Abstract
Atezolizumab (Tecentriq®), an immune checkpoint inhibitor against programmed death ligand 1 (PD-L1), is the first immunotherapy agent to be approved (for use in combination with nab-paclitaxel) in the USA, the EU (as first-line) and Japan for the treatment of advanced triple-negative breast cancer (TNBC). Approval was based on the results of the phase III IMpassion130 trial in patients with unresectable locally advanced or metastatic TNBC, in which atezolizumab plus nab-paclitaxel significantly prolonged progression-free survival (PFS) when compared to placebo plus nab-paclitaxel in the intent-to-treat (ITT) population and the PD-L1+ subgroup. Statistically significant overall survival (OS) benefits were not seen in two interim analyses and final OS data are awaited. The tolerability and safety profile of atezolizumab plus nab-paclitaxel was consistent with those of each individual drug. The most common treatment-related adverse events included neutropenia, peripheral neuropathy and reduced neutrophil count. Adverse events of special interest occurred with higher frequency in patients who received atezolizumab plus nab-paclitaxel than placebo plus nab-paclitaxel, and were mostly immune-related (e.g. immune-related rash, hypothyroidism and hepatitis). Health-related quality of life was not significantly impacted by the addition of atezolizumab to nab-paclitaxel therapy. Thus, atezolizumab plus nab-paclitaxel is a useful immunochemotherapy option for patients with unresectable locally advanced or metastatic TNBC, including those whose tumours have PD-L1 expression ≥ 1%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A first-in-class anti-PD-L1 monoclonal antibody |
Significantly prolongs PFS in the ITT and PD-L1+ populations when combined with nab-paclitaxel |
Manageable and expected tolerability profile |
Treat immune-related adverse events by withholding/discontinuing therapy or with concomitant corticosteroids |
1 Introduction
Breast cancer currently has the second highest incidence and the fifth highest mortality rate of all cancers worldwide (11.6% of new diagnoses and 627,000 deaths in 2018) [1]. Triple-negative breast cancer (TNBC) is a rare, aggressive subtype that accounts for ≈ 20% of breast cancer diagnoses and which typically has poorer prognosis and lower survival rates than other subtypes (median overall survival is < 1 year) [2,3,4]. This cancer has no oestrogen or progesterone receptor expression and no overexpression of human epidermal growth factor receptor 2 [3, 5]. Due to the heterogeneity of the disease and absence of receptor expression, the benefits of targeted therapies are limited and chemotherapy is routinely used to treat advanced TNBC [6]. Preferred regimens include anthracyclines (e.g. doxorubicin), taxanes (e.g. paclitaxel), other microtubule inhibitors (e.g. eribulin), anti-metabolites (e.g. capecitabine) and platinum agents (e.g. carboplatin) [7,8,9,10]. Effective treatments for advanced TNBC are considered a significant unmet need [4, 11].
Immunotherapy is a promising new direction for the management of TNBC, with immune checkpoint blockade demonstrating strong anti-tumour activity and prolonged patient survival [4, 12]. Compared with other breast cancer subtypes, TNBC is more likely to harbour tumour-infiltrating lymphocytes (TILs) and to express programmed death protein ligand 1 (PD-L1), mainly on TILs rather than on tumour cells [5, 13]. PD-L1 binds to programmed death protein 1 (PD-1) and B7-1 receptors in T-cells and antigen presenting cells to inhibit anti-tumour immune responses. Immune checkpoint inhibitors against PD-1/PD-L1 prevent this interaction, resulting in upregulated T-cell activity and a more robust immune reaction [12, 14]. While response to immunotherapy is usually low, current evidence suggests the addition of a cytotoxic agent may synergistically enhance the overall efficacy of the combination regimen [15].
Atezolizumab (Tecentriq®) in combination with nanoparticle albumin-bound paclitaxel (Abraxane®; hereafter referred to as nab-paclitaxel) is approved in several countries, including in the USA, EU and Japan for the treatment of unresectable locally advanced or metastatic TNBC with PD-L1 expression [16,17,18]. This article briefly summarizes the pharmacological properties of atezolizumab (Table 1) and discusses the efficacy and tolerability profiles of atezolizumab plus nab-paclitaxel in this disease setting.
