Abstract
Background
Previous systematic reviews (2008; 2016) concluded similarity in outcomes between brand-name and generic drugs in cardiology, but they included ≥ 50% comparative bioavailability studies, not designed or powered to detect a difference in efficacy or safety between drug types. We aimed to summarise best-evidence regarding the effectiveness and safety of generic versus brand-name drugs used in cardiology.
Methods
For this systematic review of the literature, scientific databases (MEDLINE and EMBASE) were searched from January 1984 to October 2018. Original research reports comparing the clinical impact of brand-name versus generic cardiovascular drugs on humans treated in a real-life setting, were selected. Meta-analyses and subgroup analyses were performed. Heterogeneity (I2) and risk of bias were tested.
Results
Among the 3148 screened abstracts, 72 met the inclusion criteria (n ≥ 1,000,000 patients, mean age 65 ± 10 years; 42% women). A total of 60% of studies showed no difference between drug types, while 26% concluded that the brand-name drug was more effective or safe, 13% were inconclusive and only 1% concluded that generics did better. The overall crude risk ratio of all-cause hospital visits for generic versus brand-name drug was 1.14 (95% confidence interval: 1.06–1.23; I2: 98%), while it was 1.05 (0.98–1.14; I2: 68%) for cardiovascular hospital visits. The crude risk ratio was not statistically significant for randomised controlled trials only (n = 4; 0.92 [0.63–1.34], I2: 35%).
Conclusion
The crude risk of hospital visits was higher for patients exposed to generic compared to brand-name cardiovascular drugs. However, the evidence is insufficient and too heterogeneous to draw any firm conclusion regarding the effectiveness and safety of generic drugs in cardiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This systematic review and meta-analysis reports that more than half of studies showed no difference in outcomes between cardiovascular generics and the brand-name drugs. |
The overall crude risk of hospital visits was higher for patients exposed to generics. |
Even though evidence is insufficient and very heterogeneous to draw any firm conclusion, results signal that more studies are required to confirm the effectiveness and safety of international generic drug licensing processes. |
1 Introduction
When a brand-name drug patent expires, generic drugs are commercialised at lower cost [1]. Health authorities regulate bioequivalence standards for generic drugs by comparative bioavailability studies. It is known and accepted that some bioavailability parameters for generic versions may vary up to 20% compared to the original reference drug [2]. This difference could potentially explain the occurrence of side effects or low efficacy for patients switched to generics [3,4,5], a fact already controversial in the literature [6, 7].
Two large systematic reviews and meta-analyses (2008; 2016) reported similar rates of hospital visits and clinical measurement outcomes between brand-name and generic users in cardiology [8, 9]. However, even though well conducted, the conclusions of these systematic reviews were based on the authors’ meta-analyses, which included ≥ 50% of comparative bioavailability studies. Those studies are not designed or powered to detect a difference in efficacy or safety. Indeed, comparative bioavailability studies are generally crossover randomised controlled trials powered to detect a difference in bioavailability between drugs. A selected group of 12–50 healthy fasting subjects are administered a single dose of the tested generic and, after a washout period, a single dose of the brand-name reference product [2, 10]. The follow-up of subjects is normally < 72 h. We believe that including comparative bioavailability studies in these systematic reviews may have led to underestimation of the true difference between the groups.
Clinicians and researchers agree on the urgency to determine if generic drugs, licensed through healthcare policies, are as effective and safe as the brand-name products [11]. In the current study, we aimed to perform a synthesis of best evidence to answer the following question: “Are generic drugs used in cardiology as effective and safe as their brand-name counterparts?” The tested hypothesis is that there would be a difference in outcomes between generic and brand-name users. Results would then differ from previously published systematic reviews on this research question [8, 9].
2 Methods
2.1 Study Design
This is a systematic review of the literature following the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions [12]. Results are reported according to The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [13].
2.2 Source of Data
The search was conducted online in MEDLINE and EMBASE. These electronic databases were searched from their inception or from January 1984 to October 2018, and an update was performed on July 2019.
2.3 Search Strategy
Similar to Kesselheim et al. [8], three main subject terms were used: (1) a term related to the type of study (e.g. clinical trial, retrospective cohorts, etc.), (2) a term related to the product of interest (e.g. generic, brand-name, etc.), (3) a term related to cardiovascular medicine (e.g. heart failure, beta-blockers, etc.; details in Supplementary Material). The search strategy was designed for MEDLINE and adapted for Embase. Abstracts containing at least one search item in each of the three main categories met criteria for the title and abstract review. References from identified studies and existing reviews were screened to complete the systematic search of studies. Every identified abstract was imported into Endnote (version X9, Thomson Reuters). Duplicates were removed.
