Abstract
The combination of targeted therapy and immunotherapy in the treatment of metastatic renal cell carcinoma (mRCC) has significantly improved outcomes for many patients. There are multiple FDA-approved regimens for the frontline setting based on numerous randomized Phase III trials. Despite these efforts, there remains a conundrum of identifying a biomarker-driven approach for these patients and it is unclear how to predict which patients are most likely to respond to these agents. This is due, in part, to an incomplete understanding of how these drug combinations work. The use of tyrosine kinase inhibitors that have multiple ‘off-target’ effects may lend themselves to the benefits observed when given in combination with immunotherapy. Further, targeting multiple clones within a patient’s heterogenic tumor that are responsive to targeted therapy and others that are responsive to immunotherapy may also explain some level of improved response rates to the combination approaches compared to monotherapies. This review highlights the 5 FDA-approved regimens for mRCC in the frontline setting and offers insights into potential mechanisms for improved outcomes seen in these combination approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Metastatic renal cell carcinoma remains an incurable disease where advances in therapies are needed. |
In this review, we highlight the latest breakthroughs in the treatment of metastatic renal cell carcinoma with immunotherapy and targeted therapies. |
These combination therapies have been widely established as standard treatments in metastatic renal cell carcinoma, but we discuss some future considerations that could further improve patient outcomes with these therapies. |
1 Introduction
Systemic therapy with immunotherapy in metastatic renal cell carcinoma (mRCC) initially started with interleukin-2 (IL-2) and interferon-gamma to provoke anti-tumor immune responses with response rates ranging from 10 to 20% and overall survival approaching 13 months [1,2,3]. Due to the potential for systemic toxicities from these immunotherapies, administration was routinely performed in an inpatient hospital setting. Long-term follow-up of mRCC patients treated with high-dose IL-2 have demonstrated sustained complete responses (CRs) and durable survival outcomes in select mRCC patients [4, 5]. Subsequent studies have identified potential correlates with tissue markers and immune parameters that may aid further biomarker selection of candidates for IL-2 in mRCC [6]. These immunotherapeutic agents such as high-dose IL-2 offered the earliest glimpses of cure in mRCC and supported the notion that renal cell carcinoma (RCC) is an immune-sensitive tumor.
The first-line treatment of mRCC was also significantly impacted with the advent of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors (TKIs); the first being sunitinib [7, 8]. The inherent biology of mRCC, which includes widespread neovascularization, makes them sensitive to VEGF-TKIs. Because the majority (70–80%) of cases diagnosed are clear cell histology, the VHL-HIF pathway has largely been a focus for therapeutic targeting in mRCC [9]. The inactivation of the VHL gene is responsible for the constellation of symptoms in von Hippel Lindau syndrome, one of which includes clear cell mRCC [10]. The VHL protein functions to degrade hypoxia-inducible factor (HIF), but when aberrant; HIF builds up and enhances the transcription of genes normally upregulated in hypoxia, such as VEGF [9]. This canonical pathway provided the basis for clinical development of VEGF-TKIs that have produced objective responses rates (ORR) of 25–42% for sunitinib, 30–31% for pazopanib, and 33% for cabozantinib, in the first-line setting [7, 11,12,13]. Although these were marked breakthroughs for patients, mRCC remains an incurable cancer where a high unmet need remains for rational development of agents and combinations in the systemic treatment of advanced disease.
Given this unmet need, and the historic understanding of mRCC being an immuno-responsive tumor, there has been a renewed interested in immunotherapies for mRCC. As mentioned previously, clear cell RCC (ccRCC) has long been known to be an immunogenic tumor based on the ability to elicit anti-tumor immune responses from systemic IL-2 and interferon-gamma therapies [1,2,3]. The tumor microenvironment of ccRCC has been characterized by a rich T-cell infiltrate, natural killer cells, and dendritic cells that altogether have been hypothesized to render ccRCC tumors conducive to immunotherapy [14]. However, anti-tumor immune responses as mediated by tumor-infiltrating lymphocytes are attenuated by regulatory T cells, myeloid-derived suppressor cells, and upregulation of immune checkpoints (i.e., CTLA-4, PD-1) in ccRCC. More recently, development of immune checkpoint inhibitors (ICIs) has reaffirmed the immunogenic nature of ccRCC. For example, as monotherapy, nivolumab, the anti-PD-1 biologic, was initially approved in the second-line setting after progression on a VEGF-TKI yielding an ORR of 25% [15]. Further, single-agent ipilimumab, a CTLA-4 inhibitor, was shown to have a modest ORR of 13%, similar to the early era immunotherapeutic cytokines [16]. When given in combination, nivolumab + ipilimumab produced response rates of 40% in the second-line setting, confirming the rationale for testing this combination in the first-line setting [17]. The CheckMate 214 study reported an initial response rate of 42% [18]. The efficacy of immune checkpoint inhibition (ICI) with nivolumab + ipilimumab was independent of biomarker selection (i.e., PD-L1). Despite response rates continuing to improve, there continues to remain a large subset of patients who derive no benefit from VEGF-TKIs alone or ICI alone. In this review, we detail the clinical development of combination ICI + ICI and VEGF TKI + ICI therapies as the next frontier of systemic therapies in mRCC (Fig. 1). We will specifically focus on trials that have led to U.S. Food and Drug Administration (FDA) approvals of ICI-based first-line combinations in advanced ccRCC. The role of immunotherapy in non-clear cell RCC has been reviewed extensively elsewhere and will not be an emphasis of this review [19, 20].
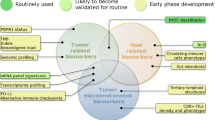
Schematic of a renal cell carcinoma with the potential FDA-approved drugs. Renal cell carcinomas have implications with receptors that include VEGF, RET, FGFR, MET, and AXL all of which are targetable with drugs. For example, VEGF is inhibited by axitinib, cabozantinib, pazopanib, and lenvatinib. RET is inhibited by lenvatinib and cabozantinib. FGFR is inhibited by pazopanib and lenvatinib. MET is inhibited by cabozantinib. AXL is inhibited by cabozantinib. Additionally, some renal cell carcinomas have PD-L1 expression which can be targeted by immune checkpoint inhibitors nivolumab, pembrolizumab, atezolizumab, and avelumab. The drug ipilimumab blocks CTLA-4 interaction of antigen presenting cells (APC) with T cells enhancing the immune response. The drug everolimus blocks mTOR, which is involved in DNA synthesis and cell growth
2 Rationale Behind Combination VEGF and Immune Checkpoint Inhibition in First-line Metastatic Renal Cell Carcinoma
The rationale to support combination VEGF and immune checkpoint inhibition in renal cell carcinoma is likely to be from distinct therapeutics with mechanisms of action that target non-redundant or discrete pathways driving a patient’s cancer. By employing an agent that works by eliminating the VEGF-driven mRCC clonal populations, and the ICI yielding additive effect by revamping the immune system to attack mRCC cells, the total amount of tumor cells targeted likely increases [21]. However, along with the effects on vascular endothelial and cancer cells, VEGF signaling can promote an immunosuppressive tumor microenvironment through effects on innate and adaptive immune cells as well as on endothelial cells [22]. Using ICIs can therefore overcome some of these potentially immunosuppressive effects of highly vascular tumors such as RCC associated with increased expression of VEGF. Use of antiangiogenics can also counter these immunosuppressive effects by increasing tumor infiltration by dendritic cells and effector T-cells and decreasing tumor infiltration by regulatory T-cells and myeloid-derived suppressor cells. Response rates for combination VEGF-ICI regimens range from 37 to 71% [23,24,25,26]. Multi-target kinase inhibitors, such as lenvatinib, not only inhibit VEGF (1–3), but also inhibit fibroblast growth factor receptors (FGFR) 1–4, platelet-derived growth factor receptor α (PDGFRα), RET, and KIT [27]. As each of these growth factor signaling pathways may have independent effects on the immune system and tumor progression, such broad targeting of TKIs may provide a mechanism for improving efficacy compared to more specific VEGF-ICI combinations using bevacizumab, a monoclonal antibody which only has affinity for VEGF 1 and 2 [28]. It should be noted that the combination of VEGF and immune checkpoint blockade may result in synergistic or supra-additive antitumor effects (i.e., beyond additive effects). However, this has not been formally tested pharmacologically across this class of agents and would need further investigation in RCC models.