2 Therapeutic Efficacy of Atezolizumab Plus Nab-Paclitaxel
The efficacy of atezolizumab in combination with nab-paclitaxel was demonstrated in the international, randomized, double-blind, active-comparator (nab-paclitaxel) controlled phase III IMpassion130 trial [5]. Patients over 18 years of age were eligible if they had metastatic or unresectable locally advanced, histologically documented TNBC. Eligible patients must have had a representative tumour specimen to evaluate PD-L1 expression status and no previous chemotherapy or targeted therapy for metastatic triple-negative disease. Previous curative therapy (radiation and chemotherapy, including taxanes) was permitted if completed ≥ 12 months before randomization. Having measurable disease (as defined by RECIST v1.1), an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–1 and adequate haematological and organ function were also prerequisites for study inclusion [5]. Main exclusion criteria included: untreated central nervous system disease, a history of autoimmune disease, brain metastases (either untreated or treated with corticosteroids), a live attenuated vaccine administered ≤ 4 weeks prior to randomization, previous use of immune checkpoint-targeting therapies, recent use of a systemic immune-stimulatory agent (≤ 4 weeks or ≤ 5 half-lives of the drug, whichever was first) or systemic immunosuppressive medication (≤ 2 weeks prior to randomization) or current glucocorticoid use [5, 19].
Patients were randomized to receive atezolizumab plus nab-paclitaxel or placebo plus nab-paclitaxel [5]. Randomization was stratified by the presence or absence of liver metastases, previous treatment with a taxane and PD-L1 expression status in tumour-infiltrating immune cells [< 1% (PD-L1 negative) or ≥ 1% (PD-L1 positive) of tumour area]. Atezolizumab at 840 mg on days 1 and 15 and nab-paclitaxel at 100 mg/m2 of body surface area on days 1, 8 and 15 of each 28-day cycle were administered intravenously until disease progression or unacceptable toxicity. In the absence of disease progression, atezolizumab and nab-paclitaxel were able to be discontinued independently. No atezolizumab dose reductions were permitted; however, nab-paclitaxel dosage modifications were acceptable for managing adverse effects. Nab-paclitaxel was to be continued for ≥ 6 cycles only in the absence of toxic effects [5].
The co-primary efficacy endpoints were investigator-assessed progression-free survival (PFS) and overall survival (OS) in the whole intent-to-treat (ITT) population and the subgroup of patients who were categorized as expressing ≥ 1% PD-L1 (henceforth the PD-L1+ population) [Table 2] [5]. OS was assessed in two interim analyses and a final analysis. A key secondary endpoint was objective response rate (ORR), assessed by the investigator using RECIST v1.1. A stepwise testing procedure was used to control type 1 error in assessing these endpoints. If significant between-group differences in PFS were seen in either the ITT or PD-L1+, or both, populations, then ORR was compared in either or both corresponding populations. The first interim OS analysis in the ITT population was conducted at the time of the primary (i.e. final) PFS analysis, irrespective of the results of the PFS and ORR analyses; if a statistical between-group difference was seen in this analysis, then the interim OS analysis was conducted in the PD-L1+ population [5].
Baseline demographic and disease characteristics were generally well balanced in the ITT population (n = 902); the median age was 55 years for the atezolizumab plus nab-paclitaxel group and 56 years for the placebo plus nab-paclitaxel group, and the majority of patients were white (67.5%) or Asian (17.8%) [5, 16]. Liver metastases were seen in 27.9% of atezolizumab plus nab-paclitaxel recipients and 26.2% of placebo plus nab-paclitaxel recipients. At least 63% of patients in either group had previously received adjuvant or neoadjuvant therapy, and over 51% had received treatment with a taxane or an anthracycline. The baseline characteristics of the PD-L1+ population were consistent with the whole ITT population [5].