2.4 Eligibility Criteria for Study Selection
Title and abstracts of each identified reference were screened independently by three reviewers (JL, MT and JMG) and selected for full-text review if they were original research reports comparing the clinical impact of brand-name versus generic cardiovascular drugs on humans treated in real-life setting (i.e. not in a pre-marketing, randomised clinical trial environment). Studies conducted with healthy subjects, using biological products or aiming at comparing pharmacokinetics parameters only, were excluded (more details in Supplementary material). Disagreements were discussed and resolved by consensus. Then, JL and MT assessed every full-text article to determine final inclusions.
2.5 Outcomes
Primary outcomes were clinical measures when available (systolic blood pressure [diastolic was not available], lipids level, heart rate, etc.) and all-cause hospital visits (including consultations at the emergency room [department], hospital admissions, specific cardiovascular disease-related hospital visits, etc.).
2.6 Data Extraction
Data were extracted by two independent reviewers (JL and MT). A pilot extraction was conducted on 10 studies of various designs for training and to ensure further agreement on data extraction. A standardised data extraction form was used (see Items in Supplementary Material). Authors were contacted for missing data.
2.7 Risk of Bias
The risk of bias of each study was assessed by two independent reviewers using recommended tools: (1) the Cochrane method for randomised controlled trials [13] and (2) ROBINS-I for non-randomised controlled trials [14]. For the former, the judgement for each entry involves assessing the risk of selection, performance, detection, attrition and reporting bias as low, high or unclear (including lack of information or uncertainty). For the latter, the judgement regarding the risk of selection, information and confusion bias was made as low, moderate, serious, critical or “no information” [14]. Unlike Kesselheim et al. [8] and Manzoli et al. [9], we did not elect to use scales that yield a summary score, as this practice is now discouraged by the Cochrane Collaboration (2019). The risk of bias was impossible to assess for some studies due to lack of information (e.g. abstract only, article in Russian language).
2.8 Statistical Analyses
Descriptive analyses of main study characteristics were performed, and the proportions of studies with the following conclusions were calculated: (1) No difference; (2) Favours generics; (3) Favours brand-name; (4) Uncertain or various differences. We also performed a subgroup analysis to compare the distribution of authors’ conclusion according to the type of publication (abstract vs full text).
Outcome data were aggregated using random effect meta-analyses. Crude association was expressed as risk ratio for hospital visit outcomes and mean difference for continuous clinical outcomes. Meta-analyses were performed using Review Manager (version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). Heterogeneity was tested with the I2 statistic and interpreted with suggested thresholds (Low: 0–40%; Moderate: 30–60%; Substantial: 50–90%; Considerable heterogeneity: 75–100%) [13]. We constructed funnel plot to assess the risk of publication bias. Subgroup analyses were planned a priori and conducted according to the following variables: study design (randomised vs non-randomised control trials), publication type (abstract vs full text), follow-up duration, source of funding (industry, non-industry or not reported) and drug classes. Statistical significance was judged according to 95% confidence intervals.
3 Results
3.1 Characteristics of Studies
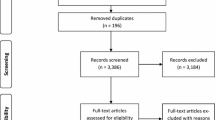
A total of 3148 abstracts were identified by the search strategy (Fig. 1). A set of 105 studies were then screened for final eligibility. Of those, 72 were included in the qualitative analysis (published as abstracts or full texts; 33 excluded due to non-English non-French language (6), duplicate studies of previously published abstracts (16) or not comparing brand-name to generic drugs (11). Among those, only 33 were included in the quantitative analysis as others had no extractable data in the abstracts or the full text for pooling. A total of 22 studies were funded by the pharmaceutical industry (Table 1). The risk of bias was modest to serious in the majority of studies (Table S1 and S2, Supplementary Material). There were 30 randomised clinical trials and 42 non-randomised clinical/observational studies. Follow-up time varied between < 1 day and 20 years (Table 1).
3.2 Patient Characteristics
The studies included a total of > 1,000,000 patients who used generic or brand-name cardiovascular drugs. Mean age was 65 ± 10 years old and 42% were women. The most commonly studied therapeutic classes were antiplatelets, statins, anticoagulants, angiotensin II receptor blockers and beta-blockers (Fig. 2). It was not possible to ascertain differences in patient characteristics according to generic or brand-name group due to unavailability of most granular data and to the heterogeneity of available information.
3.3 Comparison on Outcome Between Generics and the Brand-name
A total of 43 studies (60%) showed no difference between generic and brand-name drugs, while 19 studies (26%) concluded that the brand-name drug was more effective or safe than the generic drug. Nine studies (13%) were inconclusive and only one (1%) concluded that generics were more safe or effective than the brand-name drug.