3 Immunotherapy Trials (Table 1)
3.1 CheckMate-214 − Nivolumab + Ipilimumab Versus Sunitinib
The Phase III CheckMate-214 trial compared dual ICI, nivolumab + ipilimumab, versus standard of care VEGF-TKI, sunitinib in treatment-naïve patients [18]. In this trial, 1070 patients were randomized 1:1 and patients were included regardless of their International Metastatic RCC Database Consortium (IMDC) risk score [18, 29]. The study was powered for coprimary endpoints of overall survival (OS), objective response rate (ORR), and progression-free survival (PFS) in patients with intermediate or poor risk with exploratory analyses performed on the outcomes of favorable risk patients. For patients with intermediate and poor risk, the ORR was 42% (95% CI 37–47) with a PFS of 11.6 months and an OS that was not reached with a median follow-up of 25.2 months [18]. Of note, 9% of these patients who received the doublet ICI had a complete response (CR) compared to a 1% CR rate for intermediate- and poor-risk patients who received sunitinib. This contrasts with the patients with intermediate and poor risk who received sunitinib and had an ORR of 27% with a PFS of 8.4 months and an OS of 26.0 months [18].
Notably in favorable-risk patients, the mortality benefit was unclear. Intriguingly sunitinib showed benefit when compared to ICI in these favorable-risk patients for ORR and PFS. The ORR for favorable-risk patients who received nivolumab + ipilimumab was 29% with a median PFS of 15.3 months and an OS that was not reached [18]. For patients who received sunitinib, the ORR was 52% with a median PFS of 25.1 months and OS of 32.9 months [18]. Of note, the PD-L1 expression was lower in favorable-risk patients, but the CR rate for ICI was 11% versus 6% in this cohort [18].
The Functional Assessment of Cancer Therapy–Kidney Symptom Index-19 (FKSI-19) score for patients who received nivolumab + ipilimumab was significantly more improved than those who received sunitinib (p < 0.001) [18]. The most common grade ≥ 3 toxicities for patients who received nivolumab + ipilimumab were fatigue (4%) and diarrhea (4%) [18]. This was similarly found in patients who received sunitinib where patients experienced fatigue (9%), diarrhea (5%), and, unique to VEGF-TKIs, hypertension (16%) and palmar-plantar erythrodysesthesia (9%) [18]. Sunitinib is a receptor TKI that inhibits VEGF, PDGF, KIT, RET, FLT3, and CSF-1 [30]. In intermediate- and poor-risk patients, nearly one-third of patients were PD-L1 positive (≥ 1% via the Dako PD-L1 IHC 28-8 pharmDx test) and nivolumab + ipilimumab consistently demonstrated superior OS benefit over sunitinib across all PD-L1 subgroups. On extended follow-up of a minimum of 4 years, the OS benefit by nivolumab + ipilimumab over sunitinib was maintained with a CR rate of 10.7% versus 2.6% in the intent-to-treat (ITT) population [31]. Interestingly, the CR rate of the combination was higher at 12% versus 6.5% with sunitinib in the favorable-risk group, although again, these findings are exploratory in this subgroup.
3.2 JAVELIN Renal 101 − Avelumab + Axitinib Versus Sunitinib
The Phase III JAVELIN Renal 101 trial compared combination ICI + VEGF-TKI, avelumab + axitinib, versus standard of care VEGF-TKI, sunitinib in treatment-naïve patients. In this trial, 886 patients were randomized 1:1 and patients were included with favorable-, intermediate-, and poor-risk IMDC scores [29, 32]. Similar to sunitinib, axitinib is a receptor TKI that inhibits VEGF, PDGF, and KIT [33]. The study was powered for coprimary endpoints of PFS and OS in patients with PD-L1-positive tumors [32]. For patients with PD-L1-positive tumors who received avelumab + axitinib, the ORR was 55.2% (95% CI 49.0–61.2) with a PFS of 13.8 months and an OS hazard ratio (HR) compared to sunitinib of 0.61 (95% CI 0.47–0.79; p < 0.001) with a median follow-up of 9.9 months for avelumab + axitinib and 8.4 months for sunitinib [32]. Of the patients who received avelumab + axitinib, 5.4% of these patients achieved CR compared to a 2.1% CR rate for those who received sunitinib. This contrasts to the patients with PD-L1-positive tumors who received sunitinib and had an ORR of 25.5% with a PFS of 7.2 months [32]. An important note to make is that mortality benefit was observed in patients with all IMDC risk scores.
The most common grade ≥ 3 toxicities for patients who received avelumab + axitinib were hypertension (25.6%), diarrhea (6.7%), and hand-foot syndrome (5.8%) [32]. This was similarly found in patients who received sunitinib where patients experienced hypertension (17.1%), hand-foot syndrome (4.3%), and fatigue (3.6%) [32]. Despite axitinib being more selective for VEGF receptors than sunitinib, patients who received the combination fared better for response and outcome. The authors of this study concluded that the reason for this observation may be due to effects of the combination of VEGF-TKI + ICI compared to VEGF-TKI alone. Of note, the original primary endpoint was PFS in those with mRCC with a clear cell component irrespective of PD-L1 expression, although the protocol was amended in June 2017 to show superiority of the combination over sunitinib in PD-L1-positive tumors (≥ 1% of immune cells staining positive within the tumor area of the tested tissue sample by Ventana PD-L1 [SP263]). At a minimum of 13 months of follow-up, PFS was significantly longer in the avelumab plus axitinib arm than in the sunitinib arm. OS data were immature (PD-L1+ population HR 0.828 (95% CI 0.596–1.151); one-sided p = 0.1301) [34]. The predefined significance level was 0.021 for OS in PD-L1-positive tumors (one-sided log-rank test). On post hoc biomarker analyses of JAVELIN Renal 101, it was found that neither expression of PD-L1 nor tumor mutational burden differentiated PFS in either study arm [35].
3.3 KEYNOTE-426 − Pembrolizumab + Axitinib Versus Sunitinib
The Phase III KEYNOTE-426 trial compared combination ICI + VEGF-TKI, pembrolizumab + axitinib, versus standard of care VEGF-TKI, sunitinib in previously untreated patients with advanced ccRCC. In this trial, 1062 patients were randomized 1:1 and patients were included with favorable, intermediate, and poor risk IMDC scores [24, 29]. The study was powered for coprimary endpoints of OS reported as HR for death and PFS reported in months [24]. For patients who received pembrolizumab + axitinib, the ORR was 59.3% (95% CI 54.5–63.9) with a PFS of 15.1 months and an OS HR compared to sunitinib of 0.53 (95% CI 0.38–0.74; p < 0.0001) with a median follow-up of 12.8 months [24]. Of the patients who received pembrolizumab + axitinib, 5.8% of these patients experienced a CR compared to a 1.9% CR rate for those who received sunitinib. This contrasts with the patients who received sunitinib and had an ORR of 35.7% with a PFS of 11.1 months [24]. An important note to make is that mortality benefit was observed in patients with all IMDC risk scores.
The most common grade ≥ 3 toxicities for patients who received pembrolizumab + axitinib were hypertension (22.1%), diarrhea (9.1%), and elevation in liver enzymes (7.0–13.3%) [24]. Comparably lower rates of these same toxicities were observed in those treated with sunitinib where patients experienced hypertension (19.3%), diarrhea (4.7%), and liver enzyme elevations (2.4–3.1%) [24]. Similar to sunitinib, axitinib is a receptor TKI that inhibits VEGF, PDGF, and KIT [33]. Despite axitinib having greater selectivity than sunitinib, patients who received the combination fared better with response and outcome, which may be attributed to the known activity of ICI in patients with mRCC and the effects of combining ICI and VEGF inhibition. Axitinib + pembrolizumab showed consistent OS benefits over sunitinib across PD-L1 combined positive scores or CPS (defined as PD-L1-positive cells (tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells, multiplied by 100) by the PD-L1 IHC 22C3 pharmDx assay. With a median follow-up of 30.6 months, the OS benefit of the combination was sustained over sunitinib with a 9% CR rate for patients in the pembrolizumab plus axitinib group and 3% CR rate in the sunitinib group [36].