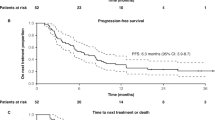
At the time of final PFS analysis (median follow-up 12.9 months; data cut-off date 7 April 2018), 79.4% of patients in the atezolizumab plus nab-paclitaxel group and 83.8% of patients in the placebo plus nab-paclitaxel group in the ITT population had disease progression or died; 7.1% and 5.3% of patients from the respective groups received palliative radiation therapy [5]. The median PFS in the atezolizumab plus nab-paclitaxel group was significantly longer (by 1.7 months) than in the placebo plus nab-paclitaxel group in the ITT population (Table 2), with a 20% lower risk of disease progression or death. The median PFS was significantly longer by 2.5 months with atezolizumab plus nab-paclitaxel than placebo plus nab-paclitaxel in the PD-L1+ population (Table 2), with a 38% lower risk of progression or death. In sensitivity analyses, investigator-assessed PFS results were confirmed by a central review [5].
In subgroup analyses of the ITT population, consistent PFS benefits were seen with atezolizumab plus nab-paclitaxel relative to placebo plus nab-paclitaxel in the majority of subgroups based on stratification factors and other baseline characteristics [5]. Hazard ratios (HRs) for median PFS in the ITT population favoured atezolizumab plus nab-paclitaxel recipients who were PD-L1 positive, ≥ 65 years old, white or Japanese, had not previously received neoadjuvant or adjuvant chemotherapy (but with previous taxane treatment), did not have liver metastasis, had an ECOG performance score of 0 or metastatic disease with 0–3 sites. Similarly, in the PD-L1+ population, PFS benefits were seen with atezolizumab plus nab-paclitaxel in the > 40 years old, black and without prior treatments for advanced disease subgroups. Benefits were seen irrespective of ECOG performance status, baseline disease status, number of metastatic sites, presence of bone metastases, or whether the patient had lymph node-only disease [5].
Statistical significance was not reached for median OS in the first interim OS analysis, when atezolizumab plus nab-paclitaxel recipients were compared with placebo plus nab-paclitaxel recipients in the ITT population (Table 2) [5]. The second interim analysis in the ITT population yielded similar results, with a median OS of 21.0 months in the atezolizumab plus nab-paclitaxel group and 18.7 months in the placebo plus nab-paclitaxel group (HR 0.86; 95% CI 0.73–1.02) [20]. In the PD-L1+ population, median OS in the respective groups was 25.0 and 18.0 months (HR 0.71; 95% CI 0.54–0.94); however this data was not formally tested, as statistical significance was not reached for OS in the ITT population at first interim OS analysis [5].
In a post-hoc subgroup analysis (not pre-specified or properly powered) of Japanese patients (n = 65), the efficacy profile for atezolizumab plus nab-paclitaxel was consistent with that in the overall population [21]. Median PFS was 7.4 months in the atezolizumab plus nab-paclitaxel group and 4.6 months in the placebo plus nab-paclitaxel group (HR 0.47; 95% CI 0.25–0.90). In PD-L1 + Japanese patients (n = 65), median PFS was 10.8 and 3.8 months, respectively (HR 0.04; 95% CI < 0.01–0.35) [21]. Similarly, a post-hoc analysis of all Asian patients (n = 145) reported an efficacy profile consistent with that seen in the ITT and PD-L1 + populations in the IMpassion130 trial (abstract [22]).
The addition of atezolizumab to nab-paclitaxel did not appear to impact health-related quality of life (HRQOL), as assessed by the European Organisation for Research and Treatment of Cancer Quality-of-life Questionnaire Core 30 (QLQ-C30) and the breast cancer module (QLQ-BR23) (abstract [23]). HRQOL scores were similar in the atezolizumab plus nab-paclitaxel and placebo plus nab-paclitaxel groups at baseline and throughout the treatment period in the ITT and PD-L1 + populations. There were no significant between-group differences in median time to deterioration (first ≥ 10-point decrease from baseline that was sustained for 2 cycles) of HRQOL, physical and role functioning [23].
