Abstract
Tolvaptan [Jynarque® (USA); Jinarc® (EU, Canada); Samsca® (Japan)] is a highly selective vasopressin V2 receptor antagonist approved for the treatment of autosomal dominant polycystic kidney disease (ADPKD). In the phase III TEMPO 3:4 trial, 3 years’ treatment with tolvaptan slowed the increase in total kidney volume (TKV) and the decline in renal function relative to placebo. The composite secondary endpoint of time to investigator-assessed clinical progression also favoured tolvaptan over placebo. Although tolvaptan did not demonstrate a sustained disease-modifying effect on TKV over the longer term in the TEMPO 4:4 extension trial, the effect of tolvaptan in slowing renal function decline was maintained for a further 2 years. The phase III REPRISE trial confirmed the efficacy of tolvaptan in patients with later-stage ADPKD. Most of the adverse events commonly observed with tolvaptan (e.g. polyuria, nocturia, polydipsia, thirst) are consistent with its pharmacological activity. In the TEMPO trials, tolvaptan was also associated with idiosyncratic hepatotoxicity which was reversible on discontinuation of the drug. Although the use of tolvaptan requires careful consideration and balancing of benefits and risks, it provides a valuable treatment option to slow the progression of ADPKD in patients at risk of or with evidence of rapidly progressing disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highly selective vasopressin V2 receptor antagonist |
Slows increase in TKV and decline in renal function |
Most common adverse events are aquaretic in nature and related to its mechanism of action |
Associated with idiosyncratic elevated liver enzymes |
1 Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a chronic, progressive disease characterized by the development and growth of renal cysts [1]. ADPKD is associated with multiple renal and extra-renal manifestations, including pain, renal function abnormalities, cardiovascular and cerebrovascular complications, and the development of cysts in the liver and other organs [1, 2]. Most patients with ADPKD eventually develop end-stage renal disease (ESRD) and require renal replacement therapy (i.e. kidney transplantation or dialysis) [1]. ADPKD is the most common genetic kidney disease [2]. It is caused by a heterozygous mutation in one of two genes: PKD1, which accounts for 80–85% of cases, and PKD2. The condition is more severe in patients with PKD1 mutations than in those with PKD2 mutations [2].
Several signalling pathways have been implicated in the pathogenesis of ADPKD, including intracellular dysregulation of calcium, accumulation of cyclic adenosine monophosphate (cAMP), and activation of mitogen-activated protein and mammalian target of rapamycin kinases [3]. The antidiuretic hormone arginine vasopressin (AVP) stimulates cAMP production in the distal nephron and collecting ducts by binding to V2 receptors [4]. cAMP agonists, including AVP, play an important role in cyst growth by promoting cyst cell proliferation and cyst fluid secretion [4]. Antagonism of AVP at the V2 receptor results in free water excretion (aquaresis), reduced urine osmolality, suppression of AVP-induced cAMP production and decreased kidney cyst cell proliferation [4, 5].
Tolvaptan [Jynarque® (USA); Jinarc® (EU, Canada); Samsca® (Japan)] is a highly selective vasopressin V2 receptor antagonist approved for the treatment of ADPKD. The pharmacological properties of tolvaptan have been reviewed in detail [6] and are summarized in Table 1. This review focuses on the clinical use of tolvaptan in adults at risk of or with evidence of rapidly progressing ADPKD [7, 8]. Tolvaptan (Samsca®) is also approved in adults for the treatment of hyponatraemia secondary to the syndrome of inappropriate antidiuretic hormone secretion (SIADH); discussion of this indication is beyond the scope of this review.
2 Therapeutic Efficacy of Tolvaptan in ADPKD
The efficacy of oral tolvaptan for the treatment of ADPKD was investigated in the randomized, double-blind, placebo-controlled, multicentre, phase III TEMPO 3:4 trial [9] (Sect. 2.1) and its long-term extension, TEMPO 4:4 [10] (Sect. 2.1.2). The efficacy of tolvaptan in patients with later-stage ADPKD who had more advanced disease than those in TEMPO 3:4 was investigated in the randomized, double-blind, placebo-controlled, multicentre, phase III REPRISE trial [11] (Sect. 2.2.1) and in a prospective study in Japanese patients [12] (Sect. 2.2.2). In addition, the efficacy of tolvaptan in the real-world setting is discussed (Sect. 2.3).
2.1 TEMPO 3:4
TEMPO 3:4 included patients aged 18–50 years with ADPKD, rapidly progressive kidney growth [total kidney volume (TKV) ≥ 750 mL] by MRI, and an estimated creatinine clearance (CLCR) of ≥ 60 mL/min [9]. After stratification by presence or absence of hypertension, CLCR (< 80 vs. ≥ 80 mL/min), TKV (< 1000 vs. ≥ 1000 mL) and geographic area, patients were randomized to receive tolvaptan or placebo. The initial dosage of tolvaptan was split (45 mg in the morning and 15 mg in the afternoon), with weekly increases to 60 and 30 mg and then 90 and 30 mg, as tolerated. After the titration phase, patients continued to receive the highest tolerable dosage of tolvaptan for 36 months. The primary endpoint was the annual rate of percentage change in TKV [9].
Over 3 years, tolvaptan significantly slowed the increase in TKV compared with placebo [9]. The mean percentage change in TKV per year was significantly lower with tolvaptan than with placebo (Table 2); the ratio of the geometric means of growth rate was 0.97 (95% CI 0.97–0.98; p < 0.001). The analysis of the primary endpoint was confirmed by a mixed-model repeated-measures analysis in which the least squares mean change in TKV over 3 years was 9.6% with tolvaptan versus 18.8% with placebo (p < 0.001). The between-group difference (– 9.2%) represented a 49% treatment effect in the intent-to-treat population. Of note, the treatment effect was more pronounced during the first year of treatment than during the second or third years [9].
The beneficial effect of tolvaptan on TKV was seen in all prespecified stratification subgroups: sex, age (< 35 vs. ≥ 35 years), baseline TKV (< 1500 vs. ≥ 1500 mL), baseline estimated CLCR (< 80 vs. ≥ 80 mL/min) and presence or absence of hypertension [9]. In post hoc analyses of TEMPO 3:4, tolvaptan significantly (p < 0.01) reduced the rate of TKV growth relative to placebo across CKD stages 1 to 3 [13], in patients with more severe disease (i.e. image class 1C–E) [14] and in all albumin-to-creatinine ratio (ACR) subgroups (< 1.5, 1.5–2.99, 3.0–14.99 and ≥ 15.0 mg/mmol) [15], although the treatment effect was more pronounced in patients with higher baseline albuminuria (p < 0.05 for interaction) [15].
