopurinol-related adverse effects in patients receiving higher than CLCR-based doses is currently underway.
3.1.1.2 Drug Interactions
There are a number of important drug interactions with allopurinol. Perhaps the most important is the interaction with azathioprine, whose active metabolite 6-mercaptopurine is partly inactivated by xanthine oxidase. Thus, inhibition of xanthine oxidase by allopurinol can result in increased 6-mercaptopurine concentrations and consequent pancytopenia [18]. The dose of both allopurinol and azathioprine should be reduced and careful monitoring is required. The safest approach is to avoid allopurinol in those patients on azathioprine.
Perhaps a less recognised interaction that is of particular relevance in the elderly is that between allopurinol and furosemide. Although it is well known that furosemide increases serum urate, we have shown that patients receiving both allopurinol and furosemide have higher serum urate concentrations despite higher plasma oxypurinol concentrations [19]. Furthermore, patients receiving furosemide require higher doses of allopurinol relative to renal function to achieve the target serum urate [17]. Thus, furosemide attenuates the hypouricaemic effects of allopurinol and oxypurinol. Clinicians should therefore review the need furosemide on a regular basis.
3.1.1.3 Effect on Co-Morbidities
Patients with gout frequently have multiple co-morbidities including hypertension, cardiovascular disease, renal impairment, diabetes mellitus, obesity and hyperlipidaemia. There is increasing interest in the effects of allopurinol (and other ULTs) on these conditions in patients with and without gout.
The relationship between urate and chronic kidney disease is complex. Hyperuricaemia is an indicator of kidney function as its excretion declines along with a decline in renal function. Whether urate has a causative role in progression of renal disease is less clear. Allopurinol has been reported to slow the progression of renal disease, although adequately powered prospective randomised controlled trials in patients without gout are yet to be undertaken [20].
Gout is associated with an increased risk of cardiovascular disease and death, particularly in those with a high cardiovascular risk [21, 22]. Allopurinol has been reported to have beneficial effects in cardiovascular disease including improved exercise capacity in chronic stable angina [23] and a reduction in risk of myocardial infarction [24]. In patients with gout, allopurinol has been associated with a significant reduction in readmissions or death after heart failure [25] and may reduce blood pressure [26, 27]. The exact mechanisms of these beneficial effects of allopurinol remain uncertain but may relate to xanthine oxidase inhibition per se or urate lowering. While the results of further large-scale clinical trials examining the risks and benefits of allopurinol on cardiovascular disease are underway, for patients with gout the beneficial effects provide yet another reason to adhere to therapy.
3.1.2 Febuxostat
Febuxostat is a newer xanthine oxidase inhibitor approved by the US Federal Drug Administration (FDA) for the treatment of gout in 2009. Unlike allopurinol, it is primarily metabolised in the liver. A number of clinical trials have demonstrated the urate-lowering efficacy of febuxostat [10, 28]. A post-hoc analysis of those patients >65 years of age enrolled in the CONFIRMS study, which compared febuxostat 40 or 80 mg daily and fixed dose allopurinol 200 or 300 mg daily depending on renal function, has been undertaken. Of the 2,269 patients enrolled, 375 were >65 years of age. At the 6-month study end, 47.3 % of those on allopruinol, 61.7 % of those on febuxostat 40 mg daily and 82.0 % of those receiving febuxostat 80 mg daily achieved serum urate <0.36 mmol/L [29]. Although febuxostat was superior to allopurinol in urate lowering, it is important to note that no allopurinol dose escalation was undertaken. The rate of adverse events in the elderly population was similar to that observed in the entire CONFIRMS study population. Febuxostat provides an alternative therapeutic option for patients who have had severe allopurinol adverse reactions such as AHS, although it has also been rarely associated with hypersensitivity vasculitis [30].
The most common adverse effects associated with febuxostat are diarrhoea, nausea and abnormal liver function tests. Post-marketing cases of hepatic failure have been reported (http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm243770.htm).
Febuxostat is currently substantially more expensive than allopurinol, which may in part limit its uptake. Using the Markov health state model, it has been shown to be cost effective as a second-line agent in patients who fail to achieve treatment targets or have adverse effects with allopurinol [31].
3.1.2.1 Use in Renal Impairment
In patients with mild to moderate renal impairment (CLCR >30 ml/min), no dose adjustment is required. There is limited data in patients with CLCR <30 ml/min and it should be avoided in these patients at present. Data is limited to small case series which report low-dose febuxostat (10–20 mg daily) is effective in lowering serum urate in patients on haemodialysis [32]. In patients with renal transplants and asymptomatic hyperuricaemia, small studies have shown significant reductions in serum urate without major adverse events [33, 34]. As with any new therapy, post-marketing surveillance can reveal rare adverse effects. Neutropaenia and rhabdomyolysis in patients with chronic kidney disease receiving febuxostat have been reported [35, 36].