3.4 CheckMate 9ER – Nivolumab + Cabozantinib Versus Sunitinib
The Phase III CheckMate 9ER trial compared combination ICI + VEGF-TKI, nivolumab + cabozantinib, versus standard of care VEGF-TKI, sunitinib in previously untreated patients with mRCC and a clear-cell component. In this trial, 651 patients were randomized 1:1 and patients were included with favorable, intermediate, and poor risk IMDC scores [25, 29]. Similar to sunitinib, cabozantinib is a receptor TKI that inhibits VEGF, AXL, RET, MET, ROS1, TYRO3, MER, KIT, TRKB, FLT3, and TIE-2 [37]. The study’s primary endpoint was PFS with secondary endpoints of OS and ORR [25]. For patients who received nivolumab + cabozantinib, the ORR was 55.7% with a PFS of 16.6 months (95% CI 12.5–24.9) and an OS HR compared to sunitinib of 0.51 (95% CI 0.41–0.64; p < 0.001) with a median follow-up of 18.1 months [25]. Of the patients who received nivolumab + cabozantinib, 8.0% had a CR compared to a 4.6% CR rate for those who received sunitinib [25]. This is in contrast to the patients who received sunitinib and had an ORR of 27.1% with a PFS of 8.3 months [25]. The median duration of response for patients in the nivolumab + cabozantinib arm was 20.2 months compared to 11.5 months in the sunitinib arm, while the median time to response was 2.8 months with nivolumab plus cabozantinib and 4.2 months with sunitinib [25]. An important note to make is that mortality benefit was observed in patients with all IMDC risk scores, regardless of PD-L1 expression (PD-L1 IHC 28-8 pharmDx assay), and regardless of bone metastases [25].
The most common grade ≥ 3 toxicities for patients who received nivolumab + cabozantinib were hypertension (12.5%), hand-foot syndrome (7.5%), and diarrhea (6.9%) [25]. These adverse events were seen in comparable rates to those treated with sunitinib: hypertension (13.1%), hand-foot syndrome (7.5%), and diarrhea (4.4%) [25]. Additionally, the FKSI-19 score for patients who received nivolumab + cabozantinib was significantly higher over 91 weeks than those who received sunitinib (p < 0.05) [25].
Both the breadth of tyrosine kinases that cabozantinib blocks and the effects of nivolumab likely contributed to the superior outcome when compared to sunitinib. Many growth factor receptors have been implicated in the pathogenesis, tumorigenesis, and progression of mRCC [38]. Specifically, AXL and MET inhibition have been shown to overcome RCC resistance to sunitinib [39]. The tyrosine kinase, TIE-2, has also been implicated in the resistance to sunitinib, which may explain why those who received cabozantinib fared better [40]. The benefits of cabozantinib compared to sunitinib in the front-line setting have already led to the FDA approval of cabozantinib monotherapy from the Phase III CABOSUN trial [12]. The addition of nivolumab, which clearly adds another mechanism of antitumor activity and increased immune response and surveillance to treat mRCC, likely targets additional mechanisms of immune escape otherwise not targeted with sunitinib alone, and targets the subpopulation of patients who may not have otherwise responded to single-agent cabozantinib in CABOSUN, since the ORR was 33% compared to 12% in those patients who received sunitinib [12]. Although this strategy appears to be tolerated, a more targeted approach and personalized treatment focusing on specific immune checkpoints and other targetable molecules may help to improve outcomes and responses further [41,42,43,44].
3.5 CLEAR – Pembrolizumab + Lenvatinib Versus Sunitinib
The Phase III CLEAR trial compared combination ICI + VEGF-TKI (pembrolizumab + lenvatinib) versus VEGF-TKI + mTOR inhibitor (lenvatinib + everolimus) versus standard of care VEGF-TKI (sunitinib) in treatment-naïve patients with advanced RCC and a clear-cell component. Similar to sunitinib, lenvatinib is a receptor TKI that inhibits VEGF, FGFR, RET, PDGFR, and KIT [45]. In this trial, 1069 patients were randomized 1:1:1 and patients were included with favorable, intermediate, and poor risk IMDC scores [26, 29]. The study’s primary endpoint was PFS with secondary endpoints of OS. For patients who received pembrolizumab + lenvatinib, the median PFS was 23.9 months compared to 9.2 months in patients who received sunitinib, with a HR for disease progression or death of 0.39 (95% CI 0.32–0.49; p < 0.001) [26]. The median OS was not reached in either treatment groups, but the HR for death was 0.66 (95% CI 0.49–0.88; p = 0.005) for those who received pembrolizumab + lenvatinib compared to those who received sunitinib [26]. There was a significantly longer PFS in those patients who received lenvatinib + everolimus of 14.7 months compared to sunitinib, but not a significantly longer OS HR for these patients with HR of 1.15 (95% CI 0.88–1.50; p = 0.30) [26]. Response rates for the three cohorts were 71.0%, 53.5%, and 36.1% for patients who received pembrolizumab + lenvatinib, lenvatinib + everolimus, and sunitinib, respectively [26]. Of the patients who received pembrolizumab + lenvatinib, 16.1% had a CR compared to 9.8% who received lenvatinib + everolimus and 4.2% who received sunitinib [26]. The median duration of response for patients in the pembrolizumab + lenvatinib arm was 25.8 months compared to 16.6 months for those patients who received lenvatinib + everolimus and 14.6 months in the sunitinib arm [26]. An important note to make is that mortality benefit was observed in patients with all IMDC risk scores and regardless of PD-L1 expression (CPS PD-L1 IHC 22C3 pharmDx assay). The median time to response was 1.94 months with lenvatinib + pembrolizumab.
The most common grade ≥ 3 toxicities for patients who received pembrolizumab + lenvatinib were hypertension (27.6%), diarrhea (9.7%), and weight loss (8.0%) [26]. This was similarly found in patients who received sunitinib where patients experienced hypertension (18.8%), diarrhea (5.3%), and fatigue (4.4%) [26]. Additionally, patients who received lenvatinib + everolimus experienced higher rates of these toxicities than sunitinib: hypertension (22.5%), diarrhea (11.5%), and weight loss (7.3%) [26]. A distinct feature of lenvatinib compared to other TKIs is that it exhibits a novel binding mechanism to VEGFR2 causing it to bind more rapidly as well as dissociate slower than sunitinib [46]. In addition to the off-target effects of lenvatinib, the novel pharmacodynamics that it displays may attribute to its superior efficacy.
4 Clinical Considerations and Future Directions
The addition of ICI to single-agent VEGF-TKIs has clearly improved outcomes for patients [15, 18, 25, 26]. There are now 5 FDA-approved ICI-based combinations in the first-line mRCC space: nivolumab + ipilimumab, axitinib + pembrolizumab, axitinib + avelumab, cabozantinib + nivolumab, and lenvatinib + pembrolizumab. With an ever-crowded space, the role of biomarkers to inform treatment and selection of optimal therapy is an area that continues to grow within the management of mRCC [47,48,49]. Nevertheless, the addition of immunotherapeutic drugs in the frontline setting has continued to move the field forward and offer improved outcomes for patients. We end with a discussion on several key areas of ongoing investigation that are critical to optimizing treatment outcomes with immunotherapy-based combinations in mRCC.
4.1 Choice of Regimen
With the widespread implementation of these first-line ICI-based combinations in mRCC, there is growing debate into the preferred first-line regimen. First, it should be noted that the Phase III IMmotion151 trial of first-line atezolizumab and bevacizumab demonstrated a superior PFS benefit to sunitinib [23]. However, there was a lack of significant OS benefit with the combination compared to sunitinib, which has not resulted in an FDA approval for this regimen in mRCC. Similarly, the combination of axitinib + avelumab has not demonstrated an OS benefit over sunitinib as OS data remain immature [32]. As a result, consensus guidelines do not recommend atezolizumab + bevacizumab or axitinib + avelumab as first-line systemic options in mRCC given the absence of a survival signal in the Phase III setting [50]. This essentially leaves four FDA-approved first-line ICI-based combinations available for intermediate-poor risk mRCC: nivolumab + ipilimumab, axitinib + pembrolizumab, cabozantinib + nivolumab, and lenvatinib + pembrolizumab. This dwindles down to three regimens in favorable risk patients given the lack of benefit demonstrated by nivolumab + ipilimumab over sunitinib in this IMDC risk group based on CheckMate 214 [18]. Ultimately, the differential activity of these combinations are likely due to multiple factors including heterogeneity in patient populations, study design, and mechanisms of action (Fig. 1) [22]. For example, although across studies the majority of patients treated with ICI combinations had received prior nephrectomies and had ≥ 2 sites of metastases with lung being the most common site, the CLEAR cohort had a slightly higher percentage of IMDC favorable risk patients (31%) and a lower rate of sarcomatoid features (7.9%) than the cohorts from CheckMate 214 and CheckMate 9ER [18, 25, 26]. Alternatively, the KEYNOTE-426 cohort had the highest percent of IMDC favorable risk patients on the study arm (32%) across studies, but 17.9% of these patients had tumors with sarcomatoid features, which historically, portend a more aggressive variant [24]. However, a nuanced discussion of subgroups and cross-study comparisons could provide more granularity of scenarios suitable for specific regimens (with the limitations of cross-trial comparisons acknowledged).