The composite secondary endpoint of time to investigator-assessed clinical progression favoured tolvaptan over placebo, with significantly fewer ADPKD events per 100 person-years (PY) of follow-up (Table 2) [9]. This was confirmed by the analysis of time to first event [hazard ratio (HR) 0.83; 95% CI 0.72–0.94; p = 0.005]. The outcome of the composite endpoint was largely driven by lower rates of worsening renal function and kidney pain with tolvaptan versus placebo; no treatment effects were observed for hypertension or albuminuria (Table 2) [9]. A post hoc exploratory analysis demonstrated that tolvaptan was associated with a significantly (p < 0.001) lower incidence of kidney pain events than placebo in all subgroups defined according to pain severity and independent of patient characteristics predisposing for kidney pain [16]. The effect of tolvaptan on kidney pain was partly due to a reduction in the incidence of renal complications (e.g. urinary tract infections, kidney stones and haematuria) [16].
Over 3 years, tolvaptan was associated with a significantly slower loss of renal function than placebo (as assessed by the change in the slope of renal function) (Table 2) [9]. The increase in mean serum creatinine was 0.16 mg/dL with tolvaptan and 0.23 mg/dL with placebo (p < 0.001). The annual change in the estimated glomerular filtration rate (eGFR) slope was – 2.72 mL/min/1.73 m2 per year in the tolvaptan group versus – 3.70 mL/min/1.73 m2 per year in the placebo group (p < 0.001) [9].
The beneficial effects of tolvaptan on renal function were seen in all prespecified stratification subgroups; however, these effects were nominally (p < 0.001) greater in patients with hypertension or a TKV of ≥ 1500 mL and in patients aged ≥ 35 years [9]. A post hoc analysis of the composite secondary endpoint demonstrated a significant (p < 0.05) effect in favour of tolvaptan in patients with stage 1 or 3 CKD, but not in patients with stage 2 CKD [13]. Analyses of eGFR decline demonstrated a significant (p < 0.001) tolvaptan treatment effect in patients with more advanced disease (CKD stage 2–3) [13], in patients with more severe disease (image class 1C–E) [14] and in all ACR subgroups [15]. In additional post hoc analyses, 3 years of treatment with tolvaptan significantly reduced albuminuria (p < 0.001) [15], BP (p value not stated) (abstract [17]) and urinary excretion of monocyte chemotactic protein-1 (p < 0.0001) [18] relative to placebo.
2.1.1 Patients with ADPKD in Japan
Tolvaptan slowed the increase in TKV relative to placebo in a subpopulation of patients with ADPKD in Japan (n = 177) from TEMPO 3:4 [19]. The mean percentage change in TKV per year was significantly (p < 0.001) smaller with tolvaptan than with placebo (+ 1.3 vs. + 5.0%). The between-group difference (– 3.7%) was indicative of a 75% treatment effect. The ratio of the geometric means of growth rate was 0.96 (95% CI 0.95–0.98; p < 0.001) [19].
Unlike the total TEMPO 3:4 study population, tolvaptan did not reduce the risk of ADPKD events in the subpopulation in Japan [19]. There were 41 events per 100 PY in the tolvaptan group and 52 events per 100 PY in the placebo group (HR 0.77; 95% CI 0.55–1.08). Although tolvaptan was associated with a significantly (p = 0.001) lower rate of worsening renal function than placebo (1 vs. 8 events per 100 PY; HR 0.17; 95% CI 0.06–0.49), no treatment effects were observed for kidney pain, hypertension or albuminuria. The change in the slope of renal function favoured tolvaptan over placebo. The estimated slope was – 4.8 (mg/mL)−1 per year in the tolvaptan group versus – 6.3 (mg/mL)−1 per year in the placebo group (p = 0.012), corresponding to a 23% improvement in the rate of renal function decline (as measured by the reciprocal of serum creatinine level) [19].
2.1.2 Longer-Term Efficacy (TEMPO 4:4)
Tolvaptan did not demonstrate a sustained disease-modifying effect on TKV over the longer term, but showed a sustained beneficial effect on eGFR in TEMPO 4:4, a 2-year, open-label extension of TEMPO 3:4 [10]. TEMPO 4:4 enrolled 871 patients who had successfully completed TEMPO 3:4 during the previous 6 months. They had an eGFR of ≥ 30 mL/min/1.73 m2 within 45 days prior to the baseline visit. All patients received tolvaptan as a 45, 60 or 90 mg dose in the morning and a 15 or 30 mg dose ≈ 9 h later [10].
The change in TKV from TEMPO 3:4 baseline to TEMPO 4:4 month 24 (primary endpoint) was 29.9% in ‘early-treated’ patients (i.e. those who received tolvaptan in TEMPO 3:4) and 31.6% in ‘delayed-treated’ patients (i.e. those who received placebo in TEMPO 3:4); the between-group difference was not significant [10]. The slope of TKV growth during TEMPO 4:4 was 6.16% per year with early treatment and 4.96% per year with delayed treatment (p = 0.05) and did not meet the criteria for non-inferiority. After adjusting for imbalanced baseline characteristics and other confounding covariates, the TKV treatment difference at the end of TEMPO 4:4 increased from – 1.70 to – 4.15% in favour of early treatment (p = 0.04). Post hoc analyses in patients with more severe (i.e. image class 1C–E or with truncating PKD1 mutations) or more advanced (i.e. CKD stage 2–3) disease showed a lower TKV increase with early treatment [10].
With regard to the change in eGFR from TEMPO 3:4 baseline to TEMPO 4:4 month 24 (key secondary endpoint), the nominally significant (p < 0.001) difference between early and delayed treatment was maintained at each timepoint, reaching 3.15 mL/min/1.73 m2 at the end of TEMPO 4:4 [10]. The slope of eGFR during TEMPO 4:4 was – 3.26% per year with early treatment and – 3.14% per year with delayed treatment (treatment difference – 0.11 mL/min/1.73 m2; 95% CI –0.75, 0.52); this endpoint met the protocol predefined criterion for non-inferiority (upper limit of 95% CI less two-thirds of the eGFR slope difference in TEMPO 3:4). Post hoc analyses demonstrated that the treatment effect of tolvaptan on eGFR observed at the end of TEMPO 3:4 was maintained during TEMPO 4:4 in patients with more severe or more advanced disease [10].