The only extractable clinical and hospital visits data for the meta-analyses were platelet function (including relative or absolute value of platelet aggregation when available), systolic blood pressure, international normalised ratio and hospital visits (all-cause and cardiovascular hospitalisations or emergency department visits). The overall crude risk ratio of all-cause hospital visits was 1.14 (1.06–1.23), while it was 1.05 (0.98–1.14) for cardiovascular hospital visits (Fig. 3a). The pooled “Hospital visits” outcome yielded a crude risk ratio of 1.10 (1.04–1.15). Platelet function (Fig. 3b), international normalised ratio (Fig. 3c) and systolic blood pressure (Fig. 3d) were similar between generic and brand-name groups. Heterogeneity was substantial to considerable in all meta-analyses (I2: 60–96%; Fig. 3), except for the systolic blood pressure (I2: 15%).
In the first sensitivity analysis, the crude risk ratio of all-cause hospital visits was statistically significant for non-randomised controlled trials (1.10 [1.05–1.16]), while it was not for randomised controlled trials (0.92 [0.63–1.34], Fig. S1-A). Following sensitivity analyses revealed that the risk ratio of hospital visits was: (1) still statistically significant when excluding abstract-only studies (1.10 [1.04–1.16], Fig. S1–B) and, (2) statistically significant in both studies with a follow-up of ≤ 2 months (3.13 [1.14–8.55]) or those with > 2 months (1.09 [1.04–1.15]), Fig. S1–C). The risk ratio of hospital visits was not statistically significant in studies funded by the industry (1.07 [0.96–1.19], Fig. S1–D), while it was statistically significant in studies not funded by the industry (1.11 [1.03–1.19]) or those for which the funding was not disclosed (1.09 [1.03–1.16]). Last, the risk ratio of hospital visits differed according to drug classes: 1.08 for antiplatelets [0.90 to 1.29], 0.93 for statins [0.86–1.02], 1.09 for anticoagulants [1.03–1.14] and 1.20 [1.17–1.23] for “others” (three angiotensin II receptor blockers and one beta-blocker, Fig. S1–E).
3.4 Risk of Publication Bias
The comparison between the distribution of proportions among authors’ conclusions while excluding abstracts versus our main analysis, yielded statistically significant differences in proportions (p = 0.0164). The Funnel plots are modestly asymmetrical (Figs S2, Supplementary Material).
4 Discussion
4.1 Major Findings
In this systematic review, 60% of the studies showed similar effectiveness or safety between generic and brand-name cardiovascular drugs; approximately 30% showed brand-name drugs to be superior. There was no difference in platelet functions, international normalised ratio and systolic blood pressure between generic and brand-name drug users. However, we found a crude 10% higher risk of all-cause hospital visits compared to brand-name users. To our knowledge, this is the first systematic review of the literature reporting some differences in hospital visits between generics and brand-name drugs in cardiology.
Our results differ from previously published systematic reviews assessing equivalence of generic and brand-name drugs in cardiology [8, 9]. These reviews did not detect any difference in outcomes for generic versus brand-name cardiovascular drugs users, while we report that there may be some differences. Two main reasons could explain this discrepancy. First, we think previously published systematic reviews underestimated the true difference between groups due to the type of studies included. Indeed, half of the studies included in the Kesselheim et al. article [8] were comparative bioavailability studies. As mentioned earlier, those studies are not powered or designed to detect lack of efficacy/adverse events and definitely cannot be used to ascertain that generic drugs are equivalent to brand-name drugs in real-life settings. The subsequent paper of Manzoli et al. included only randomised clinical trials (n = 74), 42 of which are comparative bioavailability studies conducted in healthy subjects [9]. The second reason explaining discrepancies between our results and previously published similar systematic reviews is the addition of new scientific evidence in recent years. Among others, a research group from Ontario (Canada) published two well-conducted cohort studies comparing hospital admissions and mortality between generic and brand-name atorvastatin [7] and clopidogrel users [6]. No differences between groups were found in those studies but the substitution itself was not assessed. On the other hand, a group from Quebec (Canada) published 3 articles containing 5 time series analyses and over 500,000 patients using generic or brand-name losartan, valsartan, candesartan, warfarin or clopidogrel [3,4,5]. Those studies reported an 8–21% increase in rates of emergency room consultations or hospital admissions for the population switched to generic versions compared to patients who remained on the brand-name drug. The authors report that it was not possible to adjust the rates or the segmented regression models for potential confounders with this study design, but they performed many sensitivity analyses to test the robustness of the results.
4.2 Confounding Bias?
The risk ratio of all-cause hospital visits was statistically significant for non-randomised controlled trials, but not for randomised controlled trials, suggesting that results from non-randomised controlled trials are biased by confounders. If true, this would probably lead to an overestimation of the risk ratio of hospital visits between generic and brand-name users. Other factors seemed to impact the risk ratio estimated in our meta-analysis (follow-up duration, drug classes). Unfortunately, we were not able to perform meta-regression due to the heterogeneity of the information available in the articles and to the small amount of studies per subgroup.