3.1.2.2 Use in Hepatic Impairment
Febuxostat is metabolised in the liver via the uridine diphosphate glucuronosyltransferase enzyme system and oxidation via the cytochrome P450 system. In patients with mild to moderate hepatic impairment (Child–Pugh class A or B), no dose adjustment is required [37]. There is limited data in patients with more severe hepatic impairment and caution is recommended in this group of patients.
3.1.2.3 Drug Interactions
Whether there is an interaction between febuxostat and azathioprine has not been tested. However, given that the mechanism of the interaction between allopurinol and azathioprine is mediated by xanthine oxidase inhibition, it is likely that there will be the same interaction between azathioprine and febuxostat. A recent case report highlights this interaction which results in an increase in the active metabolites of azathioprine [38]. Thus, in the management of gout the febuxostat/azathioprine combination should be avoided.
Clinically important interactions with other commonly prescribed medications, including NSAIDS (ibuprofen, naproxen, indomethacin), captopril, warfarin, digoxin, colchicine and hydrochlorothiazide, have not been reported [39–42].
3.1.2.4 Effect on Co-Morbidities
The effects of febuxostat on renal function are also the subject of ongoing investigation. In a post-hoc analysis of the FOCUS (Febuxostat Open-label Clinical Trial of Urate-lowering efficacy and Safety) study, a reduction in serum urate was associated with maintenance or improvement in renal function [43]. Whether this effect is related to a reduction in serum urate, xanthine oxidase inhibition or other mechanisms remains unclear. In patients without gout, clinical trials are currently underway to determine the effects of febuxostat on renal function [44].
The cardiovascular safety of febuxostat has also been questioned. In the phase III clinical trials of febuxostat, a small number of major cardiovascular adverse events were reported [10]. A causal relationship between cardiovascular adverse events and febuxostat has not been determined. There are currently several large prospective clinical trials examining the cardiovascular safety of allopurinol and febuxostat. The CARES (Cardiovascular Safety of Febuxostat and Allopurinol in Patients With Gout and Cardiovascular Comorbidities) study is enrolling males and females ≥50 and ≥55 years of age, respectively, with gout with a history of major cardiovascular or cerebrovascular disease. This study aims to enrol 7,500 patients to determine the rates of major cardiovascular outcomes (death, non-fatal myocardial infarction, non-fatal stroke and unstable angina with urgent revascularization) in those receiving allopurinol (up to 600 mg daily in those with estimated CLCR ≥60 ml/min or 400 mg/day in those with estimated CLCR 30–60 ml/min) or febuxostat (up to 80 mg daily) [45]. The FAST (Febuxostat versus Allopurinol Streamlined Trial) is another cardiovascular safety study enrolling patients ≥60 years of age with at least one other additional cardiovascular risk factor who require ULT. Patients will be randomised to receive febuxostat (up to 120 mg daily) or allopurinol (up to 900 mg/day). The primary outcome measure is first cardiovascular event including non-fatal myocardial infarction, non-fatal stroke and cardiovascular death and the secondary outcomes include all-cause mortality, heart failure and new or worsening angina [46]. These two studies should provide important information about the safety and efficacy of both febuxostat and allopurinol in the predominantly elderly population.
3.2 Uricosuric Agents
The other main group of established ULTs are the uricosuric agents, which lower serum urate by increasing renal urate excretion (Fig. 2). This group includes benzbromarone, which is not widely available in some countries due to concerns about hepatotoxicity, and probenecid.
3.2.1 Benzbromarone
Benzbromarone is a potent uricosuric agent that acts via the renal urate transporters URAT1 and GLUT9. It is an effective ULT alone and in combination with allopurinol [47, 48]. Benzbromarone is not widely available due to concerns over hepatotoxicity, which can be fatal, particularly when high doses are used (300 mg daily) [49]. The risk of hepatotoxicity may be reduced by gradual dose increase and regular monitoring of liver function tests [50]. Previous studies in human liver microsomes demonstrate that the cytochrome P450 enzyme CYP2C9 has an important role in the conversion of benzbromarone to 6-hydroxy benzbromarone [51]. CYP2C9 is subject to extensive genetic polymorphism and poor metaboliser alleles have been identified including CYP2C9*2 (rs1799853, 430T>C, Arg144Cys) and CYP2C9*3 (rs1057910, 1075A>C, Ile359Leu), which occur in 3–14 % of Caucasians, 1–7 % of Asians, and 3–5 % of Polynesians [52, 53]. In a pharmacokinetic study of benzbromarone, a CYP2C9*3 homozygote had significantly impaired benzbromarone clearance compared with CYP2C9*3 heterozygotes and CYP2C9*1 homozygotes, suggesting that genetic variation in CYP2C9 may impair the metabolism of benzbromarone [54]. Whether these genetic polymorphisms are associated with an increased risk of hepatotoxicity with benzbromarone is unknown.
Because of the potential risks associated with its use, we reserve benzbromarone for those who have failed to achieve the target serum urate with allopurinol and probenecid and limit the benzbromarone dose to 100 mg daily.