2.2 In Patients with ADPKD With Later-Stage Disease
2.2.1 REPRISE
Tolvaptan was associated with a slower eGFR decline than placebo in patients with later-stage ADPKD, according to the results of the REPRISE trial [11]. The trial included patients aged 18–55 years with a baseline eGFR of 25–65 mL/min/1.73 m2 and patients aged 56–65 years with an eGFR of 25–44 mL/min/1.73 m2 plus eGFR decline of > 2.0 mL/min/1.73 m2 per year. Following an 8-week pre-randomization period that included sequential single-blind placebo and tolvaptan run-in phases (during which each patient’s ability to take tolvaptan without an acceptable level of side effects was assessed), patients who were able to tolerate tolvaptan 60 or 90 mg in the morning and 30 mg in the afternoon were randomized to receive tolvaptan (n = 683) or placebo (n = 687) for 12 months. Randomization was stratified by age (≤ 55 vs. > 55 years), baseline eGFR (≤ 45 vs. > 45 mL/min/1.73 m2) and TKV (≤ 2000 vs. > 2000 mL vs. unknown). At baseline, most patients had stage 3a (30%), 3b (45%) or 4 (19%) CKD. The primary endpoint was the change in eGFR from baseline to follow-up, with adjustment for the exact duration each patient was in the trial (interpolated to 1 year) [11].
At 1 year, the mean change in eGFR was –2.34 mL/min/1.73 m2 in the tolvaptan group and –3.61 mL/min/1.73 m2 in the placebo group (difference 1.27 mL/min/1.73 m2; 95% CI 0.86–1.68; p < 0.001) [11]. The key secondary endpoint of the mean slopes of the change in eGFR, adjusted for the duration of the trial (interpolated to 1 year) and the acute effect of tolvaptan, was – 3.16 mL/min/1.73 m2 in the tolvaptan group and – 4.17 mL/min/1.73 m2 in the placebo group (difference 1.01 mL/min/1.73 m2; 95% CI 0.62–1.40; p < 0.001). For both the primary and secondary endpoints, the beneficial effects of tolvaptan were seen in prespecified subgroups defined according to sex, baseline eGFR, CKD stage (except for stage 2) and geographic region (USA vs. other), as well as in the subgroups of patients who were aged ≤ 55 years and who were White, but not in the smaller subgroups of patients who were aged > 55 years, who were non-White or who had stage 2 CKD [11].
2.2.2 Patients with ADPKD in Japan
The efficacy of tolvaptan in Japanese patients with later-stage ADPKD was not significantly different to that seen in Japanese patients with earlier-stage ADPKD, according to the results of a prospective cohort study (n = 54) [12]. All patients received tolvaptan 45 mg in the morning and 15 mg in the evening. The dosage was adjusted as tolerated, up to a maximum of 120 mg/day. Patients were categorized as earlier CKD stage (eGFR ≥ 45 mL/min/1.73 m2; n = 28) or later CKD stage (eGFR < 45 mL/min/1.73 m2; n = 26). The primary endpoints were the change in height-adjusted TKV (htTKV) and eGFR at 1 year. The median absolute change in htTKV was 75 mL/year in later CKD stage and 36 mL/year in earlier CKD stage (p = 0.054). The corresponding relative changes in htTKV were 8.2 and 5.7% per year. The median absolute change in eGFR at 1 year was – 2.8 mL/min/1.73 m2 in later CKD stage and – 3.3 mL/min/1.73 m2 in earlier CKD stage. The corresponding relative changes in eGFR were – 9.7 and – 6.8% per year. Consistent with the results of the primary endpoint, baseline eGFR was not significantly correlated with absolute or relative change in eGFR. However, there was a significant (p = 0.03) negative correlation between baseline eGFR and absolute change in htTKV [12].
2.3 Real-Word Experience
Real-world experience has confirmed the efficacy of tolvaptan for the treatment of ADPKD (available as abstracts). In several small (n < 100) studies, the efficacy of tolvaptan was generally consistent with that seen in pivotal clinical trials [20,21,22,23,24,25,26]. Among patients treated with tolvaptan in the SUISSE ADPKD cohort (n = 76), the median and mean changes from baseline in eGFR after 4 weeks of treatment were – 5.8 and – 5.6 mL/min/1.73 m2, respectively [21]. eGFR values remained stable at the end of follow-up (1 year); the mean slope of the linear regression for eGFR was 0.005 mL/min/1.73 m2 [21]. Patients in the larger AD(H)PKD registry (n < 450) experienced increased urine volume and urine osmolality during treatment with tolvaptan [27]. Adherence to therapy was ≈ 80% and > 75% of patients reported little to no problems with tolvaptan therapy in everyday life [27].
A retrospective study conducted in Japan showed that the efficacy of tolvaptan in patients with later-stage disease was similar to that seen in patients with earlier-stage disease [20]. The changes from baseline in eGFR after 6, 12 and 24 months of treatment with tolvaptan were – 2.9, – 4.2 and – 8.7 mL/min/1.73 m2, respectively, in patients with stage 2–3b CKD (n = 14) and – 1.3, – 2.1 and – 4.8 mL/min/1.73 m2, respectively, in patients with stage 4 CKD (n = 6), with no significant differences at any time point. The ratio of change in TKV at 12 months was also not significantly different between patients with stage 2–3b and stage 4 CKD [20].
3 Tolerability of Tolvaptan
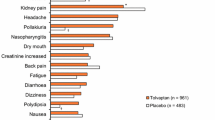
Adverse events (AEs) were common in patients with ADPKD in the TEMPO 3:4 trial, occurring in 98% of tolvaptan recipients and 97% of placebo recipients [9]. The most common (> 30% incidence) AEs with tolvaptan were thirst (55 vs. 21% with placebo), polyuria (38 vs. 17%) and hypertension (32 vs. 36%). AEs related to aquaresis (e.g. polyuria, nocturia, polydipsia and thirst) occurred significantly (p < 0.001) more often in the tolvaptan group than in the placebo group, while AEs related to ADPKD (e.g. kidney pain, urinary tract infection and haematuria) were significantly (p < 0.05) more common with placebo [9].