3 Tolerability of Atezolizumab Plus Nab-Paclitaxel
Atezolizumab plus nab-paclitaxel had manageable safety and tolerability profiles consistent with that known for the individual agents when used in patients with locally advanced or metastatic TNBC in the IMpassion130 trial, with no new safety signals identified [5, 16]. The median duration of treatment in the atezolizumab plus nab-paclitaxel group was 24.1 and 22.1 weeks, respectively, for the two drugs [5]. Nab-paclitaxel was given for a median duration of 21.8 weeks in the placebo plus nab-paclitaxel group (versus 22.1 weeks for placebo). Analyses were conducted in the safety population (defined as patients who received ≥ 1 dose of study drug: 452 atezolizumab plus nab-paclitaxel recipients and 438 placebo plus nab-paclitaxel recipients) [5].
Treatment-emergent adverse events (AEs) of any grade occurred in 99.3% of atezolizumab plus nab-paclitaxel recipients and 97.9% of placebo plus nab-paclitaxel recipients; grade 3–4 treatment-emergent AEs occurred in 48.7% and 42.2% of patients, respectively [5]. Most reported AEs were considered treatment-related: treatment-related AEs of any grade occurred in 96.5% of atezolizumab plus nab-paclitaxel recipients and 93.6% of placebo plus nab-paclitaxel recipients, and grade 3–4 treatment-related AEs occurred in 39.6% and 30.1% of patients, respectively. The most common (incidence > 1%) any-grade treatment-related AEs in both groups were alopecia, nausea, fatigue, anaemia and diarrhoea. The most common grade 3–4 treatment-related AEs (incidence ≥ 2% in the atezolizumab plus nab-paclitaxel group) were neutropenia, reduced neutrophil count, peripheral neuropathy, peripheral sensory neuropathy and fatigue (Fig. 1), most of which were generally manageable with treatment disruptions or discontinuations [5].
Most common (incidence > 1%) grade 3–4 treatment-related adverse events in patients with unresectable locally advanced or metastatic triple-negative breast cancer in the IMpassion130 trial [5]. AnP atezolizumab plus nab-paclitaxel, PnP placebo plus nab-paclitaxel, pts patients
Serious AEs occurred in 22.8% of atezolizumab plus nab-paclitaxel recipients and 18.3% of placebo plus nab-paclitaxel recipients; approximately half of these were considered treatment-related (12.4% and 7.3%) [5]. Fatal AEs were reported in six atezolizumab plus nab-paclitaxel recipients (autoimmune hepatitis, pneumonia, septic shock, aspiration, pulmonary embolism, mucosal inflammation and death) and three placebo plus nab-paclitaxel recipients (acute myocardial infarction, hepatic failure and death not otherwise specified). Of these, three deaths (autoimmune hepatitis, mucosal inflammation and septic shock) and one death (hepatic failure) from the respective groups were considered treatment-related [5].
The incidence of AEs leading to dose interruption of atezolizumab or placebo was numerically higher in the atezolizumab plus nab-paclitaxel group (30.8%) than the placebo plus nab-paclitaxel group (23.5%); the incidence of dose interruption or reduction of nab-paclitaxel due to an AE was 43.1% and 39.3% in the respective groups [5]. In the respective treatment groups, AEs led to discontinuation of atezolizumab or placebo in 6.4% and 1.4% of patients, and nab-paclitaxel in 15.9% and 8.2% of patients [5].
Adverse events of special interest (AESIs) of any grade occurred in 57.3% and 41.8% of patients in the atezolizumab plus nab-paclitaxel and placebo plus nab-paclitaxel groups, respectively [5]. Treatment-emergent AESIs were mostly immune-related, suggesting an immune relation to atezolizumab toxicity. The most common (incidence ≥ 3%) AESIs of any grade in the respective groups were immune-related rash (34.1% vs 26%), hypothyroidism (17.3% vs 4.3%) and hepatitis (15.3% vs 14.2%). A small number of AESIs were grade 3–4 (7.5% and 4.3% of patients in each group), with the highest proportion reported as immune-related hepatitis in 5.1% and 3% of patients, respectively. There were two fatal AESIs: autoimmune hepatitis in the atezolizumab plus nab-paclitaxel group and hepatic failure in the placebo plus nab-paclitaxel group [5]. Most grade 2–3 AESIs can be managed by withholding treatment until symptoms improve, while grade 3–4 AESIs usually require treatment with corticosteroids and permanent discontinuation of atezolizumab plus nab-paclitaxel therapy [16, 17].