3.2.1.1 Use in Renal Impairment
Benzbromarone retains its urate-lowering efficacy even in patients with significantly impaired renal function. It has been used effectively in patients with CLCR as low as 17 ml/min [55, 56]. However, its ability to reduce serum urate declines as renal function declines and when GFR is <20 ml/min it may only produce a reduction in serum urate of ~20 % [56, 57].
3.2.1.2 Use in Hepatic Impairment
As noted above, benzbromarone has been associated with severe hepatotoxicity. The risk of hepatotoxicity is increased in patients with existing liver disease or previous episodes of hepatotoxicity. Benzbromarone should not be used in patients with known liver disease and should be used with caution in patients with a history of hepatic impairment. Patients who have an excess intake of alcohol are also likely to be more at risk of liver toxicity and consideration to an alternative ULT should be given.
3.2.1.3 Drug Interactions
There are a number of important drug interactions with benzbromarone. The anticoagulant effect of warfarin is increased with benzbromarone [58] and a reduction in warfarin dose of ~30 % should be considered. Benzbromarone is a moderate inhibitor of CYP2C9 [59] and may therefore increase concentrations of drugs primarily metabolised by this enzyme. For example, sulphonylurea and phenytoin levels may increase and blood glucose and phenytoin concentrations should be checked, respectively. Fluconazole inhibits the metabolism of benzbromarone and this combination should be avoided. Benzbromarone hepatotoxicity could theoretically be additive with other hepatotoxic drugs, thus caution should be given to its use in combination with other hepatotoxic drugs.
3.2.2 Probenecid
Probenecid also inhibits URAT1 and GLUT9, thereby increasing renal urate excretion. Probenecid is generally reserved for those who cannot tolerate or fail to achieve the target serum urate on a xanthine oxidase inhibitor. It has been shown to be less effective than benzbromarone when used as monotherapy in this situation [47]. The combination of allopurinol and probenecid can provide additional urate lowering over either agent alone [60]. This is despite a reduction in plasma oxypurinol concentrations in patients on the combination of allopurinol and probenecid [61]. Probenecid is generally well tolerated although gastrointestinal adverse effects can occur [47]. Deposition of urate crystals within the kidney and formation of uric acid stones is a risk with all uricosuric agents [62].
3.2.2.1 Use in Renal Impairment
The urate-lowering ability of probenecid may reduce as renal function declines [62, 63]. In a more recent study, there was no difference in the percentage of patients who achieved target serum urate with estimated GFR <50 ml/min/1.73 m2 compared with those with estimated GFR ≥50 ml/min/1.73 m2 [64]. There is a lack of data on the use of probenecid in renal impairment and no studies to determine the threshold CLCR below which probenecid would be expected to have a clinically insignificant urate-lowering effect.
3.2.2.2 Drug Interactions
Probenecid interacts with a number of antibiotics including penicillin and β-lactam antibiotics. Indeed, in some clinical situations probenecid is specifically used to increase plasma antibiotic concentrations. Aspirin, which is commonly used in the elderly, can reduce renal urate excretion even in a low dose [65]. There does not appear to be a clinically significant effect on the urate-lowering effects of probenecid by aspirin [66].
3.3 Recombinant Uricases—Pegloticase
Recombinant uricases are a new class of ULT which may rapidly decrease both serum urate and number and size of tophi. They are usually reserved for the more refractory patients although studies are ongoing to determine their optimal administration. The necessity for intravenous administration, the frequent development of neutralising antibodies and their significant cost currently preclude their widespread use.
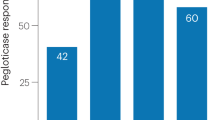
Humans lack the enzyme uricase which metabolises uric acid to the more water-soluble allantoin. Thus, uric acid is the final product of purine metabolism (Fig. 1). Pegloticase is a recombinant polyethylene—glycol conjugated uricase that is FDA approved for gout in patients who have failed other conventional ULTs. It rapidly and profoundly reduces serum urate in the majority of patients and can lead to resolution of gouty tophi [67, 68]. It can therefore cause a significant number of gout flares despite the use of prophylactic therapy such as colchicine or NSAIDs [67]. Pegloticase must be administered by intravenous infusion every 2 weeks and infusion reactions are common, occurring in 20–40 % of patients. Anti-pegloticase antibodies may develop in ~40 % and are associated with a loss of urate-lowering effect [69]. This loss of urate-lowering effect preceded an infusion reaction in 79 % [69]. Concomitant use of immunosuppression with mycophenolate mofetil, cyclosporine A, azathioprine or tacrolimus alone or in various combinations may reduce the risk of developing anti-peglitocase antibodies as evidenced by a study which included seven organ-transplant recipients [70]. The relationship between increased risk of cardiovascular events and pegloticase remains unclear. As noted previously, cardiovascular risk factors are common in patients with gout. In the phase II clinical trials, there was a non-significant increase in cardiovascular events in those patients that received pegloticase as compared with placebo [67]. More recently, an analysis of the FDA adverse event reporting system found that cardiovascular events occurred more frequently than was statistically expected [71]. Further data are required about the cardiovascular safety of pegloticase. Currently the cost of pegloticase may limit its use in many countries.