In TEMPO 3:4, the most common serious AEs in the tolvaptan group were ALT elevation (0.9 vs. 0.4% with placebo), AST elevation (0.9 vs. 0.4%), chest pain (0.8 vs. 0.4%) and headache (0.5 vs. 0%) [9]. AEs leading to discontinuation occurred in 15% of patients treated with tolvaptan and 5% of patients treated with placebo; 8% of tolvaptan recipients discontinued treatment as a result of aquaretic symptoms and 1% discontinued due to liver function abnormalities [9]. Tolvaptan is known to decrease uric acid clearance by the kidney [7]. In TEMPO 3:4, increased serum uric acid levels (> 10 mg/dL) were reported in 6% of patients receiving tolvaptan and 2% of patients receiving placebo [7]. The incidence of gout as an AE was 3% in the tolvaptan group and 1% in the placebo group [9]. Uric acid levels should be assessed prior to the initiation of tolvaptan and as indicated during treatment [7].
AEs were also common in patients with later-stage ADPKD in the REPRISE trial [11]. The most common (> 20%) AEs during the 5-week single-blind tolvaptan period were polyuria (32%), thirst (29%) and nocturia (21%). The incidence of AEs during the 1-year double-blind period was 85% in the tolvaptan group and 82% in the placebo group. The incidence of serious AEs was 13% in the tolvaptan group and 9% in the placebo group. AEs leading to discontinuation occurred in 10% of tolvaptan recipients and 2% of placebo recipients; 2% of tolvaptan recipients discontinued treatment as a result of aquaretic AEs and 2% discontinued due to hepatic enzyme abnormalities [11].
The long-term safety profile of tolvaptan in two open-label extension trials was generally similar to that observed in TEMPO 3:4 [10, 28]. In TEMPO 4:4, 92% of ‘early-treated’ patients and 97% of ‘delayed-treated’ patients experienced at least one treatment-emergent AE (TEAE) over 24 months [10]. The incidence of serious TEAEs was 16% with tolvaptan and 18% with placebo. TEAEs leading to discontinuation occurred in 5% of tolvaptan recipients and 15% of placebo recipients [10]. The TEMPO Extension Japan Trial included 135 Japanese patients who participated in TEMPO 3:4 (and were not eligible to enter TEMPO 4:4, which was conducted outside of Japan) [28]. Almost all (> 99%) patients experienced AEs; however, most were of mild severity and were observed during the first 3 months of treatment [28].
3.1 Aquaretic Adverse Events
Aquaresis associated with tolvaptan may cause dehydration, hypovolaemia and hypernatraemia [7, 8]. In TEMPO 3:4, 750 of 961 tolvaptan recipients reported at least one aquaretic AE [29]. A post hoc analysis of tolvaptan-related discontinuations from TEMPO 3:4 and TEMPO 4:4 demonstrated that patients who discontinued tolvaptan due to an aquaretic AE (n = 72) were younger (mean 36.2 vs. 38.9 years; p = 0.006), had higher baseline renal function (eGFR 88.2 vs. 80.9 mL/min/1.73 m2; p = 0.006) and had higher fasting urine osmolality (mean 554 vs. 492 mOsm/kg; p = 0.045) than those who reported an aquaretic AE but continued to receive tolvaptan (n = 573). Moreover, patients who discontinued tolvaptan due to an aquaretic AE were more likely to be male (57 vs. 41%; p < 0.05), were younger (mean 36.2 vs. 40.0 years; p = 0.007), had higher baseline renal function (eGFR 88.2 vs. 78.7 mL/min/1.73 m2; p = 0.005), had lower baseline htTKV (mean 915 vs. 1032 mL/m; p = 0.03) and were faster to discontinue treatment (median 96 vs. 372 days; p < 0.0001) than patients who discontinued tolvaptan for another (non-aquaretic) reason (n = 105) [29]. Potentially clinically important elevation of sodium levels (> 150 mmol/L) was seen in 4% of tolvaptan recipients and 1% of placebo recipients [9]. The incidence of hypernatraemia as an AE was 3% with tolvaptan and 1% with placebo [9].
The aquaretic effect of tolvaptan did not appear to diminish over time [10, 28]. In TEMPO 4:4, aquaretic TEAEs associated with long-term tolvaptan therapy included thirst (47% with early treatment vs. 50% with delayed treatment), polyuria (41 vs. 55%), nocturia (26 vs. 34%) and polydipsia (11 vs. 16%) [10]. The most common AEs in the TEMPO Extension Japan Trial were aquaretic AEs, namely thirst (77%), pollakiuria (57%) and polyuria (38%); almost all of these AEs occurred during the first 3 months of tolvaptan therapy [28].
To avoid dehydration, patients should be instructed to drink sufficient amounts of water at the first signs of thirst [7, 8]. Tolvaptan is contraindicated in patients who are unable to perceive or respond to thirst [7, 8]. Patients should drink 1–2 glasses of water before bedtime (regardless of perceived thirst), replenishing fluids following each episode of nocturia [7]. Abnormal sodium levels should be corrected prior to the initiation of tolvaptan [7, 8]. If a patient develops abnormal sodium levels during treatment with tolvaptan or becomes dehydrated or hypovolaemic (and fluid intake cannot be increased), the drug should be interrupted until serum sodium, hydration status and volume status are within the normal ranges [8].
3.2 Hepatotoxicity
Tolvaptan has been associated with idiosyncratic hepatic toxicity, characterized by elevated transaminases and, rarely, concomitant elevations in total bilirubin [7]. The drug can cause serious and potentially fatal liver injury, including acute liver failure requiring liver transplantation [8]. Tolvaptan has a special warning for idiosyncratic hepatic toxicity in the EU [7] and carries a boxed warning for serious liver injury in the USA [8]. In TEMPO 3:4, ALT and AST elevations > 3 × the upper limit of normal (ULN) were observed in 4.4 and 3.1% of tolvaptan recipients and 1.0 and 0.8% of placebo recipients [7]. Potentially clinically important bilirubin elevations (> 1.5 × ULN) were seen in 0.9% of tolvaptan recipients and 1.9% of placebo recipients [9]. Two patients in the tolvaptan group had elevated ALT or AST enzymes (> 3 × ULN) with concurrent elevations in total bilirubin (> 2 × ULN); these abnormalities resolved after discontinuation of tolvaptan [9].
In REPRISE, 74 of 681 patients (11%) patients in the tolvaptan group and 36 of 685 patients (5%) in the placebo group had hepatic AEs during the double-blind period [11]. Serious hepatic AEs occurred in 5% of tolvaptan recipients and 1% of placebo recipients. ALT elevations > 3 × ULN were observed in 6% of tolvaptan recipients and 1% of placebo recipients; these levels normalized following the interruption or discontinuation of tolvaptan [11].
In an interim analysis of the Canadian ADPKD patient registry C-MAJOR (n = 250) and hepatic safety monitoring and distribution program (n = 1143), 3% of 941 patients receiving tolvaptan had elevated liver function tests (> 3 × ULN) during a mean follow-up of 64.1 weeks; however, there were no cases of drug-induced liver injury [30].