4.3 Publication Bias?
It is interesting to note that only 70% of eligible records made it to publication of a full text article (Table 1). There was a statistically significant difference in proportions when comparing our qualitative analyses including and excluding abstracts. Nevertheless, it is reassuring to see that the overall qualitative interpretation of our results was similar in both classifications. In comparison, in 2016, Flacco et al. reported that fewer than 50% of registered protocols comparing a generic to a brand-name drug have ever had fully published results [15], and most of those were comparative bioavailability studies funded by generic manufacturers. The presence of a publication bias among generic versus brand-name scientific evidences is therefore possible even if it is difficult to detect in the Funnel plots. The presence of such a bias would be associated with an underestimation of true difference in outcomes between generic and brand-name drug users in cardiology.
4.4 Strengths and Limitations
Our review included the whole range of oral cardiovascular drug classes, even though some drug classes are underrepresented [e.g. I(f) current inhibitor]. Generalisability of the results should mostly apply to well-represented drug classes like antiplatelet, anticoagulants and statins. Another strength of this review is the inclusion of comprehensive real-world evidence on clinical equivalence. The related limitation of this feature is revealed by meta-analyses, reporting very high heterogeneity of included studies (only 15 studies with hospital visits extractable data for meta-analysis, and among those, only 4 reporting specific cardiovascular-related hospital visits), affecting the ability to draw firm conclusions on study results. Another limitation of our meta-analysis is the possibility of confounding bias; those are crude results and meta-regression was not possible as discussed above. As well, only 33 of 72 included records could be included in meta-analyses, mostly due to the presence of many abstracts with unextractable data. Meta-analyses may thus not fully reflect the literature (published and unpublished). Therefore, pooled differences in outcomes should be interpreted cautiously, notably regarding platelet aggregation for which no standardised value was used to pool studies. Despite these limitations, the results of our review are based on an exhaustive literature search and rigorous methodology.
4.5 Implications
The field of generic drug equivalence is very challenging due, among others things, to (1) the globalisation of raw ingredients/finished products (pills) manufacturers, governed by various jurisdictions worldwide, (2) the variable enforcement power of health authorities regarding good manufacturing practices [2], and the sparsity of sufficiently powered randomised controlled trials. Nevertheless, this systematic review highlights that the safety and effectiveness of generic cardiovascular drugs is uncertain. The biological plausibility of experiencing adverse events or lack of efficacy after switching from the brand-name to a generic version (or vice versa) has already been published [5, 16], but is still subject to debate [11]. Healthcare professionals, as well as patients, should be aware of the potential effects of generic substitution and report any adverse event or lack of efficacy to health authorities, such as Health Canada MedEffect system or the Food and Drug Administration Adverse Event Reporting System [17, 18]. The results of this review suggest that international generic drug licensing processes [2, 10] may need to be further challenged.
5 Conclusion
In the current analysis, 60% of the studies showed similar effectiveness or safety between generic and brand-name cardiovascular drugs; approximately 30% showed brand-name drugs to be superior. Overall, our results differ from previously published systematic reviews, but evidence is insufficient and too heterogeneous to support that generics are as effective and safe as brand-name drugs in cardiology. In particular, the crude risk ratio was not statistically significant between groups from randomised controlled trials only. More studies are then required to reassure patients, clinicians, researchers, payers and governments that actual generic drugs licensing processes are safe for the population taking generic drugs.
References
Association Canadienne du Médicament Générique. Les faits. 2017. http://generiquescanadiens.ca/les-faits/medicaments-generiques/. Accessed 27 septembre 2017
Santé Canada. Ligne directrice - Normes en matière d’études de biodisponibilités comparatives: Formes pharmaceutiques de médicaments à effets systémiques. 2018. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medicaments/demandes-presentations/lignes-directrices/biodisponibilite-bioequivalence/normes-matiere-etudes-biodisponibilite-comparatives-formes-pharmaceutiques-medicaments-effets-systemiques.html. Accessed 29 octobre 2018
Leclerc J, Blais C, Rochette L, et al. Did Generic Clopidogrel Commercialization Affect Trends of ER Consultations and Hospitalizations in the Population Treated with Clopidogrel? Drugs Aging. 2019.
Leclerc J, Blais C, Rochette L, et al. Trends in hospital visits for generic and brand-name warfarin users in Quebec, Canada; a population-based time series analysis. Am J Cardiovasc Drugs. 2018;19(3):287–97.
Leclerc J, Blais C, Rochette L, et al. Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec, Canada: a population-based time series analysis. Circ Cardiovasc Qual Outcomes. 2017;10:1–9.