Although a definitive preference for one regimen over another in the first-line space has not been established, several factors can guide clinicians in choice. In mRCC patients with bulky, symptomatic disease for which a response is needed, VEGF-TKI + ICI combinations have generally produced more robust ORRs (as high as 71%) when compared to ICI + ICI combinations (39.1% with nivolumab + ipilimumab) [26, 51]. However, in intermediate-poor risk disease (where the majority of mRCC cases fall) and in those without a need for a tumor debulking effect from therapy, nivolumab + ipilimumab has the longest median follow-up of all combinations with a promising CR rate of nearly 11% and among the longest duration of responses (not reached at the time of follow-up of at minimum 4 years) [31, 51]. Regardless, of response rates, when responses were achieved, all VEGF-TKI + ICI combinations have demonstrated prolonged median duration of responses usually > 20 months [25, 26, 36]. It should be mentioned that lenvatinib + pembrolizumab has the shortest median time to response of 1.94 months compared to the 2.8 months for nivolumab + ipilimumab [26, 51]. However, this difference in time to response could very well be impacted by protocol-defined timepoints of first response evaluation where it was 8 weeks after randomization with lenvatinib + pembrolizumab and 12 weeks after randomization with other ICI combinations [18, 24,25,26]. When a longer tumor response is not needed, nivolumab + ipilimumab has generated robust durable response and CR rates of those having achieved the elusive CR, 32.2% remained on therapy and 45.8% discontinued therapy and did not require subsequent systemic therapy at minimum 4-year follow-up [51]. Therefore, nivolumab + ipilimumab is an attractive option to provide a clinically meaningful treatment-free interval in mRCC patients.
From a quality-of-life perspective, both cabozantinib + nivolumab and nivolumab + ipilimumab demonstrated significantly improved health-related quality-of-life (HRQoL) metrics compared to sunitinib in their respective first-line mRCC studies [25, 31]. In a more recent analysis of CheckMate 9ER, patient-reported outcomes were maintained or improved with cabozantinib + nivolumab with a significantly delayed time to deterioration when compared to sunitinib [52]. Lenvatinib + pembrolizumab had largely similar HRQoL scores to sunitinib, while axitinib + pembrolizumab did not cause a significant deterioration in HRQoL when compared to sunitinib but did not meet the threshold for minimally important improvement in HRQoL [53, 54]. Final HRQoL analyses from these last 2 studies are pending and whether their differences from CheckMate 9ER or CheckMate 214 are attributable to differences in toxicity profiles and ways in which clinicians should translate these findings to the clinic remain unknown. A key take-away, however, is that none of these ICI combinations cause a significant deterioration in HRQoL compared to the control arm in mRCC patients.
In more selected subsets of mRCC patients, there is evidence that cabozantinib may have a unique tropism for bone metastases with promising activity in this mRCC subgroup [55]. Indeed, with cabozantinib + nivolumab, HRs for survival as low as 0.54 were seen in subgroup analyses of mRCC patients with bone metastases [25]. In those with brain metastases, another mRCC subgroup with poor prognoses, nivolumab + ipilimumab showed limited activity in this subgroup with no objective response reported in patients with brain metastases that were multiple or larger than 1 cm [56]. However, we and others have shown an impressive CNS penetration ability of cabozantinib likely owing to expression of one of its therapeutic targets cMET in the brain, with objective intracranial response rates as high as 55% [57, 58]. Specifically, in one study the expression of MET was found to be in 35% of brain metastases compared to 0% of primary RCC tumors [59]. Of note, limited-to-no data exist on the intracranial activity of other VEGF-TKIs such as pazopanib, sorafenib, or axitinib in this subgroup. In mouse models, pazopanib showed limited ability to penetrate the CNS whereas only 1.5% of the concentration in plasma was able to reach the brain [60]. This is in contrast to preclinical models showing the ability of cabozantinib to cross the blood–brain barrier whereby analysis of whole-brain lysates showed 20% of peak plasma levels [61]. Based on this evidence, cabozantinib + nivolumab may be a preferred first-line regimen for these select mRCC subgroups.
4.2 Novel ICI-based Combinations
There continues to be development of additional ICI-based combination options such as cabozantinib with atezolizumab, which targets PD-L1 [62]. This trial was a dose escalation study and showed that patients who received a higher dose of cabozantinib (60 mg) compared to a lower dose (40 mg) had similar ORR (58% vs 53%) but a higher CR rate (11% vs 3%) when given in combination with atezolizumab [62]. It is unclear whether the role of PD-L1 inhibition versus PD-1 inhibition makes a significant impact on patient outcomes; however, there is some evidence that PD-L1 blockade may be more effective in signal inhibition [63]. Building on the success of ICI + ICI and ICI + VEGF-TKI doublets, investigations are ongoing involving ICI-based “triplets” such as quavonlimab (MK-1308) + pembrolizumab + lenvatinib, MK-4280 + pembrolizumab + lenvatinib, and pembrolizumab + belzutifan + lenvatinib [64]. The COSMIC-313 Phase III trial is ongoing and will evaluate the efficacy of first-line cabozantinib + nivolumab + ipilimumab versus nivolumab + ipilimumab in IMDC intermediate-poor risk mRCC (NCT03937219). Recent studies have also supported a role of the gut microbiome in modulating antitumor immune responses to ICIs [65]. Based on this, preliminary data from a Phase IB trial showed that ORR was significantly higher with the addition of CBM-588 (the key constituent being Clostridium butyricum) to nivolumab + ipilimumab than nivolumab + ipilimumab alone in the first-line treatment of mRCC subjects [66]. Beyond pairing with systemic agents, the Phase I/II RAPPORT trial recently demonstrated that in patients with clear-cell mRCC pretreated with ≤ 2 prior systemic therapies and with 1–5 oligometastases, combining a single fraction of 20 Gy stereotactic ablative body radiotherapy (SABR) to all metastatic sites followed by pembrolizumab for 8 cycles demonstrated feasibility and promising overall response and local control rates [67]. In short, given the success of ICI + ICI or ICI + VEGF-TKI combinations in the mRCC, efforts are ongoing to enhance outcomes through strategies employing an ICI backbone, and results of these endeavors are eagerly anticipated.
4.3 Rechallenge with ICI in mRCC Pretreated with ICI
Efforts to identify the role of immunotherapy rechallenge in those who have previously been treated with ICI may also provide insight into this potential treatment strategy [68, 69]. In patients who received ICI in an earlier line of therapy, rechallenge continues to yield benefit in mRCC patients [69]. Patients who were given pembrolizumab + lenvatinib and were treatment-naïve had an ORR at 24 weeks of 72.7% compared to an ORR at 24 weeks of 55.8% in those who previously received ICI. This was higher than the 41.2% ORR in patients who were previously treated but not with ICI [69]. Additionally, patients who were given pembrolizumab + lenvatinib and were treatment-naïve had a median duration of response lasting 24.2 months compared to the 12.5 months in those who previously received ICI and the 9.0 months in those who were previously treated but not with ICI [69]. This study showed that, despite having already being exposed to ICI, there is the potential for mRCC patients to continue to derive meaningful benefit from rechallenging with ICI in a later line of therapy. However, the success of ICI rechallenge may be dependent on mechanism of action (i.e., pairing with a VEGF-TKI) as salvage nivolumab + ipilimumab in those who achieved SD or PD after initial ICI therapy in mRCC resulted in no CRs and a low conversion rate to PR (4%) [70].