4 Dosage and Administration of Atezolizumab Plus Nab-Paclitaxel
Atezolizumab in combination with nab-paclitaxel is approved in the USA [16], the EU [17] and Japan [18] for the treatment of adult patients with unresectable locally advanced or metastatic TNBC whose tumours express PD-L1 (PD-L1 stained tumour-infiltrating immune cells of any intensity covering ≥ 1% of the tumour area), as determined by a validated test (must be approved by the Food and Drug Administration in the USA [16]). In the EU, patients must not have received prior chemotherapy for metastatic disease [17]. Approval in the USA was accelerated using PFS data, and continued approval may be contingent upon verification and description of clinical benefit in further confirmatory trial(s) [16]. The recommended dose of atezolizumab is 840 mg as an intravenous infusion over 60 min on days 1 and 15 of a 28-day cycle, followed by 100 mg/m2 (based on body surface area) nab-paclitaxel on days 1, 8 and 15 [16, 17]. Treatment is to be continued until disease progression or unacceptable toxicity, and either drug can be discontinued for toxicity independently of each other [16].
Consult local prescribing information for detailed information regarding warnings, precautions, dosage modifications, AESIs (Sect. 3) and use in special populations.
5 Place of Atezolizumab Plus Nab-Paclitaxel in the Management of Advanced TNBC
Atezolizumab (in combination with nab-paclitaxel) is the first immunotherapy agent (an immune checkpoint inhibitor) to be approved in several countries worldwide to treat adult patients with unresectable locally advanced or metastatic TNBC that expresses PD-L1 (Sect. 4). Approval was based on results from the phase III IMpassion130 trial, in which median PFS was significantly improved by atezolizumab plus nab-paclitaxel when compared to placebo plus nab-paclitaxel in the ITT and PD-L1+ populations (Sect. 2). PFS benefits were particularly notable in PD-L1+ patients; hence, PD-L1 expression status can be seen as a strong predictor of clinical benefit, supporting its use as a predictive biomarker for patients with advanced TNBC [11, 24]. The addition of atezolizumab to nab-paclitaxel was also associated with improvements in OS (interim analysis) and ORR, although statistical significance was not reached (Sect. 2).
Atezolizumab plus nab-paclitaxel had a manageable tolerability profile in patients with unresectable locally advanced or metastatic TNBC, and no new safety signals were identified in the IMpassion130 trial (Sect. 3). Treatment-related grade 3–4 AEs were most commonly neutropenia, reduced neutrophil count, peripheral neuropathies and fatigue, and were generally manageable with treatment disruptions or discontinuations (Sect. 3). Immune checkpoint inhibitors, including atezolizumab, are associated with a higher risk of immune-related AEs [25]. In IMpassion130, the majority of AESIs were immune-related and were similar to AESIs reported from clinical trials of atezolizumab in other approved indications [16, 17]. These AESIs are typical of, and unique to, checkpoint inhibitors [25].
There is increasing evidence that heavy antibiotic usage in cancer patients (either prophylactically or in response to infections) can alter gut microbiomes and negatively affect therapeutic response [25, 25]. Research into the effects of concomitant antibiotics in patients with advanced TNBC receiving atezolizumab combination therapy would be of interest.
Taxanes (e.g. paclitaxel or docetaxel) are the most commonly used chemotherapy treatment for advanced TNBC [7, 8, 25]. Nab-paclitaxel demonstrates similar efficacy and better tolerability when compared to paclitaxel [19], and does not require glucocorticoid pre-medication (Table 1). Consequently, it is the preferred taxane in combination with other cancer therapies [7, 8]. Nonetheless, there is currently no standard of care regimen for TNBC, and there are no direct comparisons of atezolizumab plus nab-paclitaxel with therapies other than nab-paclitaxel. A network meta-analysis, subject to its limitations, estimated greater treatment effects (OS and PFS) for atezolizumab plus nab-paclitaxel than for paclitaxel or docetaxel, when each were compared with nab-paclitaxel alone [25]. While the addition of an immune checkpoint inhibitor to first-line chemotherapy can lead to meaningful clinical benefits, particularly in patients who express PD-L1, there is a need for confirmatory real-world efficacy and tolerability data.