Hepatic AEs did not appear to be progressive over the longer term [10, 28]. In TEMPO 4:4, the incidence of ALT elevations > 3 × ULN in early-treated patients was similar to that observed in delayed-treated patients (3 vs. 4%) [10]. One (delayed-treated) patient in TEMPO 4:4 had an increase in ALT level of > 3 × ULN and a concomitant increase in total bilirubin of > 2 × ULN; these abnormalities resolved after discontinuation of tolvaptan [10]. In the TEMPO Extension Japan Trial, hepatic events were reported in 14 (10%) patients, eight of whom experienced ALT or AST elevations > 3 × ULN [28]. These abnormalities resolved with (n = 6) or without (n = 2) interruption of tolvaptan [28].
In the EU, tolvaptan is contraindicated in patients with elevated liver enzymes and/or signs or symptoms of liver injury prior to initiation of treatment that meet the requirements for permanent discontinuation [7]. In the USA, tolvaptan is contraindicated in patients with a history, signs or symptoms of significant liver impairment or injury [8]. To reduce the risk of significant and/or irreversible liver injury, monitoring of liver transaminases and bilirubin is required prior to the initiation of tolvaptan and at prespecified intervals during treatment [7, 8]. Administration of tolvaptan should be interrupted at the onset of signs or symptoms of hepatic injury or if abnormal liver enzymes are detected. The drug may be restarted upon stabilization or resolution of symptoms and/or laboratory abnormalities [7, 8]. In the EU, dosage adjustments are not required in patients with mild or moderate hepatic impairment (Child-Pugh classes A and B) [7]. However, the benefits and risks of tolvaptan should be evaluated carefully in patients with severe hepatic impairment, and liver enzymes should be monitored regularly [7].
4 Dosage and Administration of Tolvaptan
Tolvaptan is approved for the treatment of ADPKD in several countries, including the USA, Canada, Japan and those of the EU. In the EU, tolvaptan is indicated to slow the progression of cyst development and renal insufficiency of ADPKD in adults with stage 1–4 CKD at initiation of treatment with evidence of rapidly progressing disease [7]. In the USA, tolvaptan is indicated to slow renal function decline in adults at risk of rapidly progressing ADPKD [8]. The initial dosage of tolvaptan is 60 mg/day, administered as a split-dose regimen of 45 mg upon waking (≥ 30 min before the morning meal [7]) and 15 mg 8 h later (with or without food [7]) [7, 8]. The dosage is then titrated to 90 mg/day (60 plus 30 mg) and then to 120 mg/day (90 plus 30 mg), with intervals of ≥ 1 week between titrations [7, 8]. The EU SPC states that patients should be maintained on the highest tolerable dosage [7].
The efficacy and tolerability of tolvaptan in paediatric patients has not been established [7, 8]. In the EU, contraindications to tolvaptan include anuria, volume depletion, hypernatraemia, pregnancy and breastfeeding [7]. In the USA, tolvaptan is contraindicated in patients taking strong CYP3A inhibitors, and in patients with uncorrected abnormal blood sodium levels, hypovolaemia, anuria or uncorrected urinary outflow obstruction [8]. Consult local prescribing information for detailed information regarding dosage adjustments, contraindications, warnings and precautions, drug interactions and use in specific patient populations.
5 Place of Tolvaptan in the Management of ADPKD
For many years, the focus of treatment in patients with ADPKD has been on reducing morbidity and mortality associated with the manifestations of the disease [31]. Supportive therapies recommended to slow the rate of disease progression include optimization of BP, sufficient fluid intake, salt-reduced diets, caffeine restriction, smoking cessation and the avoidance of nephrotoxic agents (e.g. NSAIDs) [31]. The highly selective vasopressin V2 receptor antagonist tolvaptan is currently the only pharmacological agent approved for the treatment of ADPKD. Recently updated Canadian guidelines recommend tolvaptan for the treatment of patients with ADPKD who fulfil the enrolment criteria of the TEMPO 3:4 or REPRISE trials [32]. Experts in the USA [33] and Europe [34] have also provided evidence-based recommendations and guidance for the use of tolvaptan in ADPKD. The UK National Institute for Health and Care Excellence (NICE) recommends tolvaptan as a treatment option for adults with ADPKD only if they have stage 2 or 3 CKD at the initiation of treatment with evidence of rapidly progressing disease [35].
Tolvaptan was initially approved in the EU to slow the progression of cyst development and renal insufficiency of ADPKD in adults with stage 1–3 CKD at the initiation of treatment with evidence of rapid disease progression [7]. This approval was based on the results of the TEMPO 3:4 trial, in which the drug significantly slowed the increase in TKV over a 3-year period relative to placebo (Sect. 2.1). Although the change in TKV was most prominent during the first year of treatment, possibly due to an acute reduction in cyst fluid secretion, the benefit of tolvaptan observed during years 2 and 3 was consistent with the inhibition of cyst-cell proliferation [9]. The composite secondary endpoint of time to clinical progression also favoured tolvaptan over placebo, an effect largely driven by lower rates of kidney pain and worsening renal function (Sect. 2.1).
Tolvaptan is also approved in the USA to slow renal function decline in adults at risk of rapidly progressing ADPKD [8]. The US approval was supported by data from TEMPO 3:4 (Sect. 2.1), as well as new data from the REPRISE trial (Sect. 2.2.1). REPRISE was designed to confirm the efficacy and tolerability of tolvaptan in patients with later-stage ADPKD and to address US FDA concerns regarding potential hepatotoxicity and missing data due to the high withdrawal rate in the TEMPO 3:4 tolvaptan group [36]. REPRISE confirmed the efficacy of tolvaptan in slowing renal function decline (Sect. 2.2.1), a key secondary endpoint of TEMPO 3:4, and extended the results of TEMPO 3:4 to patients with more advanced CKD [37]. The results of the REPRISE trial also led to an extension of the existing EU label for tolvaptan, to include treatment of ADPKD in patients with stage 4 CKD [7]. In REPRISE, a randomized withdrawal design was used to enrich the trial population for patients who were able to tolerate tolvaptan. While this design reduced the number of early withdrawals (as evidenced by a 96% study completion rate in both treatment groups [11]) and permitted robust assessment [36], it may have limited the wide-ranging applicability of the results [11].