3.3.1 Use in Renal Impairment
There is limited experience with pegloticase in patients with renal impairment. A post-hoc analysis of two phase III pegloticase clinical trials has recently been reported. Of the 212 patients enrolled, 103 (49 %) had chronic kidney disease, stage 3 (estimated [e]GFR 30–59 ml/min/1.72 m2) or 4 (eGFR 15–29 ml/min/1.72 m2), no patients with chronic kidney disease stage 5 were enrolled. Importantly there was no significant change in renal function during the 6-month study period and the efficacy and safety of pegloticase did not appear to be affected by renal function. Furthermore, there was no association between response to pegloticase and renal function [72].
4 Pipeline Urate-Lowering Therapies
There are a number of new ULTs at various stages of development at this time (Table 1).
4.1 Lesinurad (RDEA594)
Lesinurad inhibits the urate transporters URAT1 and OAT4 in the proximal tubule of the kidney. Inhibition of these key urate transporters normalises the low renal urate excretion that is the most common cause of hyperuricaemia in gout. Lesinurad has been shown to lower serum urate but there is very limited data in patients with renal impairment [73]. In a phase IIb study of 208 patients with gout and CLCR >60 ml/min with serum urate ≥0.36 mmol/L despite allopurinol 200–600 mg/day, patients were randomised to addition of lesinurad 200 mg, 400 mg or 600 mg daily or placebo. After 4 weeks, lesinurad plus allopurinol resulted in a significant reduction in mean serum urate of 30 %, 22 % and 16 % for the 600 mg, 400 mg and 200 mg lesinurad doses, respectively, with only a 3 % reduction in the placebo group [74]. Similarly, in a phase Ib trial, the addition of lesinurad to febuxostat resulted in 100 % of the 20 subjects enrolled achieving the target serum urate of <0.36 mmol/L [75]. Thus, this combination of drugs with different mechanisms of action for urate lowering appears to be an effective new strategy in the management of gout. Further long-term safety and efficacy data, particularly in those with impaired renal function and other co-morbidities, is required.
4.2 Levotofisopam
Levotofispam is a 2,3-benzodiazepine derivative already approved for treatment of anxiety and autonomic instability. Although the mechanism of action remains unclear, it is thought to act as a uricosuric agent. In a phase IIa trial of 13 patients with gout, levotofisopam 50 mg three times daily for 7 days resulted in a mean serum urate reduction of 48.8 %. The mean baseline serum urate was 0.48 mmol/L (range 0.42–0.58 mmol/L) and all patients achieved a serum urate <0.36 mmol/L on day 7 [76]. Like other ULTs, acute gout flare was common, occurring in 23 % of patients.
4.3 Ulodesine (BCX4208)
Ulodesine is a purine nucleoside phosphorylase inhibitor, an enzyme in the urate production pathway a step high than xanthine oxidase. It therefore inhibits uric acid production. Short-term studies have shown it to be effective as monotherapy and to provide additional urate lowering when combined with allopurinol [77]. The most common side effects in short-term studies using ulodesine 40–240 mg daily have been diarrhoea, headache and a reduction in lymphocytes [78].
4.4 Arhalofenate (MBX-102)
Arhalofenate is a peroxisome proliferator-activated receptor-ligand (PPAR)-γ modulator initially investigated as a potential therapy for type 2 diabetes. Initial phase II studies in 955 patients revealed a dose-dependent reduction in mean serum urate of 13 %, 22 % and 29 % in the arhalofenate 200 mg, 400 mg and 600 mg daily groups, respectively. Similar reductions in serum urate were observed in patients with mild-moderate renal impairment and in those receiving concomitant diuretics [79]. The urate-lowering effect of arhalofenate is due to its ability to inhibit the renal urate transporters URAT1 and OAT4 [80]. Further phase II studies of arhalofenate alone and in combination with allopurinol or febuxostat are underway.
4.5 Topiroxostat (FYX-051)
Topiroxostat is a ULT which binds and inhibits xanthine oxidase in a different fashion to allopurinol and febuxostat [81]. In a 22-week, double-blind, randomised controlled trial of topiroxostat 160 mg daily versus placebo in patients with renal impairment (GFR ≥30 to <60 ml/min/1.72 m2) there was a significantly greater percentage reduction in serum urate in the topiroxostat group compared with placebo (−45.38 ± 21.80 % vs. 0.08 ± 9.92 %). Ninety percent of those receiving topiroxostat achieved a serum urate <0.36 mmol/L by week 22. There was no significant effect on renal function [82]. Further larger long-term studies are required.
4.6 Tranilast
Tranilast was initially developed for the treatment of allergy in Japan. Short-term studies in healthy volunteers have shown a reduction in serum urate with tranilast [83]. It has been shown to inhibit the renal urate transports URAT1 and GLUT9, resulting in increased renal urate excretion [84]. Further clinical trials of the urate-lowering effects of tranilast alone and in combination with other ULTs are underway.