In a different approach, the Phase III PDIGREE is actively exploring a response-adapted therapeutic strategy in the first-line treatment of metastatic ccRCC (NCT03793166). Here, enrolled subjects are treated with induction ipilimumab 1 mg/kg and nivolumab 3 mg/kg intravenously once every 3 weeks. Based on 3-month radiographic assessment (after completing nivolumab + ipilimumab), patients will be separated into 3 groups: complete responders will receive maintenance nivolumab, patients with progressive disease will switch to cabozantinib 60 mg oral daily, and patients with non-complete response/non-progressive disease are randomized to nivolumab versus nivolumab with cabozantinib 40 mg oral daily. This study will seek to inform the effectiveness of maintenance or salvage strategies with various approaches including VEGF inhibition alone, ICI alone, or combination VEGF-TKI + ICI following ICI exposure.
4.4 Post-ICI-based Combination Treatment Sequencing
Furthermore, the second-line treatment space is a continually evolving arena with the mTOR inhibitor everolimus with lenvatinib, cabozantinib alone, and nivolumab alone being among many options available in pretreated mRCC patients [15, 71, 72]. It is unclear whether the role of ICI + VEGF-TKI in the frontline setting impacts the benefit patients derive from using another VEGF-TKI in the second line; however, data from the sunitinib/pazopanib frontline setting era showed benefit in single agent cabozantinib (HR for death 0.66; 95% CI 0.53–0.83; p = 0.00026) [72]. For the likely smaller subset of patients who will receive VEGF-TKI alone in the upfront setting, second-line immunotherapy remains an established option where nivolumab alone had significant benefit (HR for death 0.73; 98.5% CI 0.57–0.93; p = 0.002), with the caveat that these patients were ICI-naïve [15]. Adding mTOR inhibition to VEGF-TKI with lenvatinib + everolimus in patients who had progressed on single agent VEGF-TKI therapy or after stopping this therapy within 9 months showed a significant benefit for PFS over everolimus alone (HR for progression, 0.40, 95% CI, 0.24–0.68; p = 0.0005) [71]. In the Phase III first-line mRCC setting, lenvatinib + everolimus showed improved PFS and ORR but not OS to sunitinib [26]. Therefore, in those who are lenvatinib-naïve following treatment with ICI-based therapies, lenvatinib + everolimus could be considered in those with preserved performance status (PS) who could tolerate the fairly high toxicity profile. One could argue if the patient is fit enough, everolimus alone should not be prioritized above lenvatinib + everolimus based on its inferiority to the combination.
As important as it is to combine the most effective treatments upfront to improve OS, the debate of optimal sequencing following first-line treatment with an ICI-based combination in mRCC is a relevant one as > 50% of subjects received subsequent systemic therapy in the recent CLEAR trial [26]. Here, the majority (50.7%) received a subsequent anti-angiogenic, 13.6% received a subsequent ICI, and only 2.8% received an mTOR inhibitor as subsequent therapy. Cabozantinib remains a viable second-line and beyond therapy given its OS benefit proven over everolimus in a randomized Phase III trial in mRCC patients treated with ≥ 1 prior VEGF-TKI [72]. Therefore, in mRCC patients who are treated with a non-cabozantinib-based ICI first-line combination, cabozantinib remains a preferred second-line option recognized by consensus guidelines [50]. This is consistent with the KEYNOTE-426 trial, where following axitinib + pembrolizumab, cabozantinib was the most commonly used second-line agent in this population [24]. In CheckMate 214, cabozantinib, sunitinib, pazopanib, and axitinib were among the most commonly used agents following first-line nivolumab + ipilimumab [51]. However, this study was conducted prior to the CABOSUN trial, which demonstrated the superiority of cabozantinib over sunitinib leading to its FDA approval in the first-line setting in December 2017 [12]. In those treated with first-line cabozantinib + nivolumab, the most commonly used subsequent-line therapy was axitinib [25] and expert panels agree that axitinib remains a viable option in this setting given positive PFS data in VEGF-refractory disease [50]. It should be noted that both axitinib and cabozantinib have shown activity following ICI therapies in mRCC [73, 74]. In the third-line space, aside from using systemic agents that the patient has not been exposed to in prior treatment settings, tivozanib has shown a PFS benefit over sorafenib in mRCC patients treated with 2–3 prior therapies (including ICI) with a promising safety profile [75]. Therefore, tivozanib remains a reasonable third- or fourth-line mRCC option particularly in those for whom toxicities are of concern. However, it should be noted that none of these agents have demonstrated superiority over the other in the refractory setting. Regardless of standard-of-care systemic therapy options following treatment with ICI-based combinations, clinical trials should always be considered irrespective of treatment setting.
4.5 Biomarker Development to ICI-based Combinations in mRCC
Identifying specific biomarkers or biomarker signatures that can help predict response to ICI + VEGF-TKI combinations is also underway. There have been various groups looking for single-gene biomarkers to predict response such as PD-L1, TMB, PBRM1, SMARCA4, and others; however, none have yielded significant impact in predicting outcomes in patients with mRCC [76,77,78,79]. PD-L1 expression has been among the more studied biomarkers for ICI-based therapies in mRCC where incidence of PD-L1-positive tumors has been documented to be as high as 63% [32]. However, interpretation of its usefulness as a predictive biomarker has been difficult due to lack of standardization with use of different assays and criteria for PD-L1 positivity across Phase III studies [18, 24,25,26, 32, 80]. For example, CheckMate 214 and CheckMate 9ER used the Dako PD-L1 IHC 28-8 pharmDx test (defines PD-L1 expression as the percent of positive tumor cell membrane staining in a minimum of 100 tumor cells), the KEYNOTE-426 and CLEAR trials used the PD-L1 IHC 22C3 pharmDx assay (defines PD-L1 expression by CPS, which is the number of PD-L1-staining cells [tumor cells, lymphocytes, and macrophages] divided by total number of viable tumor cells, multiplied by 100), JAVELIN Renal 101 used the Ventana PD-L1 (SP263) assay (defines PD-L1 positivity by ≥ 1% of immune cells staining positive within the tumor area of the tested tissue sample), and IMmotion 151 used the Roche PD-L1 (SP142) assay (defines PD-L1 expression by PD-L1 staining of any intensity in tumor-infiltrating immune cells covering a percentage of tumor area occupied by tumor cells, associated intra-tumoral, and contiguous peri-tumoral desmoplastic stroma) [18, 24,25,26, 32, 80]. Enthusiasm for PD-L1 as a biomarker has been dampened as the benefit to ICI-based combinations was demonstrated over sunitinib across Phase III first-line studies irrespective of PD-L1 expression. Furthermore, in two Phase III studies (JAVELIN Renal 101 and IMmotion 151), ICI-based combinations did not demonstrate improved OS for patients with PD-L1-positive mRCC over sunitinib [32, 80]. Meta-analyses of recent randomized control trials of ICIs in mRCC including subgroup analyses of survival outcomes to PD-L1 expression on tumor samples have also corroborated that the usefulness of PD-L1 as a predictive biomarker was not clearly demonstrated [81]. As a result of the constellation of findings thus far, PD-L1 expression has not been established as a standard biomarker to be routinely used in the selection of mRCC candidates for ICI-based therapies.
Despite the shortcomings of PD-L1, efforts to identify multi-gene signatures have identified patients who may benefit from ICI-based combinations in mRCC. In the open-label Phase II BIONIKK study comparing front-line nivolumab versus nivolumab + ipilimumab versus sunitinib or pazopanib, patients were stratified into 4 groups based on a 35-gene mRNA expression pattern [82]. In this study, there were significant differences for ORR to receiving nivolumab or nivolumab + ipilimumab or sunitinib or pazopanib and these differences were noted based on the various signature groups and there was no correlation between the molecular subgroups and IMDC score [83]. Similarly, these efforts were performed on an angiogenesis and immunomodulatory 26-gene expression signature on the JAVELIN Renal 101 trial where there was no association between PD-L1 nor TMB on PFS [35]. Interestingly, patients with high expression of angiogenesis markers differentiated PFS in patients who received sunitinib, but not in patients who received axitinib + avelumab [35]. Additional efforts have analyzed the role of 7-molecular subtypes associated with prognosis in patients with mRCC who received atezolizumab + bevacizumab, VEGF monoclonal antibody, in the Phase III IMmotion151 trial [84]. Gene signatures of patients who responded to sunitinib were enriched for hypoxia (VEGF)-associated signaling; however, there was no specific gene signature that differentiated responders to atezolizumab + bevacizumab compared to non-responders [84].