Ko DT, Krumholz HM, Tu JV, et al. Clinical outcomes of plavix and generic clopidogrel for patients hospitalized with an acute coronary syndrome. Circ Cardiovasc Qual Outcomes. 2018;11:e004194.
Jackevicius C, Tu JV, Krumholz HM, et al. Comparative effectiveness of generic atorvastatin and lipitor(R) in patients hospitalized with an acute coronary syndrome. J Am Heart Assoc. 2016;5(4):e003350.
Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA J Am Med Assoc. 2008;300:2514–26.
Manzoli L, Flacco ME, Boccia S, et al. Generic versus brand-name drugs used in cardiovascular diseases. Eur J Epidemiol. 2016;31:351–68.
Davit B, Braddy AC, Conner DP, et al. International guidelines for bioequivalence of systemically available orally administered generic drug products: a survey of similarities and differences. The AAPS J. 2013;15:974–90.
Alter D. When do we decide that generic and brand-name drugs are clinically equivalent? Perfecting decisions with imperfect evidence. circulation: cardiovascular quality and outcomes. 2017;10.
Cochrane. Guides and handbooks. 2017. http://training.cochrane.org/handbooks. Accessed 16 novembre 2017
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JAC, Hernan M, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:1–7.
Flacco ME, Manzoli L, Boccia S, et al. Registered randomized trials comparing generic and brand-name drugs: a survey. Mayo Clin Proc. 2016;91:1021–34.
Leclerc J. Surveillance des consultations à l’urgence et des hospitalisations chez les utilisateurs de médicaments génériques et originaux en cardiologie, au Québec. Université Laval; 2017. p. 447.
Santé Canada. MedEffet Canada. 2019. https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/medeffet-canada.html. Accessed 27 juin 2019
U.S. Food and Drug Administration (FDA). FDA Adverse Event Reporting System. 2019. https://www.fda.gov/drugs/fda-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-electronic-submissions. Accessed 12 juin 2019
Sharoky M, Perkal M, Tabatznik B, et al. Comparative efficacy and bioequivalence of a brandname and a generic triamterene-hydrochlorothiazide combination product. Clini Pharm. 1989;8:496–500.
Saseen JJ, Porter JA, Barnette DJ, et al. Postabsorption concentration peaks with brand-name and generic verapamil: a double-blind crossover study in elderly hypertensive patients. J Clin Pharmacol. 1997;37:526–34.
Neutel JM, Smith DHG. A randomized crossover study to compare the efficacy and tolerability of Barr warfarin sodium to the currently available Coumadin. Cardiovasc Rev Rep. 1998;19:49–59.
Swenson CN, Fundak G. Fundak. Observational cohort study of switching warfarin sodium products in a managed care organization. Am J Health-syst Pharm AJHP. 2000;57:452–5.
Assawawitoontip S, Wiwanitkit V. A randomized crossover study to evaluate LDL-cholesterol lowering effect of a generic product of simvastatin (Unison company) compared to simvastatin (ZocorTM) in hypercholesterolemic subjects. J Med Assoc Thail. 2002;85:S118–24.
Milligan PE, Banet GA, Waterman AD, et al. Substitution of generic warfarin for Coumadin in an HMO setting. Ann Pharmacother. 2002;36:764–8.
Ol’binskaia LI, Danilogorskaia IA. Efficacy, safety, and pharmaco-economical aspects of the therapy for dyslipidemia with brand-name and generic statins. Terapevticheskii arkhiv. 2003;75:47–50.
Witt DM, Tillman DJ, Evans CM, et al. Evaluation of the clinical and economic impact of a brand name-to-generic warfarin sodium conversion program. Pharmacotherapy. 2003;23:360–8.
Ashraf T, Ahmed M, Talpur MS, et al. Competency profile of locally manufactured clopidogrel Lowplat and foreign manufactured clopidogrel Plavix in patients of suspected ischemic heart disease (CLAP-IHD). JPMA J Pak Med Assoc. 2005;55:443–8.
Lee HL, Kan CD, Yang YJ. Efficacy and tolerability of the switch from a branded toa generic garfarin sodium product: an observer-blinded, randomized, crossover study. Clin Ther. 2005;27:309–19.
Pereira JA, Holbrook AM, Dolovich L, et al. Are brand-name and generic warfarin interchangeable? Multiple N-of-1 randomized, crossover trials. Ann Pharmacother. 2005;39:1188–93.
Ahrens W, Hagemeier C, Muhlbauer B, et al. Hospitalization rates of generic metoprolol compared with the original beta-blocker in an epidemiological database study. Pharmacoepidemiol Drug Saf. 2007;16:1298–307.