4.7 Other URAT1 Inhibitors
RDEA 3170 is another potent oral URAT1 inhibitor under development [85]. Similarly, pre-clinical studies of the selective URAT1 inhibitor UR-1102 have shown dose-dependent reductions in serum urate in monkeys [86]. Further human studies are awaited.
4.8 Dual URAT1 and Xanthine Oxidase Inhibitors
KUX1151 and RLBN1001 are dual xanthine oxidase and URAT1 inhibitors under clinical investigation [87].
5 Conclusions
Once a decision has been made to commence ULT in patients with gout, the therapeutic goal, irrespective of which agent is used, is to achieve a sustained lowering of urate to <0.36 mmol/L, with a lower target of <0.30 mmol/L in those patients with tophi. First-line therapy for the majority of patients will be allopurinol, which needs to be gradually increased to achieve the therapeutic target. Emerging evidence suggests that this approach can also be tailored for those patients with renal impairment. Febuxostat, a relatively new xanthine oxidase inhibitor, is a welcome alternative, particularly in those patients intolerant or refractory to allopurinol or where allopurinol use is contraindicated. Uricosuric agents will be indicated in selected individuals. Pegloticase, a recombinant uricase, may dramatically reduce both hyperuricaemia and tophi but its use is currently restricted to severe cases and is usually administered in a specialist centre.
There are a number of pipeline drugs currently in development. Lesinurad, a urate transporter inhibitor, is currently in phase III trials. The potential of other novel agents that inhibit urate transporters or xanthine oxidase via alternative mechanisms are being actively analysed in current clinical trials.
This recent interest in ULTs is gratifying and is in part driven by recognition that hyperuricaemia may be an independent risk factor in cardiovascular and renal disease. Nonetheless, the introduction of these newer agents should lead to better management of gout in elderly and other high-risk patient groups, particularly in those refractory to or intolerant of standard therapies.
References
Mikuls T, Farrar J, Bilker W, Fernandes S, Schumacher HR, Saag K. Gout epidemiology: results from the UK general practice research database, 1990–1999. Ann Rheum Dis. 2005;64:267–72.
Khanna D, Fitzgerald J, Khanna P, Sangmee B, Singh M, Neogi T, et al. American College of Rheumatology Guidelines for the Management of Gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricaemia. Arthritis Care Res. 2012;64(10):1431–46.
Loebl WY, Scott JT. Withdrawal of allopurinol in patients with gout. Ann Rheum Dis. 1974;33(4):304–7.
Gast L. Withdrawal of longterm antihyperuricemic therapy in tophaceous gout. Clin Rheumatol. 1987;6(1):70–3.
Perez-Ruiz F, Atxotegi J, Hernando I, Calabozo M, Nolla J. Using serum urate levels to determine the period free of gouty symptoms after withdrawal of long-term urate-lowering therapy: a prospective study. Arthritis Care Res. 2006;55(5):786–90.
Hutton I, Gamble G, Gow P, Dalbeth N. Factors associated with recurrent hospital admissions for gout: a case-controlled study. J Clin Rheumatol. 2009;15(6):271–4.
Dalbeth N, Collis J, Gregory K, Clark B, Robinson E, McQueen F. Tophaceous joint disease strongly predicts hand function in patients with gout. Rheumatology. 2007;46:1804–7.
Becker M, Schumacher HR, Benjamin K, Gorevic P, Greenwald M, Fessel J, et al. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36(5):1041–8.
Sivera F, Andrés M, Carmona L, Kydd A, Moi J, Seth R, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73(2):328–35.
Becker M, Schumacher HR, Espinoza L, Wells A, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricaemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:R63. doi:10.1186/ar2978.
Hande K, Noone R, Stone W. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47–56.
Hung S, Chung W, Liou L, Chu C, Lin M, Huang H, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci. 2005;102(11):4134–9.
Vazquez-Mellado J, Meono Morales E, Pacheco-Tena C, Burgos-Vargas R. Relationship between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis. 2001;60:981–3.
Dalbeth N, Kumar S, Stamp LK, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricaemia in patients with gout. J Rheumatol. 2006;33(8):1646–50.
Yun J, Marcaida M, Eriksson K, Jamin H, Fontana S, Pichler W, et al. Oxypurinol directly and immediately activates the drug-specific T cells via the preferential ise of HLA-B*58:01. J Immunol. 2014;192:2984–93.
Stamp L, Taylor W, Jones P, Dockerty J, Drake J, Frampton C, et al. Starting dose, but not maximum maintenance dose, is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012;64(8):2529–36.
Stamp L, O’Donnell J, Zhang M, James J, Frampton C, Barclay M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in chronic gout, including in those with renal impairment. Arthritis Rheum. 2011;63(2):412–21.
Venkat Raman G, Sharman V, Lee H. Azathioprine and allopurinol: a potentially dangerous combination. J Int Med. 1990;228:69–71.