Tolvaptan demonstrated efficacy across a broad range of patient subgroups, including Japanese patients (Sect. 2). Of note, there was a 1.5% difference in the annual rate of TKV growth between the overall TEMPO 3:4 trial population and the Japanese subpopulation (Sect. 2.1.1). While there is no definitive explanation for this difference, the authors suggest that it may be due to differences in height and bodyweight between the two populations [19]. Even in Japanese patients, the treatment effect of tolvaptan in slowing renal function decline in patients with later-stage ADPKD was similar to that in patients with earlier-stage disease (Sect. 2.2.2), as observed in the REPRISE trial [12].
Patients in the TEMPO 3:4 and REPRISE trials were advised to maintain good hydration [9, 11]. There has been some discussion in the literature regarding the efficacy of high fluid intake alone for the treatment of ADPKD, with preclinical and clinical trials demonstrating inconclusive results [38]. Additional trials are ongoing, including PREVENT-ADPKD, which will investigate the long-term efficacy and safety of prescribed fluid intake (to reduce urine osmolality to ≤ 270 mOsmol/kg) for the prevention of kidney failure in 180 patients with stage 1–3 CKD due to ADPKD [39]. However, head-to-head studies directly comparing tolvaptan and fluid intake alone in patients with ADPKD are needed. The 2-year WATSAPP trial plans to directly compare the efficacy of increased water intake (3–4 L/day) versus tolvaptan (30–90 mg as tolerated) in 1500 patients with ADPKD [40].
Data from real-world studies of tolvaptan in patients with ADPKD were generally consistent with those seen in clinical trials (Sect. 2.3). However, it should be noted that patient numbers in these studies were small. Larger-scale, long-term observational studies in the real-world setting would be of great interest.
The long-term efficacy of tolvaptan was demonstrated in the TEMPO 4:4 extension trial (Sect. 2.1.2). The beneficial effects of tolvaptan on eGFR seen in TEMPO 3:4 were maintained for a further 2 years. However, tolvaptan was not associated with sustained slowing of TKV growth over the longer term (Sect. 2.1.2). The absence of an overall effect of tolvaptan on TKV may have been due to unforeseen trial design limitations (e.g. loss of randomization from TEMPO 3:4 due to unequal distribution of withdrawals) or to the larger effect of tolvaptan during the first year of treatment [10]. Another contributing factor may have been the gender imbalance between the early- and delayed-treated groups at TEMPO 4:4 baseline (which further increased at month 24). It has been shown that males have faster TKV growth rates than women over time [10]. Indeed, after adjustment for baseline imbalances, there was a significant difference in favour of early treatment (Sect. 2.1.2). Nevertheless, questions have been raised regarding the effect of tolvaptan on TKV and whether it might become negligible over many years of treatment [41]. The impact of tolvaptan on long-term disease progression requires further investigation.
Although 75% of patients receiving tolvaptan in TEMPO 3:4 reported that they could tolerate their current dose for the rest of their life [29], the potential benefits of tolvaptan are not without risks. AEs were common in the TEMPO 3:4 and REPRISE trials (Sect. 3.1). Consistent with its mechanism of action, tolvaptan was associated with high rates of aquaretic AEs. Of note, patients in earlier stages of disease progression appear to be more sensitive to these AEs. Tolvaptan also carries a special warning regarding the risk of hepatotoxicity (Sect. 3.2). To mitigate this risk, all patients receiving tolvaptan are required to undergo regular liver function testing. Two patients from TEMPO 3:4 and one patient from TEMPO 4:4 had idiosyncratic elevations of liver enzymes which were reversible with discontinuation of tolvaptan (Sect. 3.2). All three patients met the criteria for Hy’s law, defined as ALT > 3 × ULN and total bilirubin > 2 × ULN [42]. The identification of Hy’s law cases indicates the potential for a drug to cause liver injury capable of progressing to liver failure [42]. In these three instances where patients met Hy’s law criteria, the independent hepatic adjudication committee deemed the drug-induced liver injury was probably due to tolvaptan. No patients in REPRISE met the criteria for Hy’s law; this may have been due to more frequent monitoring and early interruption of therapy [11]. Potential mechanisms of tolvaptan-induced liver injury and biomarkers of patient susceptibility are being investigated, which may help to identify ADPKD patients at risk of hepatotoxicity [43]. The risk of hepatotoxicity associated with the use of tolvaptan in a real-world setting is currently being evaluated in a 6-year, prospective, post authorization safety study [44]. Another post-marketing surveillance study will assess liver function over an 8-year period in the real-world clinical setting in Japan (NCT02847624) [45].
The long-term safety profile of tolvaptan in the extension trials was generally similar to that observed in TEMPO 3:4 (Sect. 3). While the aquaretic effect of tolvaptan did not seem to diminish over time (Sect. 3.1), hepatic AEs did not appear to be progressive over the longer term (Sect. 3.2). The long-term safety of tolvaptan in patients with ADPKD who participated in TEMPO 4:4 or other tolvaptan trials is currently being investigated in a large (n ≈ 2500), open-label, multicentre, phase IIIb trial (NCT02251275) [45].
Evidence suggests that early treatment to slow ADPKD progression may reduce disease burden, healthcare utilization and costs [46, 47]. In a Spanish setting, the ADPKD Outcomes Model (using TEMPO 3:4 baseline characteristics) estimated that treatment with tolvaptan would prevent 4% of ESRD cases, delay the mean time to ESRD by 4.5 years and extend life expectancy by 3.7 years per patient [47]. Treatment with tolvaptan was also estimated to avoid 5% of kidney transplants and reduce the mean dialysis time by 5 months per patient [47]. In a cost-consequence analysis of 1000 ADPKD patients, tolvaptan was associated with lower on-treatment kidney pain-related costs [48]. Two weeks after tolvaptan withdrawal, monthly CKD-related costs were lower with tolvaptan than with placebo, indicating slower CKD progression. However, it should be noted that this analysis did not account for the cost of tolvaptan [48]. Studies investigating the cost effectiveness of tolvaptan in patients with ADPKD are limited. According to the UK NICE committee, the incremental cost-effectiveness ratio per quality-adjusted life-year gained under tolvaptan in ADPKD adults with stage 2–3 CKD was approximately ₤23,500 [35]. The committee concluded that tolvaptan represented a cost-effective use of health system resources in this patient population [35]. Further robust pharmacoeconomic data are needed.
In conclusion, although the use of tolvaptan requires careful consideration and balancing of benefits and risks, tolvaptan provides a valuable treatment option to slow the progression of ADPKD in patients at risk of or with evidence of rapidly progressing disease.