4.6 Novel Molecular Targets in mRCC
The addition of next-generation sequencing (NGS) into the clinic for patients with mRCC may also help to identify novel targets [85, 86]. Further work into differential expression of various immune regulatory molecules based on genomic alterations may also provide rational insight into trial design and combination strategies of targeted therapies and immunotherapies (Table 2) [43, 44, 87]. Additionally, employing transcriptomics may also help elucidate targetable alterations and provide insight into the ability to target these alterations with higher success [88]. In addition to identifying targetable alterations, single biomarkers are unlikely to be as impactful and using a multiple gene signature may help to improve responses and durability of responses [82].
As the use of immunotherapy in mRCC continues to become a mainstay, an area of interest is the concept of hyperprogressive disease (HPD). Although there are no reports of patients with mRCC who received ICI experiencing HPD, and this concept is not consensual, it is increasingly being described in patients with solid tumors treated with ICIs [89, 90]. Therefore, further understanding of this phenomenon and its existence in mRCC would be important to know with ICI therapy. Here, genomic profiling of tumors may shed light on molecular mechanisms of HPD and efforts to identify those who would most benefit from ICI.
Future efforts into optimizing sequencing of immunotherapies and rechallenge using other modalities in mRCC, such as mTOR inhibition or HIF inhibition, may help improve outcomes as well as identify novel mechanism targets [91]. The HIF-2 alpha inhibitor, belzutifan, in particular has recently been FDA approved in von Hippel–Lindau (VHL) disease-associated RCC [92]. In this Phase II, single-arm trial, belzutifan administered orally at 120 mg daily in patients with VHL-associated RCC demonstrated an encouraging 49% (95% CI 36–62%) in patients who had previously been treated with surgical therapies [93]. The most frequent adverse events included anemia and fatigue. Given this agent’s promising activity and targeting of a canonical pathway in RCC, it is not surprising that there are now ongoing clinical trials of belzutifan in combination with ICI + VEGF-TKI in the first-line treatment of mRCC (NCT04736706).
5 Conclusions
Combination VEGF-TKI + ICI and ICI + ICI regimens have now been widely established as new first-line standards in the treatment of mRCC. Although there is ongoing debate as to the optimal regimen in the first-line setting, ICI-based combinations represent, thus far, the pairing of the most active agents in RCC to improve outcomes in otherwise incurable metastatic disease. Important considerations that warrant further investigation to enhance the efficacy of immunotherapy in RCC include development of novel combinations with an ICI backbone, biomarker development to guide selection of optimal ICI-based therapies, sequencing following post-ICI therapy, and rechallenge strategies with ICI-based therapies following ICI exposure.
References
Schmidinger M, Steger G, Wenzel C, Locker GJ, Budinsky AC, Brodowicz T, et al. Sequential administration of interferon-gamma, GM-CSF, and interleukin-2 in patients with metastatic renal cell carcinoma: results of a phase II trial. J Immunother (1991). 2001;24(3):257–62.
Escudier B, Farace F, Angevin E, Triebel F, Antoun S, Leclercq B, et al. Combination of interleukin-2 and gamma interferon in metastatic renal cell carcinoma. Eur J Cancer. 1993;29A(5):724–8. https://doi.org/10.1016/s0959-8049(05)80354-5.
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–96. https://doi.org/10.1200/JCO.2002.20.1.289.
Clark JI, Curti B, Davis EJ, Kaufman H, Amin A, Alva A, et al. Long-term progression-free survival of patients with metastatic melanoma or renal cell carcinoma following high-dose interleukin-2. J Investig Med. 2021;69(4):888–92. https://doi.org/10.1136/jim-2020-001650.
Fishman M, Dutcher JP, Clark JI, Alva A, Miletello GP, Curti B, et al. Overall survival by clinical risk category for high dose interleukin-2 (HD IL-2) treated patients with metastatic renal cell cancer (mRCC): data from the PROCLAIM(SM) registry. J Immunother Cancer. 2019;7(1):84. https://doi.org/10.1186/s40425-019-0567-3.
Hannan R, Mohamad O, Diaz de Leon A, Manna S, Pop LM, Zhang Z, et al. Outcome and immune correlates of a phase II trial of high-dose interleukin-2 and stereotactic ablative radiotherapy for metastatic renal cell carcinoma. Clin Cancer Res. 2021;27(24):6716–25. https://doi.org/10.1158/1078-0432.Ccr-21-2083.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. https://doi.org/10.1056/NEJMoa065044.
Maia MC, Adashek J, Bergerot P, Almeida L, Dos Santos SF, Pal SK. Current systemic therapies for metastatic renal cell carcinoma in older adults: a comprehensive review. J Geriatr Oncol. 2018;9(3):265–74. https://doi.org/10.1016/j.jgo.2017.11.011.
Cowey CL, Rathmell WK. Using molecular biology to develop drugs for renal cell carcinoma. Expert Opin Drug Discov. 2008;3(3):311–27. https://doi.org/10.1517/17460441.3.3.311.
van Leeuwaarde RS, Ahmad S, Links TP, Giles RH. Von Hippel-Lindau Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al., editors. GeneReviews((R)). Seattle (WA) 1993. https://www.ncbi.nlm.nih.gov/books/NBK1463/.
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized Phase III trial. J Clin Oncol. 2010;28(6):1061–8. https://doi.org/10.1200/JCO.2009.23.9764.
Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35(6):591–7. https://doi.org/10.1200/JCO.2016.70.7398.
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31. https://doi.org/10.1056/NEJMoa1303989.
Díaz-Montero CM, Rini BI, Finke JH. The immunology of renal cell carcinoma. Nat Rev Nephrol. 2020;16(12):721–35. https://doi.org/10.1038/s41581-020-0316-3.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. https://doi.org/10.1056/NEJMoa1510665.
Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–30. https://doi.org/10.1097/CJI.0b013e318156e47e.
Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 Study. J Clin Oncol. 2017;35(34):3851–8. https://doi.org/10.1200/JCO.2016.72.1985.
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. https://doi.org/10.1056/NEJMoa1712126.
Geynisman DM, Plimack ER. Systemic therapy for advanced non-clear-cell renal cell carcinoma: slow but definite progress. Eur Urol. 2021;80(2):171–3. https://doi.org/10.1016/j.eururo.2021.04.031.
Zarrabi K, Walzer E, Zibelman M. Immune checkpoint inhibition in advanced non-clear cell renal cell carcinoma: leveraging success from clear cell histology into new opportunities. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13153652.
Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017;171(7):1678-91 e13. https://doi.org/10.1016/j.cell.2017.11.009.
Hirsch L, Flippot R, Escudier B, Albiges L. Immunomodulatory roles of VEGF pathway inhibitors in renal cell carcinoma. Drugs. 2020;80(12):1169–81. https://doi.org/10.1007/s40265-020-01327-7.
Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, Phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–15. https://doi.org/10.1016/S0140-6736(19)30723-8.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27. https://doi.org/10.1056/NEJMoa1816714.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41. https://doi.org/10.1056/NEJMoa2026982.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300. https://doi.org/10.1056/NEJMoa2035716.
Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747. https://doi.org/10.1155/2014/638747.
Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13(16):1845–57. https://doi.org/10.2174/092986706777585059.
Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16(3):293–300. https://doi.org/10.1016/S1470-2045(14)71222-7.
Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–96. https://doi.org/10.1200/JCO.2006.06.3602.
Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, Phase 3 trial. Lancet Oncol. 2019;20(10):1370–85. https://doi.org/10.1016/s1470-2045(19)30413-9.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15. https://doi.org/10.1056/NEJMoa1816047.
Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R D. 2011;11(2):113–26. https://doi.org/10.2165/11591240-000000000-00000.
Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8):1030–9. https://doi.org/10.1016/j.annonc.2020.04.010.
Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the Phase 3 JAVELIN Renal 101 trial. Nat Med. 2020;26(11):1733–41. https://doi.org/10.1038/s41591-020-1044-8.
Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, Phase 3 trial. Lancet Oncol. 2020;21(12):1563–73. https://doi.org/10.1016/s1470-2045(20)30436-8.
Gibney GT, Aziz SA, Camp RL, Conrad P, Schwartz BE, Chen CR, et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. 2013;24(2):343–9. https://doi.org/10.1093/annonc/mds463.
Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–97. https://doi.org/10.1038/onc.2015.343.
Rautiola J, Lampinen A, Mirtti T, Ristimaki A, Joensuu H, Bono P, et al. Association of angiopoietin-2 and Ki-67 expression with vascular density and sunitinib response in metastatic renal cell carcinoma. PLoS ONE. 2016;11(4): e0153745. https://doi.org/10.1371/journal.pone.0153745.
Adashek JJ, Kato S, Gumas S, Lee S, Okamura R, Sicklick J, et al. 86MO Personalized molecularly matched therapies for carcinomas of unknown primary is associated with improved outcomes. Ann Oncol. 2020;31:S275–6. https://doi.org/10.1016/j.annonc.2020.08.207.
Adashek JJ, Subbiah V, Kurzrock R. From Tissue-Agnostic to N-of-One Therapies: (R)Evolution of the Precision Paradigm. Trends Cancer. 2020. https://doi.org/10.1016/j.trecan.2020.08.009.
Adashek JJ, Szeto CW, Reddy SK, Spiess PE. 1235PDifferential expression of immunoregulatory molecules and highly-associated cancer genes may provide novel insights into strategic trial design for therapeutics. Ann Oncol. 2019. https://doi.org/10.1093/annonc/mdz253.061.
Adashek JJ, Szeto CW, Reddy SK, Spiess PE. Real-world data validation for differential expression of immunoregulatory molecules and targetable cancer genes may provide therapeutic insights into agnostic-driven trial designs. Journal of Clinical Oncology. 2020;38(5_suppl):10-. doi: https://doi.org/10.1200/JCO.2020.38.5_suppl.10.
Capozzi M, De Divitiis C, Ottaiano A, von Arx C, Scala S, Tatangelo F, et al. Lenvatinib, a molecule with versatile application: from preclinical evidence to future development in anti-cancer treatment. Cancer Manag Res. 2019;11:3847–60. https://doi.org/10.2147/CMAR.S188316.
Okamoto K, Ikemori-Kawada M, Jestel A, von Konig K, Funahashi Y, Matsushima T, et al. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett. 2015;6(1):89–94. https://doi.org/10.1021/ml500394m.
Adashek JJ, Salgia MM, Posadas EM, Figlin RA, Gong J. Role of biomarkers in prediction of response to therapeutics in metastatic renal-cell carcinoma. Clin Genitourin Cancer. 2019;17(3):e454–60. https://doi.org/10.1016/j.clgc.2019.01.004.
Adashek JJ, Genovese G, Tannir NM, Msaouel P. Recent advancements in the treatment of metastatic clear cell renal cell carcinoma: A review of the evidence using second-generation p-values. Cancer Treat Res Commun. 2020;23: 100166. https://doi.org/10.1016/j.ctarc.2020.100166.
Adashek JJ, Goloubev A, Kato S, Kurzrock R. Missing the target in cancer therapy. Nature Cancer. 2021;2(4):369–71. https://doi.org/10.1038/s43018-021-00204-w.
Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Lam TB, et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: nivolumab plus cabozantinib joins immune checkpoint inhibition combination therapies for treatment-naïve metastatic clear-cell renal cell carcinoma. Eur Urol. 2021;79(3):339–42. https://doi.org/10.1016/j.eururo.2020.12.005.
Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the Phase III CheckMate 214 trial. ESMO Open. 2020;5(6): e001079. https://doi.org/10.1136/esmoopen-2020-001079.
Cella D, Motzer RJ, Suarez C, Blum SI, Ejzykowicz F, Hamilton M, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, Phase 3 trial. Lancet Oncol. 2022. https://doi.org/10.1016/s1470-2045(21)00693-8.
Bedke J, Rini B, Plimack E, Stus V, Gafanov R, Hawkins R, et al. Health-related quality-of-life (HRQoL) analysis from KEYNOTE-426: pembrolizumab (pembro) plus axitinib (axi) vs sunitinib for advanced renal cell carcinoma (RCC). EAU20 Virtual Congress, 17–26 July 2020. 2020:Game-changing Session 4.
Motzer RJ, Porta C, Alekseev B, Rha SY, Choueiri TK, Mendez-Vidal MJ, et al. Health-related quality-of-life (HRQoL) analysis from the Phase 3 CLEAR trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) for patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2021;39(15_suppl):4502. https://doi.org/10.1200/JCO.2021.39.15_suppl.4502.
Escudier B, Powles T, Motzer RJ, Olencki T, Arén Frontera O, Oudard S, et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J Clin Oncol. 2018;36(8):765–72. https://doi.org/10.1200/jco.2017.74.7352.
Flippot R, Dalban C, Laguerre B, Borchiellini D, Gravis G, Négrier S, et al. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: results of the GETUG-AFU 26 NIVOREN multicenter phase II study. J Clin Oncol. 2019;37(23):2008–16. https://doi.org/10.1200/jco.18.02218.
Hirsch L, Martinez Chanza N, Farah S, Xie W, Flippot R, Braun DA, et al. Clinical Activity and safety of cabozantinib for brain metastases in patients with renal cell carcinoma. JAMA Oncol. 2021;7(12):1815–23. https://doi.org/10.1001/jamaoncol.2021.4544.
Uche A, Sila C, Tanoura T, Yeh J, Bhowmick N, Posadas E, et al. Brain complete response to cabozantinib prior to radiation therapy in metastatic renal cell carcinoma. Case Rep Urol. 2019;2019:6769017. https://doi.org/10.1155/2019/6769017.
Derosa L, Teuff GL, Khordahi M, Chanez B, Zalcman E, Gravis G, et al. Inter and intra-tumor heterogeneity of PD-L1 and MET expression in metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2017;35(15_suppl):4569. https://doi.org/10.1200/JCO.2017.35.15_suppl.4569.
Minocha M, Khurana V, Mitra AK. Determination of pazopanib (GW-786034) in mouse plasma and brain tissue by liquid chromatography-tandem mass spectrometry (LC/MS-MS). J Chromatogr B Analyt Technol Biomed Life Sci. 2012;901:85–92. https://doi.org/10.1016/j.jchromb.2012.06.004.
Zhang Y, Guessous F, Kofman A, Schiff D, Abounader R. XL-184, a MET, VEGFR-2 and RET kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and NSCLC. IDrugs. 2010;13(2):112–21.
Pal SK, McGregor B, Suarez C, Tsao CK, Kelly W, Vaishampayan U, et al. Cabozantinib in combination with atezolizumab for advanced renal cell carcinoma: results from the COSMIC-021 study. J Clin Oncol. 2021. https://doi.org/10.1200/JCO.21.00939.
De Sousa LA, Battin C, Jutz S, Leitner J, Hafner C, Tobias J, et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep. 2019;9(1):11472. https://doi.org/10.1038/s41598-019-47910-1.
Plimack ER, Hammers HJ, Choueiri TK, Rini BI, Motzer RJ, Suttner L, et al. A Phase 1b/2 umbrella study of investigational immune and targeted combination therapies as first-line therapy for patients with advanced renal cell carcinoma (RCC). J Clin Oncol. 2021;39(15_suppl):TPS4594-TPS. https://doi.org/10.1200/JCO.2021.39.15_suppl.TPS4594.
Gong J, Chehrazi-Raffle A, Placencio-Hickok V, Guan M, Hendifar A, Salgia R. The gut microbiome and response to immune checkpoint inhibitors: preclinical and clinical strategies. Clin Transl Med. 2019;8(1):9. https://doi.org/10.1186/s40169-019-0225-x.
Meza LA, Dizman N, Bergerot PG, Dorff TB, Lyou Y, Frankel PH, et al. First results of a randomized Phase IB study comparing nivolumab/ipilimumab with or without CBM-588 in patients with metastatic renal cell carcinoma. J Clin Oncol. 2021;39(15_suppl):4513. https://doi.org/10.1200/JCO.2021.39.15_suppl.4513.
Siva S, Bressel M, Wood ST, Shaw MG, Loi S, Sandhu SK, et al. Stereotactic radiotherapy and short-course pembrolizumab for oligometastatic renal cell carcinoma-the RAPPORT trial. Eur Urol. 2021. https://doi.org/10.1016/j.eururo.2021.12.006.