Stamp L, Barclay M, O’Donnell J, Zhang M, Drake J, Frampton C, et al. Furosemide increases plasma oxypurinol without lowering serum urate—a complex drug interaction: implications for clinical practice. Rheumatology. 2012;51(9):1670–6.
Bose B, Badve S, Hiremath S, Boudville N, Brown F, Cass A, et al. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2014;29:406–13.
Krishnan E, Baker J, Furst D, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688–96.
Baker J, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: Recent developments and where do they leave us? Am J Med. 2005;118:816–26.
Norman A, Ang D, Ogston S, Lang C, Struthers A. Effect of high dose allopurinol on exercise in patients withy chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375:2161–7.
Grimaldi-Bensouda L, Alpérovitch A, Aubrun E, Danchin N, Rossignol M, Abenhaim L, et al. Impact of allopurinol on the risk of myocardial infarction. Ann Rheum Dis. 2014. doi:10.1136/annrheumdis-2012-202972.
Thansaaoulis G, Brophy J, Richard H, Pilote L. Gout, allopurinol use and heart failure outcomes. Arch Intern Med. 2010;170(15):1358–64.
Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal function. Int Urol Nephrol. 2007;39(4):1227–33.
Feig D, Soletsky B, Johnson R. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension. JAMA. 2010;300(8):924–32.
Becker M, Schumacher HR, Wortmann R, MacDonald P, Eustace D, Palo W, et al. Febuxostat compared with allopurinol in patients with hyperuricaemia and gout. N Engl J Med. 2005;353:2450–61.
Jackson RL, Hunt B, MacDonald PA. The efficacy and safety of febuxostat for urate lowering in gout patients ≥65 years of age. BMC Geriatr. 2012;12:11. doi:10.1186/1471-2318-12-11.
Chohan S, Becker M. Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J Rheumatol. 2011;38(9):1957–9.
Beard S, von Scheele B, Nuki G, Pearson I. Cost-effectiveness of febuxostat in chronic gout. Eur J Health Econ. 2013. doi:10.1007/s10198-013-0486-z.
Horikoshi R, Akimoto T, Inoue M, Morishita Y, Kusano E. Febuxostat for hyperuricaemia: experience with patients on chronic hemodialysis treatment. Clin Exp Nephrol. 2013;17:149–50.
Tojimbara T, Nakajima I, Yashima J, Fuchinoue S, Teraoka S. Efficacy and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase for the treatment of hyperuricemia in kidney transplant recipients. Transplant Proc. 2014;46:511–3.
Sofue T, Inui M, Hara T, Nishijima Y, Moriwaki K, Hayashida Y, et al. Efficacy and safety of febuxostat in the treatment of hyperuricemia in stable kidney transplant recipients. Drug Des Dev Ther. 2014;8:245–53.
Kang Y, Kim M, Jang H, Bae E, Yun S, Cho H, et al. Rhabdomyolysis associated with initiation of febuxostat therapy for hyperuricaemia in a patient with chronic kidney disease. J Clin Pharm Ther. 2014;39:328–30.
Kobayashi S, Ogura M, Hosoya T. Acute neutropenia associated with initiation of febuxostat therapy for hyperuricaemia in patients with chronic kidney disease. J Clin Pharm Ther. 2013;38:258–61.
Khosravan R, Grabowski B, Mayer M, Wu J, Joseph-Ridge N, Vernillet L. The effect of mild and moderate hepatic impairment on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase. J Clin Pharmacol. 2006;46(1):88–102.
Dore M, Frenette A, Mansour A, Troyanov Y, Begin J. Febuxostat as a novel option to optimize thiopurines’ metabolism in patients with inadequate metabolite levels. Ann Pharmacother. 2014;48(5):648–51.
Wu J, Joseph-Ridge N, et al. Pharmacokinetic interactions of concomitant administration of febuxostat and NSAIDs. J Clin Pharmacol. 2006;46(8):855–66.
Grabowski B, Khosravan R, Wu J, et al. Effect of hydrochlorothiazide on the pharmacokinetics and pharmacodynamics of febuxostat, a non-purine selective inhibitor of xanthine oxidase. Br J Clin Pharmacol. 2010;70(1):57–64.
Khosravan R, Grabowski B, Wu J, et al. Effect of food or antacid on pharmacokinetics and pharmacodynamics of febuxostat in healthy subjects. Br J Clin Pharmacol. 2008;65(3):355–63.
Mukoyoshi M, Nishimura S, Hoshide S, et al. In vitro drug-drug interaction studies with febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: plasma protein binding, identification of metabolic enzymes and cytochrome P450 inhibition. Xenobiotica. 2008;38(5):496–510.
Whelton A, MacDonald P, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7–13.
Hosoya T, Kimura K, Itoh S, Inaba M, Uchida S, Tomino Y, et al. The effect of febuxostat to prevent a further reduction in renal function of patients with hyperuricemia who have never had gout and are complicated by chronic kidney disease stage 3: study protocol for a multicenter randomized controlled study. Trials. 2014;15:26.