Kim SH, Kim YD, Lim DS, et al. Results of a phase III, 8-week, multicenter, prospective, randomized, double-blind, parallel-group clinical trial to assess the effects of amlodipine camsylate versus amlodipine besylate in korean adults with mild to moderate hypertension. Clin Ther. 2007;29:1924–36.
Tran YBL, Frial T, Miller PSJ. Statin’s cost-effectiveness: a Canadian analysis of commonly prescribed generic and brand name statins. Can J Clin Pharmacol. 2007;14:e205–14.
Loebstein R, Katzir I, Vasterman-Landes J, et al. Database assessment of the effectiveness of brand versus generic rosiglitazone in patients with type 2 diabetes mellitus. Med Sci Monit. 2008;14:CR323–6.
Tsinamdzgvrishvili B, Trapaidze D, Loladze N, et al. Efficacy of adipin and normodipin (generic drugs of amlodipine) vs norvsc in treatment of essential hypertension. Georgian Med News. 2008;154:14–7.
Kim SH, Chung WY, Zo JH, et al. Efficacy and tolerability of two formulations of ramipril in Korean adults with mild to moderate essential hypertension: an 8-week, multicenter, prospective, randomized, open-label, parallel-group noninferiority trial. Clin Ther. 2009;31:988–98.
Jeong YH, Koh JS, Kang MK, et al. The impact of generic clopidogrel bisulfate on platelet inhibition in patients with coronary artery stents: results of the ACCEL-GENERIC study. Korean J Intern Med. 2010;25:154–61.
Kim SH, Park K, Hong SJ, et al. Efficacy and tolerability of a generic and a branded formulation of atorvastatin 20 mg/d in hypercholesterolemic Korean adults at high risk for cardiovascular disease: a multicenter, prospective, randomized, double-blind, double-dummy clinical trial. Clin Ther. 2010;32:1896–905.
Sicras Mainar A, Navarro Artieda R. Influence of substitution of brand name for generic drugs on therapeutic compliance in hypertension and dyslipidemia. Gaceta Sanitaria. 2010;24:473–82.
Boh M, Opolski G, Poredos P, et al. Therapeutic equivalence of the generic and the reference atorvastatin in patients with increased coronary risk. Int Angiol. 2011;30:366–74.
Ghate SR, Biskupiak JE, Ye X, et al. Hemorrhagic and thrombotic events associated with generic substitution of warfarin in patients with atrial fibrillation: a retrospective analysis. Ann Pharmacother. 2011;45:701–12.
Khosravi AR, Pourmoqhadas M, Ostovan M, et al. The impact of generic form of clopidogrel on cardiovascular events in patients with coronary artery stent: Results of the OPCES study. J Res Med Sci. 2011;16:640–50.
Tsadok MA, Jackevicius CA, Rahme E, et al. Amiodarone-induced thyroid dysfunction: brand-name versus generic formulations. CMAJ. 2011;183:E817–23.
Bobrova OP, Petrova MM. Comparison of pharmacokinetics and pharmacodynamics of the original and generic enalapril in the elderly patients with arterial hypertension. Ration Pharmacother Cardiol. 2012;8:149–53.
Fukuhara C, Kaneshige C, Akiyama K, et al. Difference in efficacy between a brand-name product (Adalat CR) and a generic product (nifedipine CR “sawai”) in hypertensive patients on hemodialysis. Jpn J Clin Pharmacol Ther. 2012;43:387–92.
Grigor’eva NI. Assessment of therapeutic equivalence of original bisoprolol and its generics in patients with ischemic heart disease with concomitant chronic obstructive pulmonary disease. Kardiologiia. 2012;52:10–4.
Kwong WJ, Kamat S, Fang C. Resource use and cost implications of switching among warfarin formulations in atrial fibrillation patients. Ann Pharmacother. 2012;46:1609–16.
Martsevich SY, Kutishenko NP, Ginzburg ML, et al. The KARDIOKANON study: a way to settle the subject of clinical equivalence of generic and original drugs. Ration Pharmacother Cardiol. 2012;8:179–84.
Oberhansli M, Lehner C, Puricel S, et al. A randomized comparison of platelet reactivity in patients after treatment with various commercial clopidogrel preparations: the CLO-CLO trial. Arch Cardiovasc Dis. 2012;105:587–92.
Park YM, Ahn T, Lee K, et al. A comparison of two brands of clopidogrel in patients with drug-eluting stent implantation. Korean Circ J. 2012;42:458–63.
Solangi NA, Ahmed SP, Soomro K. Cholesterol, triglycerides and LDL lowering effects of generic products of simvastatin and HDL effect as compared to original brand of simvastatin in hypercholesterolemic subjects—A randomized study. Med Channel. 2012;18:41–4.