The National Comprehensive Cancer Network (NCCN) guidelines recommend atezolizumab plus nab-paclitaxel as the preferred treatment regimen for patients with PD-L1+ TNBC [7]. The American Cancer Society (ACS) states patients with this condition may be treated first with atezolizumab plus nab-paclitaxel [10]. As the European Society for Medical Oncology (ESMO) and UK National Institute for Health and Care Excellence (NICE) guidelines were updated prior to approval of atezolizumab in this indication, these guidelines only recommend chemotherapy (sequential or combination) for advanced disease [8, 9].
A cost-effectiveness analysis using data from IMpassion130 showed atezolizumab plus nab-paclitaxel was not cost-effective over a lifetime in the USA and in China [25]. The quality-adjusted life years (QALYs) gained with atezolizumab plus nab-paclitaxel was higher than with nab-paclitaxel alone in the ITT (1.41 vs 0.99 QALYs) and PD-L1+ (1.66 vs 0.88 QALYs) populations. From a US payer’s perspective, the incremental cost-effectiveness ratios (ICERs) of atezolizumab plus nab-paclitaxel compared with nab-paclitaxel were US$ 331,996.89/QALY (ITT) and US$ 229,359.88/QALY (PD-L1+) gained. From a Chinese healthcare system perspective, the ICERs were US$ 106,339.26/QALY (ITT) and US$ 72,971.88/QALY (PD-L1+) gained. These values were over the willingness-to-pay thresholds of US$ 150,000/QALY in the USA and US$ 29,383/QALY in China [25].
In conclusion, atezolizumab plus nab-paclitaxel prolonged PFS, had a known and manageable tolerability profile and minimal impact on patient-reported HRQOL; this combination is therefore a useful immunochemotherapy option for patients with PD-L1+ unresectable locally advanced or metastatic TNBC.
Data Selection Atezolizumab: 115 records identified
Duplicates removed | 28 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 27 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 29 |
Cited efficacy/tolerability articles | 8 |
Cited articles not efficacy/tolerability | 23 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were atezolizumab, Tecentriq MPDL3280A, RG7446, RO5541267, triple-negative breast cancer. Records were limited to those in English language. Searches last updated 4 Mar 2020 | |
References
GLOBOCAN. New global cancer data: GLOBOCAN 2018. 2018. https://www.uicc.org. Accessed 16 Mar 2020.
Abramson VG, Lehmann BD, Ballinger TJ, et al. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121(1):8–16.
Li XX, Yang J, Peng LM, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161(2):279–87.
Khosravi-Shahi P, Cabezón-Gutiérrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol. 2017;14(1):32–9.
Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21.
Neophytou C, Boutsikos P, Papageorgis P. Molecular mechanisms and emerging therapeutic targets of triple-negative breast cancer metastasis. Front Oncol. 2018. https://doi.org/10.3389/fonc.2018.00031.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer v 3.2020. 2020. https://www.nccn.org. Accessed 16 Mar 2020.
European Society for Medical Oncology. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29(8):1634–57.
National Institute for Health and Care Excellence. NICE pathways: managing advanced breast cancer. 2019. https://pathways.nice.org.uk. Accessed 16 Mar 2020.
The American Cancer Society. Treatment of triple-negative breast cancer. 2019. https://www.cancer.org. Accessed 16 Mar 2020.
Cyprian FS, Akhtar S, Gatalica Z, et al. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: a new clinical paradigm in the treatment of triple-negative breast cancer. Bosn J Basic Med Sci. 2019;19(3):227–33.
Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–70.
Molinero L, Li YJ, Chang CW, et al. Tumor immune microenvironment and genomic evolution in a patient with metastatic triple negative breast cancer and a complete response to atezolizumab. J Immunother Cancer. 2019;7(274):1–9.
Stovgaard ES, Nielsen D, Hogdall E, et al. Triple negative breast cancer—prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018;57(1):74–82.
Li ZH, Qiu YR, Lu WQ, et al. Immunotherapeutic interventions of triple negative breast cancer. J Transl Med. 2018;16(1):147.