White WB, Chohan S, Dabholkar A, Hunt B, Jackson R. Cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular comorbidities. Am Heart J. 2012;164(1):14–20. doi:10.1016/j.ahj.2012.04.011.
MacDonald T, Ford I, Nuki G, Mackenzie I, De Caterina R, Findlay E, et al. Protocol of the Febuxostat versus Allopurinol Streamlined Trial (FAST): a large prospective, randomised, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia. BMJ Open. 2014;4(7):e005354. doi:10.1136/bmjopen-2014-.
Reinders M, Van Roon E, Jansen T, Delsing J, Griep E, Hoekstra M, et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis. 2009;68:51–6.
Perez-Ruiz F, Calaabozo M, Fernardez-Lopez J, Herrero-Beites A, Ruiz-Lucea E, Garcia-Erauskin G, et al. Treatment of chronic gout in patients with renal function impairment: an open, randomised, actively controlled study. J Clin Rheumatol. 1999;5(2):49–55.
Hautekeete M, Henrion J, Naegels S, DeNeve A, Adler M, Deprez C, et al. Severe hepatotoxicity related to benzarone: a report of three cases with two fatalities. Liver. 1995;15:25–9.
Lee M-H, Graham G, Williams K, Day R. A benefit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interests of patients? Drug Saf. 2008;31(8):643–65.
Kobayashi K, Kajiwara E, Ishikawa M, Oka H, Chiba K. Identification of CYP isozymes involved in benzbromarone metabolism in human liver microsomes. Biopharm Drug Dispos. 2012;33:466–73.
Scott S, Khasawneh R, Peter I, Kornreich R, Desnick R. Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics. 2010;11:781–91.
Roberts R, Wallace M, Wright D, Cadzow M, Dalbeth N, Jones P, et al. Frequency of CYP2C9 polymorphisms in polynesian people and potential relevance to management of gout with benzbromarone. Jt Bone Spine. 2014;81(2):160–3.
Uchida S, Shimada K, Misaka S, Imai H, Katoh Y, Inui N, et al. Benzbromarone pharmacokinetics and pharmacodynamics in different cytochrome P450 2C9 genotypes. Drug Metab Pharmacokinet. 2010;25:605–10.
Zurcher R, Bock H, Thiel G. Excellent uricosuric efficacy of benzbromarone in cyclosporin-A treated renal transplant patients: a prospective study. Nephrol Dial Transplant. 1994;9:548–51.
Masbernard A, Giudicelli C. Ten years experience with benzbromarone in then management of gout and hyperuricaemia. S Afr Med J. 1981;59:701–6.
Heel R, Brogden R, Speight T, Avery G. Benzbromarone: a review of its pharmacological properties and therapeutic uses in gout and hyperuricaemia. Drugs. 1977;14(5):349–66.
Shimodaira H, Takahashi K, Kano K, Matsumoto Y, Uchida Y, Kudo T. Enhancement of anticoagulant action by warfarin-benzbromarone interaction. J Clin Pharmacol. 1996;36:168–74.
Locuson C, Wahlstrom J, Rock D, Rock D, Jones J. A new class of CYP2C9 inhibitors: probing 2C9 specificity with high-affinity benzbromarone derivatives. Drug Metab Dispos. 2003;31(7):967–71.
Reinders M, Van Roon E, Houtmann P, Brouwers J, Jansen T. Biochemical effectiveness of allopurinol and allopurinol-probenecid in previously benzbromarone-treated gout patients. Clin Rheum. 2007;26:1459–65.
Stocker S, Williams K, McLachlan A, Graham G, Day R. Pharmacokinetic and Pharmacodynamic Interaction between Allopurinol and Probenecid in Healthy Subjects. Clin Pharmacokinet. 2008;47(2):111–8.
Thompson G, Duff I, Robinson W, Mikkelsen W, Galindez H. Long term uricosuric therapy in gouty. Arthritis Rheum. 1962;5:384–96.
Bartels E, Matossian G. Gout: six-year follow-up on probenecid therapy. Arthritis Rheum. 1959;2(3):193–202.
Pui K, Gow P, Dalbeth N. Efficacy and tolerability of probenecid as urate-lowering therapy in gout; clinical experience in high-prevalence population. J Rheumatol. 2013;40(6):872–6.
Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000;43(1):103–8.
Harris M, Bryant L, Danaher P, Alloway J. Effect of low dose daily aspirin on serum urate levels and urinary excretion in patients receiving probenecid for gouty arthritis. J Rheumatol. 2000;27(12):2873–6.
Sundy J, Baraf H, Yood R, Edwards N, Gutierrez-Urena S, Treadwell E, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306(7):711–20.
Baraf H, Becker M, Gutierrez-Urena S, Treadwell E, Vazquez-Mellado J, Rehrig C, et al. Tophus burden reduction with pegloticase: results from phase 3 randomized trials and open-label extension in patients with chronic gout refractory to conventional therapy. Arthritis Res Ther. 2013;15:R137.