Srimahachota S, Rojnuckarin P, Udayachalerm W, et al. Comparison of original and generic clopidogrel 600 mg loading dose in the patients who planned undergoing coronary angiography. J Med Assoc Thai. 2012;95:1495–500.
Tsoumani ME, Kalantzi KI, Dimitriou AA, et al. Antiplatelet efficacy of long-term treatment with clopidogrel besylate in patients with a history of acute coronary syndrome: comparison with clopidogrel hydrogen sulfate. Angiology. 2012;63:547–51.
Colombo GL, Agabiti-Rosei E, Margonato A, et al. Off-patent generic medicines vs off-patent brand medicines for six reference drugs: a retrospective claims data study from five local healthcare units in the Lombardy Region of Italy. PloS one. 2013;8:e82990.
Haas AV, Martin-Doyle W, Vellanki A, et al. Statin switching: Trends in LDL-C and predictors of ATP-III goal attainment. Circulation. 2013;128:A14759.
Huang JH, Cheng HS, Chung CC, et al. Comparisons of clinical efficacy and safety between the brand- and generic-name fenofibrate in patients with hypertriglyceridemia. Exp Clin Med (Taiwan). 2013;5:136–8.
Kalo Z, Abonyi-Toth Z, Rokszin G, et al. Impact of switching on health care costs and outcomes in generic drug policies. Value Health. 2013;16:A537.
Malyhina AI, Zhuravleva MV, Starodubtsev AK, et al. The problem of medicines interchangeability Focus on perindopril. Ration Pharmacother Cardiol. 2013;9:505–10.
Martsevich SY, Tolpygina SN, Zakharova AV, et al. A comparative study of efficacy and tolerability of generic and original low-dose bisoprolol/hydrochlorothiazide combination in patients with arterial hypertension of 1-2 degrees. Results of clinical randomized crossover study. Ration Pharmacother Cardiol. 2013;9:511–8.
Szczotka B, Jazwinska-Tarnawska E, Wedlarski R, et al. Evaluation of efficacy and safety of hypertension treatment with original angiotensin-converting enzyme inhibitors. The comparison of original and generic formulations. Polski Merkuriusz Lekarski. 2013;34:140–4.
Tsivgoulis G, Christoforidou A, Tsakaldimi S, et al. Monitoring of clopidogrel-related inhibition in patients presenting with acute cerebral ischemia following generic substitution of clopidogrel for cardiovascular prevention. Stroke. 2013;44:ATP413.
Balandina Y, Tarlovskaya Y, Maksimchuk-Kolobova N. Comparison of “simvastatin” medications according to hypolipidemic effects and from pharmacoeconomic point of view. Atherosclerosis. 2014;235:e260.
Corrao G, Soranna D, Arfe A, et al. Are generic and brand-name statins clinically equivalent? Evidence from a real data-base. Eur J Intern Med. 2014;25:745–50.
Corrao G, Soranna D, Merlino L, et al. Similarity between generic and brand-name antihypertensive drugs for primary prevention of cardiovascular disease: evidence from a large population-based study. Eur J Clin Invest. 2014;44:933–9.
Gagne JJ, Choudhry NK, Kesselheim AS, et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med. 2014;161:400–7.
Kovacic JC, Mehran R, Sweeny J, et al. Clustering of acute and subacute stent thrombosis related to the introduction of generic clopidogrel. J Cardiovasc Pharmacol Ther. 2014;19:201–8.
Maskon O, Parasi NS, Hassan CHH, et al. Head to head comparison between original and generic clopidogrel using multiple electrodes platelet aggregometry in stable patients with indication for therapy with P2Y12 inhibitor. J Am Coll Cardiol. 2014;63:A230.
Seo KW, Tahk SJ, Yang HM, et al. Point-of-care measurements of platelet inhibition after clopidogrel loading in patients with acute coronary syndrome: comparison of generic and branded clopidogrel bisulfate. Clin Ther. 2014;36:1588–94.
Syvolap VV, Franskavichene LV, Golukhova EZ, et al. Switching from generic to brand clopidogrel in male patients after ST-elevated myocardial infarction. Cardiology (Switzerland). 2014;129:103–5.
Vichairuangthum K, Chotenoparatpat P. Comparison of original and generic enoxaparin for treatment of coronary artery disease patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2014;63:S41–2.
Hamilos M, Saloustros I, Skalidis E, et al. Comparison of the antiplatelet effect of clopidogrel hydrogenosulfate and clopidogrel besylate in patients with stable coronary artery disease. J Thromb Thrombolysis. 2015;40:288–93.
Komosa A, Siller-Matula JM, Kowal J, et al. Comparison of the antiplatelet effect of two clopidogrel bisulfate formulations: plavix and generic-Egitromb. Platelets. 2015;26:43–7.