Genentech. TECENTRIQ® (atezolizumab) injection, for intravenous use: US prescribing information. 2019. https://www.fda.gov. Accessed 16 Mar 2020.
European Medicines Agency. Tecentriq 840 mg concentrate for solution for infusion: EU summary of product characteristics. 2019. https://www.ema.europa.eu. Accessed 16 Mar 2020.
Pharmaceuticals and Medical Devices Agency. Tecentriq IV 840 mg/Tecentriq IV 1200 mg: prescribing information. 2019. https://www.pmda.go.jp. Accessed 16 Mar 2020.
Celgene Corporation. ABRAXANE® (paclitaxel protein-bound particles for injectable suspension) for injectable suspension (albumin-bound): US prescribing information. 2019. https://www.fda.gov. Accessed 16 Mar 2020.
Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59.
Iwata H, Inoue K, Kaneko K, et al. Subgroup analysis of Japanese patients in a phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn J Clin Oncol. 2019;49(12):1083–91.
Iwata H, Im SA, Sohn J, et al. Subgroup analysis of IMpassion130: atezolizumab + nab-paclitaxel (nab-P) in patients (pts) with advanced triple-negative breast cancer (TNBC) in Asian countries [abstract no. 370]. Ann Oncol. 2019;30(Supp 9):ix13–4.
Adams S, Dieras V, Barrios CH, et al. Patient-reported outcomes (PROs) from the phase III IMpassion130 trial of atezolizumab (atezo) plus nabpaclitaxel (nP) in metastatic triple-negative breast cancer (mTNBC) [abstract]. J Clin Oncol. 2019;37(Suppl 15):1067.
Emens LA, Loi S, Rugo HS, et al. IMpassion130: efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab + nab-paclitaxel in patients with treatment-naive, locally advanced or metastatic triple-negative breast cancer [abstract no. GS1-04 and presentation]. Cancer Res. 2018;79(Suppl 4):GS1-04-GS1-04.
Myers G. Immune-related adverse events of immune checkpoint inhibitors: a brief review. Curr Oncol. 2018;25(5):342–7.
Kuczma MP, Ding ZC, Li T, et al. The impact of antibiotic usage on the efficacy of chemoimmunotherapy is contingent on the source of tumorreactive T cells. Oncotarget. 2017;8(67):111931–42.
Gopalakrishnan V, Helmink BA, Spencer CN, et al. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570–80.
National Institute for Health and Care Excellence. Atezolizumab with nab-paclitaxel for treating PD-L1-positive, triple-negative, advanced breast cancer: committee discussion. 2019. https://www.nice.org.uk. Accessed 16 Mar 2020.
National Institute for Health and Care Excellence. Single technology appraisal: atezolizumab with nab-paclitaxel for treating PD L1-positive, triple-negative, advanced breast cancer [ID1522] committee papers. 2019. https://www.nice.org.uk. Accessed 16 Mar 2020.
Weng XH, Huang XT, Li HC, et al. First-line treatment with atezolizumab plus nab-paclitaxel for advanced triple-negative breast cancer: a cost-effectiveness analysis. Am J Clin Oncol. 2020. https://doi.org/10.1097/COC.0000000000000671. Accessed 16 Mar 2020.
Adams S, Diamond JR, Hamilton E, et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol. 2019;5(3):334–42.
Acknowledgements
During the peer review process, the manufacturer of atezolizumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Connie Kang and Yahiya Y. Syed are salaried employees of Adis International Ltd/Springer Nature, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
Additional information
for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.11987463.
The manuscript was reviewed by:K. Araki, Department of Medical Oncology, Gunma Prefectural Cancer Center, Ōta, Japan, A.M. Brufsky, University of Pittsburgh Medical Center Magee-Womens Hospital, Pittsburgh, PA, USA.
Rights and permissions
About this article
Cite this article
Kang, C., Syed, Y.Y. Atezolizumab (in Combination with Nab-Paclitaxel): A Review in Advanced Triple-Negative Breast Cancer. Drugs 80, 601–607 (2020). https://doi.org/10.1007/s40265-020-01295-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01295-y