Data Selection Tolvaptan: 309 records identified
Duplicates removed | 91 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 121 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 35 |
Cited efficacy/tolerability articles | 23 |
Cited articles not efficacy/tolerability | 39 |
Search Strategy: EMBASE, MEDLINE and PubMed from September 2015 to present. Previous Adis Drug Evaluation published in 2015 was hand-searched for relevant data. Clinical trial registries/databases and websites were also searched for relevant data. Key words were autosomal dominant polycystic kidney disease, tolvaptan, Jinarc, Jynarque, Samsca. Records were limited to those in English language. Searches last updated 14 January 2019 | |
References
EAF co-chairs, Harris T, Sandford R, et al. European ADPKD forum multidisciplinary position statement on autosomal dominant polycystic kidney disease care: European ADPKD forum and multispecialist roundtable participants. Nephrol Dial Transplant. 2018;33(4):563–73.
Simms RJ. Autosomal dominant polycystic kidney disease. BMJ. 2016;352:i679.
Chang MY, Ong AC. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2012;120(1):c25–34.
Reif GA, Yamaguchi T, Nivens E, et al. Tolvaptan inhibits ERK-dependent cell proliferation, Cl(-) secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol. 2011;301(5):F1005–13.
Meijer E, Gansevoort RT, de Jong PE, et al. Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: optimal timing and dosing of the drug. Nephrol Dial Transplant. 2011;26(8):2445–53.
Blair HA, Keating GM. Tolvaptan: a review in autosomal dominant polycystic kidney disease. Drugs. 2015;75(15):1797–806.
European Medicines Agency. Jinarc (tolvaptan): EU summary of product characteristics. 2018. http://www.ema.europa.eu. Accessed 10 Jan 2019.
US FDA. JYNARQUE (tolvaptan) tablets for oral use: US prescribing information. 2018. http://www.fda.gov. Accessed 10 Jan 2019.
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–18.
Torres VE, Chapman AB, Devuyst O, et al. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 trial. Nephrol Dial Transplant. 2018;33(3):477–89.
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377(20):1930–42.
Oguro M, Kogure Y, Hoshino J, et al. Tolvaptan in Japanese patients with later-stage autosomal dominant polycystic kidney disease. J Nephrol. 2018;31(6):961–6.
Torres VE, Higashihara E, Devuyst O, et al. Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: results from the TEMPO 3:4 trial. Clin J Am Soc Nephrol. 2016;11(5):803–11.
Irazabal MV, Blais JD, Perrone RD, et al. Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the TEMPO 3:4 clinical trial. Kidney Int Rep. 2016;1(4):213–20.
Gansevoort RT, Meijer E, Chapman AB, et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 trial. Nephrol Dial Transplant. 2016;31(11):1887–94.
Casteleijn NF, Blais JD, Chapman AB, et al. Tolvaptan and kidney pain in patients with autosomal dominant polycystic kidney disease: secondary analysis from a randomized controlled trial. Am J Kidney Dis. 2017;69(2):210–9.
Chapman A, Devusyt O, Gansevoort R, et al. Potential impact of tolvaptan on blood pressure in the TEMPO 3:4 patient population [abstract no. FP047]. Nephrol Dial Transplant. 2018;33(Suppl 1):i63.
Grantham JJ, Chapman AB, Blais J, et al. Tolvaptan suppresses monocyte chemotactic protein-1 excretion in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2016;32(6):969–75.
Muto S, Kawano H, Higashihara E, et al. The effect of tolvaptan on autosomal dominant polycystic kidney disease patients: a subgroup analysis of the Japanese patient subset from TEMPO 3:4 trial. Clin and Exp Nephrol. 2015;19(5):867–77.
Yamada K, Muramoto H, Araki H, et al. Effectiveness and safety of tolvaptan in autosomal dominant polycystic kidney disease patients with CKD stage G4: a retrospective multicenter study in Japan [abstract no. FP052]. Nephrol Dial Transplant. 2018;33(Suppl 1):i65.
Russmann S, Grimes E, Neidrig D, et al. Safety and efficacy of tolvaptan in the SUISSE ADPKD cohort [abstract no. 1386]. Drug Saf. 2018;41(11):1229.
Hattanda F, Nishio S, Takeda S, et al. Efficacy of tolvaptan on autosomal dominant polycystic kidney disease (ADPKD) patients in late stage CKD [abstract no. SP027]. Nephrol Dial Transplant. 2018;33(Suppl 1):i355.
Kai H, Tsunoda R, Kawamura T, et al. A prospective study of the efficacy and adverse effects of tolvaptan for autosomal dominant polycystic kidney disease (ADPKD) [abstract no. FP061]. Nephrol Dial Transplant. 2018;33(Suppl. 1):i68.
Cote G, Asselin-Thompstone L, Rene De Cotret P, et al. The impact of tolvaptan on glomerular filtration rate in patients with autostomal dominant polycystic kidney disease [abstract no. SA-PO476]. J Am Soc Nephrol. 2018;29(Suppl):859.
Honda K, Matsuura R, Ishimoto Y, et al. Association between initial dose of tolvaptan and reduction of total kidney volume in autostomal dominant polycystic kidney disease [abstract no. SA-PO477]. J Am Soc Nephrol. 2018;29(Suppl):859.
Kogure Y, Takayanagi K, Sato M, et al. Clinical features expecting high efficacy of tolvaptan in ADPKD patients [abstract no. SA-PO478]. J Am Soc Nephrol. 2018;29(Suppl):859.
Mueller R-U, Todorova P, Suarez V, et al. AD(H)PKD—a prospective cohort study on the use of tolvaptan in ADPKD [abstract no. SA-PO473]. J Am Soc Nephrol. 2018;29(Suppl):858.
Muto S, Okada T, Yasuda M, et al. Long-term safety profile of tolvaptan in autosomal dominant polycystic kidney disease patients: TEMPO extension Japan trial. Drug Healthc Patient Saf. 2017;9:93–104.
Devuyst O, Chapman AB, Shoaf SE, et al. Tolerability of aquaretic-related symptoms following tolvaptan for autosomal dominant polycystic kidney disease: results from TEMPO 3:4. Kidney Int Rep. 2017;2(6):1132–40.
McFarlane P, Bichet DG, Bergeron L, et al. Canadian real-life assessment of tolvaptan in autosomal dominant polycystic kidney disease (ADPKD): C-MAJOR study and hepatic safety monitoring program [abstract no. SA-PO480]. J Am Soc Nephrol. 2018;29(Suppl):860.