Choo DW, Wu FL, Wang J, et al. Comparative effectiveness of brand-name and generic warfarin on stroke and bleeding events in atrial fibrillation patients: a 6-year population-based retrospective cohort study in Taiwan. Value Health. 2016;19:A639.
Hellfritzsch M, Rathe J, Stage TB, et al. Generic switching of warfarin and risk of excessive anticoagulation: a Danish nationwide cohort study. Pharmacoepidemiol Drug Saf. 2016;25:336–43.
Malinova L, Furman N, Dolotovskaya P, et al. Switch to potent P2Y12 inhibitor in ST elevation myocardial infarction: role of platelet reactivity testing. Eur Heart J Acute Cardiovasc Care. 2016;5:352–3.
Ntalas IV, Kalantzi KI, Tsoumani ME, et al. Salts of clopidogrel: investigation to ensure clinical equivalence: a 12-month randomized clinical trial. J Cardiovasc Pharmacol Ther. 2016;21:516–25.
Tarlovskaya EI, Chudinovskih TI. Comparative prospective clinical economic study of original and generic bisoprolol in patients with coronary heart disease. Kardiologiia. 2016;56:12–7.
Hajizadeh R, Ghaffari S, Ziaee M, et al. In vitro inhibition of platelets aggregation with generic form of clopidogrel versus branded in patients with stable angina pectoris. J Cardiovasc Thorac Res. 2017;9:191–5.
Lee JH, Kim SH, Choi DJ, et al. Efficacy and tolerability of two different formulations of atorvastatin in Korean patients with hypercholesterolemia: a multicenter, prospective, randomized clinical trial. Drug Design Dev Ther. 2017;11:2277–84.
Loch A, Bewersdorf JP, Kofink D, et al. Generic atorvastatin is as effective as the brand-name drug (LIPITOR) in lowering cholesterol levels: a cross-sectional retrospective cohort study. BMC Res Notes. 2017;10:291.
Leclerc J, Blais C, Rochette L, et al. Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec, Canada: a population-based time series analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003891.
Pollak P, Herman RET, Feldman R. Therapeutic differences in 24-h ambulatory blood pressure in patients switched between bioequivalent nifedipine osmotic systems with differing delivery technologies. Clin Trans Sci. 2017;10:217–24.
Chanchai R, Kanjanavanit R, Leemasawat K, et al. Clinical tolerability of generic versus brand beta blockers in heart failure with reduced left ventricular ejection fraction: a retrospective cohort from heart failure clinic. J Drug Assess. 2018;7:8–13.
Desai RJ, Gopalakrishnan C, Dejene S, et al. Comparative outcomes of treatment initiation with brand-name versus generic warfarin: a medicare cohort study. Pharmacoepidemiol Drug Saf. 2018;27:403–4.
Dinic M, Maillard N, Bouiller M, et al. Generic vs brand-name drugs for the treatment of hypertension. J Hypertens. 2018;36:e123.
Gengo F, Westphal E, Aladeen T, et al. Generic clopidogrel: has substitution for brand name plavix been safe and effective? Neurology. 2018;90:P5.239.
Povetkin SV, Luneva JV. Study of clinical efficacy of original and generic drugs of ivabradine in patients with stable angina (comparative study). Ration Pharmacother Cardiol. 2018;14:34–9.
Acknowledgements
The authors would like to thank the librarians of the Institut national de santé publique du Québec for their help in developing the search strategy. CB has received a scholarship from the CHU de Quebec and the Fonds de recherche en santé du Québec. JMG has received a scholarship from the PAHO/WHO Collaborating Centre for Nursing Research Development and University of Sao Paolo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The principal investigator (JL) has received academic research grants from the Ministère de l’Économie et de l’Innovation du Québec (Canada), the Canadian Heart Failure Society, the Quebec Heart Failure Society and the Université du Quebec à Trois-Rivières to conduct this study.
Conflict of interest
JS is a professor of medicine at McGill University and serves as the Chief Scientific Officer at JSS Medical Research, a contract research organisation that executes clinical trials/studies for pharmaceutical and biotechnology companies, as well as universities and hospitals. JL is a professor of nursing at Université du Québec à Trois-Rivières. Within her role of professor, she provides (1) Continuous Medical Education sessions for health care professionals, accredited by the Fédération des médecins omnipraticiens du Québec and its local affiliates and (2) statistical expertise on Data Safety Monitoring Board Committees managed by JSS Medical Research. Other authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leclerc, J., Thibault, M., Midiani Gonella, J. et al. Are Generic Drugs Used in Cardiology as Effective and Safe as their Brand-name Counterparts? A Systematic Review and Meta-analysis. Drugs 80, 697–710 (2020). https://doi.org/10.1007/s40265-020-01296-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01296-x