Mason N, Kim Y, Shah S, Adashek JJ, Manley BJ, Spiess PE, et al. 667P Cost effectiveness of immune checkpoint inhibitors in combination with targeted therapies in metastatic renal cell carcinoma. Ann Oncol. 2021;32:S692. https://doi.org/10.1016/j.annonc.2021.08.063.
Lee CH, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a Phase 1b/2 study. Lancet Oncol. 2021;22(7):946–58. https://doi.org/10.1016/S1470-2045(21)00241-2.
McKay RR, McGregor BA, Xie W, Braun DA, Wei X, Kyriakopoulos CE, et al. Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: a response-based phase II study (OMNIVORE). J Clin Oncol. 2020;38(36):4240–8. https://doi.org/10.1200/jco.20.02295.
Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, Phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–82. https://doi.org/10.1016/S1470-2045(15)00290-9.
Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, Phase 3 trial. Lancet Oncol. 2016;17(7):917–27. https://doi.org/10.1016/S1470-2045(16)30107-3.
Ornstein MC, Pal SK, Wood LS, Tomer JM, Hobbs BP, Jia XS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, Phase 2 study. Lancet Oncol. 2019;20(10):1386–94. https://doi.org/10.1016/s1470-2045(19)30513-3.
Shah AY, Kotecha RR, Lemke EA, Chandramohan A, Chaim JL, Msaouel P, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with second-line VEGFR-TKI after first-line immune checkpoint inhibitors. Eur J Cancer. 2019;114:67–75. https://doi.org/10.1016/j.ejca.2019.04.003.
Pal SK, Escudier BJ, Atkins MB, Hutson TE, Porta C, Verzoni E, et al. Final overall survival results from a phase 3 study to compare tivozanib to sorafenib as third- or fourth-line therapy in subjects with metastatic renal cell carcinoma. Eur Urol. 2020;78(6):783–5. https://doi.org/10.1016/j.eururo.2020.08.007.
Iacovelli R, Nole F, Verri E, Renne G, Paglino C, Santoni M, et al. Prognostic Role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol. 2016;11(2):143–8. https://doi.org/10.1007/s11523-015-0392-7.
Labriola MK, Zhu J, Gupta RT, McCall S, Jackson J, Kong EF, et al. Characterization of tumor mutation burden, PD-L1 and DNA repair genes to assess relationship to immune checkpoint inhibitors response in metastatic renal cell carcinoma. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2019-000319.
Carril-Ajuria L, Santos M, Roldan-Romero JM, Rodriguez-Antona C, de Velasco G. Prognostic and predictive value of PBRM1 in clear cell renal cell carcinoma. Cancers (Basel). 2019. https://doi.org/10.3390/cancers12010016.
Mardinian K, Adashek JJ, Botta GP, Kato S, Kurzrock R. SMARCA4: implications of an altered chromatin-remodeling gene for cancer development and therapy. Mol Cancer Ther. 2021. https://doi.org/10.1158/1535-7163.MCT-21-0433.
Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Alekseev BY, et al. Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.5981.
Carretero-González A, Lora D, Martín Sobrino I, Sáez Sanz I, Bourlon MT, Anido Herranz U, et al. The value of PD-L1 expression as predictive biomarker in metastatic renal cell carcinoma patients: a meta-analysis of randomized clinical trials. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12071945.
Epaillard N, Simonaggio A, Elaidi R, Azzouz F, Braychenko E, Thibault C, et al. BIONIKK: a phase 2 biomarker driven trial with nivolumab and ipilimumab or VEGFR tyrosine kinase inhibitor (TKI) in naive metastatic kidney cancer. Bull Cancer. 2020;107(5S):eS22–7. https://doi.org/10.1016/S0007-4551(20)30283-6.
Vano Y, Elaidi RT, Bennamoun M, Chevreau CM, Borchiellini D, Pannier D, et al. LBA25 Results from the Phase II biomarker driven trial with nivolumab (N) and ipilimumab or VEGFR tyrosine kinase inhibitor (TKI) in naïve metastatic kidney cancer (m-ccRCC) patients (pts): the BIONIKK trial. Ann Oncol. 2020;31:S1157. https://doi.org/10.1016/j.annonc.2020.08.2254.
Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38(6):803-17 e4. https://doi.org/10.1016/j.ccell.2020.10.011.
Pal SK, Bergerot P, Dizman N, Bergerot C, Adashek J, Madison R, et al. Responses to alectinib in ALK-rearranged papillary renal cell carcinoma. Eur Urol. 2018;74(1):124–8. https://doi.org/10.1016/j.eururo.2018.03.032.
Hicks JK, Howard R, Reisman P, Adashek JJ, Fields KK, Gray JE, et al. Integrating somatic and germline next-generation sequencing into routine clinical oncology practice. JCO Precis Oncol. 2021. https://doi.org/10.1200/PO.20.00513.
Adashek JJ, Szeto CW, Veerapaneni S, Preble A, Reddy SK, Spiess PE. Abstract 2224: validated differential expression of immunoregulatory molecules that coincide with targetable mutations may provide novel insights into strategic trial design for therapeutics. Can Res. 2020;80(16 Supplement):2224. https://doi.org/10.1158/1538-7445.AM2020-2224.
Adashek JJ, Kato S, Parulkar R, Szeto CW, Sanborn JZ, Vaske CJ, et al. Transcriptomic silencing as a potential mechanism of treatment resistance. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.134824.
Adashek JJ, Kato S, Ferrara R, Lo Russo G, Kurzrock R. Hyperprogression and immune checkpoint inhibitors: hype or progress? Oncologist. 2019. https://doi.org/10.1634/theoncologist.2019-0636.
Adashek JJ, Subbiah IM, Matos I, Garralda E, Menta AK, Ganeshan DM, et al. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer. 2020;6(3):181–91. https://doi.org/10.1016/j.trecan.2020.01.005.
Pal SK, Forero-Torres A, Thompson JA, Morris JC, Chhabra S, Hoimes CJ, et al. A Phase 1 trial of SGN-CD70A in patients with CD70-positive, metastatic renal cell carcinoma. Cancer. 2019;125(7):1124–32. https://doi.org/10.1002/cncr.31912.
Deeks ED. Belzutifan: first approval. Drugs. 2021. https://doi.org/10.1007/s40265-021-01606-x.
Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N Engl J Med. 2021;385(22):2036–46. https://doi.org/10.1056/NEJMoa2103425.
Acknowledgements
Figure created with BioRender.com
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript. U.S. Department of Defense (Grant no. KC200178).
Conflicts of interest/competing interests
Edwin Posadas: Consulting or Advisory Role—CytoLumina, Genentech/Roche, Janssen Oncology, Janssen Oncology, Novartis; Speakers’ Bureau—Bayer; Research Funding—Pfizer; Patents, Royalties, Other Intellectual Property—Patent on NanoVelcro Assay for CTCs in prostate cancer; Travel, Accommodations, Expenses—TRACON Pharma. Neil Bhowmick: Leadership—Kairos Pharma Lmt., Armida Labs, Inc. Consulting: TRACON Pharma., Cellgene, Xencor. Research funding: Xencor. Stephen Freedland: Consulting or Advisory Role—Merck, Astellas, AstraZeneca, Pfizer, Janssen, Bayer, Clovis, Sanofi, Myovant, and Exact Sciences. Robert Figlin: Leadership—4Dx, Apollomics; Consulting or Advisory Role—Bristol-Myers Squibb, CBT Pharmaceuticals, Johnson & Johnson; Research Funding—Bristol-Myers Squibb (Inst), Calithera Biosciences (Inst), Exelixis (Inst), Merck (Inst), Peloton Therapeutics (Inst). Jun Gong: Consultant or Advisory Role—EMD Serono, Elsevier, Exelixis, QED Therapeutics, Natera, Basilea, HalioDx, Eisai, Janssen, Astellas and Amgen. All other authors report no other conflicts of interest in this work. JJB, RF, JG are supported by DoD KCRP Award KC200178.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
JG conceptualized the review; JA and JG wrote the initial drafts; all authors edited and approved the final version of the manuscript.
Rights and permissions
About this article
Cite this article
Adashek, J.J., Breunig, J.J., Posadas, E. et al. First-line Immune Checkpoint Inhibitor Combinations in Metastatic Renal Cell Carcinoma: Where Are We Going, Where Have We Been?. Drugs 82, 439–453 (2022). https://doi.org/10.1007/s40265-022-01683-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01683-6