Sommerer C, Zeier M. Clinical manifestation and management of ADPKD in western countries. Kidney Dis. 2016;2(3):120–7.
Soroka S, Alam A, Bevilacqua M, et al. Updated Canadian expert consensus on assessing risk of disease progression and pharmacological management of autosomal dominant polycystic kidney disease. Can J Kidney Health Dis. 2018. https://doi.org/10.1177/2054358118801589.
Chebib FT, Perrone RD, Chapman AB, et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018;29:1–13.
Gansevoort RT, Arici M, Benzing T, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA working groups on inherited kidney disorders and European renal best practice. Nephrol Dial Transplant. 2016;31(3):337–48.
National Institute for Health and Care Excellence (NICE). Tolvaptan for treating autosomal dominant polycystic kidney disease. 2015. http://www.nice.org.uk. Accessed 10 Jan 2019.
Mustafa RA, Yu ASL. Burden of proof for tolvaptan in ADPKD: did REPRISE provide the answer? Clin J Am Soc Nephrol. 2018;13(7):1107–9.
Wyatt CM, Le Meur Y. REPRISE: tolvaptan in advanced polycystic kidney disease. Kidney Int. 2018;93(2):292–5.
van Gastel MDA, Torres VE. Polycystic kidney disease and the vasopressin pathway. Ann Nutr Metab. 2017;70(Suppl 1):43–50.
Wong ATY, Mannix C, Grantham JJ, et al. Randomised controlled trial to determine the efficacy and safety of prescribed water intake to prevent kidney failure due to autosomal dominant polycystic kidney disease (PREVENT-ADPKD). BMJ open. 2018;8(1):e018794.
UKidney. Water vs tolvaptan in reducing ADPKD progression. http://ukidney.com. Accessed 10 Jan 2019.
Gross P, Schirutschke H, Paliege A. Con: tolvaptan for autosomal dominant polycystic kidney disease-do we know all the answers? Nephrol Dial Transplant. 2018;34(1):35–7.
Watkins PB, Lewis JH, Kaplowitz N, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38(11):1103–13.
Woodhead JL, Brock WJ, Roth SE, et al. Application of a mechanistic model to evaluate putative mechanisms of tolvaptan drug-induced liver injury and identify patient susceptibility factors. Toxicol Sci. 2017;155(1):61–74.
European Medicines Agency. Jinarc assessment report. 2015. http://www.ema.europa.eu. Accessed 10 Jan 2019.
US National Institutes of Health. 2018. http://www.clinicaltrials.gov. Accessed 10 Jan 2019.
Blanchette CM, Matter S, Chawla A, et al. Burden of autosomal dominant polycystic kidney disease: systemic literature review. Am J Pharm Benefits. 2015;7(2):e27–36.
Bennett H, McEwan P, Robinson P, et al. Validation of the ADPKD outcomes model to a Spanish setting and an evaluation of the impact of treatment on the burden of ESRD [abstract no. PUK23 plus poster]. Value Health. 2017;20(5):A309–A10.
Shephard C, Delavelle C, Riemer J, et al. Impact of tolvaptan on costs associated with renal pain and chronic kidney disease among patients with autosomal dominant polycystic kidney disease [abstract no. 274]. Am J Kidney Dis. 2018;71(4):584.
Aihara M, Fujiki H, Mizuguchi H, et al. Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury. J Pharmacol Exp Ther. 2014;349(2):258–67.
Lee Y, Blount KL, Dai F, et al. Semaphorin 7A in circulating regulatory T cells is increased in autosomal-dominant polycystic kidney disease and decreases with tolvaptan treatment. Clin Exp Nephrol. 2018;22(4):906–16.
Fujiki T, Ando F, Isobe K, et al. Tolvaptan activates Nrf2/HO-1 pathway through PERK phosphorylation [abstract no. SA-PO847]. J Am Soc Nephrol. 2018;29(Suppl):957.
Shoaf SE, Wang Z, Bricmont P, et al. Pharmacokinetics, pharmacodynamics, and safety of tolvaptan, a nonpeptide AVP antagonist, during ascending single-dose studies in healthy subjects. J Clin Pharmacol. 2007;47(12):1498–507.
Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301.
Boertien WE, Meijer E, de Jong PE, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65(6):833–41.
Boertien WE, Meijer E, de Jong PE, et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84(6):1278–86.
Harskamp LR, Gansevoort RT, Boertien WE, et al. Urinary EGF receptor ligand excretion in patients with autosomal dominant polycystic kidney disease and response to tolvaptan. Clin J Am Soc Nephrol. 2015;10(10):1749–56.
Minami S, Hamano T, Iwatani H, et al. Tolvaptan promotes urinary excretion of sodium and urea: a retrospective cohort study. Clin Exp Nephrol. 2018;22(3):550–61.
Al Therwani S, Malmberg MES, Rosenbaek JB, et al. Effect of tolvaptan on renal handling of water and sodium, GFR and central hemodynamics in autosomal dominant polycystic kidney disease during inhibition of the nitric oxide system: a randomized, placebo-controlled, double blind, crossover study. BMC Nephrol. 2017;18(1):268.
Kawano H, Muto S, Ohmoto Y, et al. Exploring urinary biomarkers in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2015;19(5):968–73.
Malmberg M, Sonderbaek R, Mose F, et al. Effect of tolvaptan on renal plasma flow and glomerular filtration in polycystic kidney disease [abstract no. SP110]. Nephrol Dial Transplant. 2018;33(Suppl 1):i381.
Shoaf SE, Bricmont P, Mallikaarjun S. Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int. 2014;85(4):953–61.
Shoaf SE, Kim SR, Bricmont P, et al. Pharmacokinetics and pharmacodynamics of single-dose oral tolvaptan in fasted and non-fasted states in healthy Caucasian and Japanese male subjects. Eur J Clin Pharmacol. 2012;68(12):1595–603.
Acknowledgements
During the peer review process, the manufacturer of tolvaptan was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Hannah Blair is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by:F. Chebib, Division of Nephrology and Hypertension, Mayo Clinic College of Medicine, Rochester, MN, USA; Z. Kendi Celebi, Department of Nephrology, Ankara University School of Medicine, Ankara, Turkey; G. K. Rangan, Department of Renal Medicine, Westmead Hospital, Sydney, NSW, Australia; T. I. Steinman, Nephrology Division, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Rights and permissions
About this article
Cite this article
Blair, H.A. Tolvaptan: A Review in Autosomal Dominant Polycystic Kidney Disease. Drugs 79, 303–313 (2019). https://doi.org/10.1007/s40265-019-1056-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-1056-1