Lipsky P, Calabrese L, Kavanaugh A, Sundy J, Wright D, Wolfson M, et al. Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther. 2014;16:R60.
Hershfield M, Ganson N, Kelly S, Scarlett E, Jaggers D, Sundy J. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther. 2014;16:R63.
Gentry W, Dotson M, Williams B, Hartley M, Stafford K, Bottorff M, et al. Investigation of pegloticase-associated adverse events from a nationwide reporting system database. Am J Health Syst Pharm. 2014;71(9):722–7.
Yood R, Ottery F, Irish W, Wolfson M. Effect of pegloticase on renal function in patients with chronic kidney disease: a post hoc subgroup analysis of 2 randomized, placebo-controlled, phase 3 clinical trials. BMC Res Notes. 2014;7:51.
Perez-Ruiz F, Hingorani V, Welp J, Sheedy B, Manhard K, Shen Z, et al. Efficacy and safety of a range of doses of RDEA594, a novel uricosuirc agent, as a single agent in hyperuricaemic gout patients: multicentre, randomized, double-blind, placebbo controlled, phase 2 experience. Ann Rheum Dis. 2010;69(Suppl 3):121.
Perez-Ruiz F, Sundy J, Krishnan E, Hingorani V, Welp J, Suster M, et al. Efficacy and safety of lesinurad (RDEA594), a novel uricosuric agent, given in combination with allopurinol in allopurinol-refractory gout patients: randomized, double-blind, placebo-controlled, phase 2B study. Ann Rheum Dis. 2011;70(Suppl 3):104.
Fleischmann R, Kerr B, Yeh L-T, Suster M, Shen Z, Polvent E, et al. Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatology. 2014. doi:10.1093/rheumatology/ket487.
Noveck R, Wang Z, Forsthoefel A, Sigmon K, Hall P, Keogh J, et al. Levotofisopam has uricosuric activity and reduces serum urate levels in patients with gout. Arthritis Rheum. 2012;64(10 (Suppl)):S356.
Hollister A, Becker M, Terkeltaub R, Waugh A, Lyman S, Flynt A, et al. BCX4208 shows synergistic reductions in serum uric acid in gout patients when combined with allopurinol. Ann Rheum Dis. 2011;70(Suppl 3):183.
Dobo S, Flynt A, Hollister A, Sheridan W. BCX4208, A novel urate-lowering therapy, was generally safe and well tolerated in two 3-week studies in gout subjects. Ann Rheum Dis. 2011;70(Suppl 3):188.
Saha G, Karpf D, Choi Y, Roberts B. Arhalofenate, a potential novel treatment for hyperuricemia, with or without metabolic co-morbidities, in patients with gout: meta-analysis of urate lowering in four phase 2 studies in type 2 diabetes. Arthritis Rheum. 2011;63(10 (suppl)):S1014.
Choi Y, Larroca V, Lucman A, Vicena V, Abarca N, Rantz T, et al. Arhalofenate is a novel dual-acting agent with uricosuric and anti-inflammatory properties. Arthritis Rheum. 2012;64(10 (supple)):S697.
Okamoto K, Nishino T. Crystal structures of mammalian xanthine oxidoreductase bound with various inhibitors: allopurinol, febuxostat, and FYX-051. J Nippon Med Sch. 2008;75(1):2–3.
Hosoya T, Ohno I, Nomura S, Hisatome I, Uchida S, Fujimori S, et al. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin Exp Nephrol. 2014. (in press).
Sundy J, Kitt M. Tranilast, a novel, potential treatment for the chronic management of hyperuricaemia in patients with gout, reduces serum uric acid in healthy subjects. Ann Rheum Dis. 2010;69(Suppl 3):607.
Mandal A, Emerling D, Serafini T, Mount D. Tranilast inhibits urate transport mediated by URAT1 and GLUT9. Arthritis Rheum. 2010;62(Suppl 10):164.
Miner J, Tan P. RDEA3170, a novoel, high affinity URAT1 inhibitor binds to a central domain with URAT1. Ann Rheum Dis. 2012;71(Suppl 3):446.
Ahn S, Horiba N, Ohtomo, Lee K, Kim K, Kim B, et al. The therapeutic efficacy of the novel uricosuric agent UR-1102 for hyperuricaemia and gout. Ann Rheum Dis. 2013;72(Suppl 3):704.
Warrell R, Klukovits A, Barnes K, Satyanarayana C, Cheeseman C, Piwinski J. Novel bifunctional inhibitors of xanthine oxidase and URAT1 induce profound hypouricaemia in human subjects. Ann Rheum Dis. 2014. doi:10.1136/annrheumdis-2014-eular.2265.
Acknowledgments
LKS has received consulting fees from Astra Zeneca. PC declares no conflicts of interest. No funding was used to support the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stamp, L.K., Chapman, P.T. Urate-Lowering Therapy: Current Options and Future Prospects for Elderly Patients with Gout. Drugs Aging 31, 777–786 (2014). https://doi.org/10.1007/s40266-014-0214-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-014-0214-0