Abstract
Type 1 diabetes is characterised by insulin deficiency caused by autoimmune destruction of the pancreatic beta cells. The treatment of type 1 diabetes is exogenous insulin in the form of multiple daily injections or continuous subcutaneous insulin infusion. Advances in diabetes technology have been exponential in the past few decades, culminating in studies to develop an automated artificial pancreas, also known as the closed-loop system. This has recently led to a commercially available, hybrid artificial pancreas in the USA and Europe. This review article aims to provide an overview of the rationale for an artificial pancreas system and an update of the current state of artificial pancreas development. We explore the different types of artificial pancreas systems being studied, including the use of adjunctive therapy, and the use of these systems in different groups of users. In addition, we discuss the potential psychosocial impact and the challenges and limitations of implementing artificial pancreas use into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Type 1 diabetes is treated with exogenous insulin, and recent advances in diabetes technology have culminated in the development of a commercially available, hybrid artificial pancreas (AP). |
Adjunctive therapy to an insulin-only AP including glucagon, amylin analogues and glucagon-like peptide 1 receptor agonists has shown promise. |
An AP has also been investigated in various cohorts including in pregnancy and type 2 diabetes and in critically ill individuals, but larger studies with evaluations of the psychosocial impact of an AP in these different cohorts are warranted. |
1 Introduction
Type 1 diabetes mellitus (T1D) is characterised by insulin deficiency caused by autoimmune destruction of the pancreatic islet beta cells. Worldwide, 425 million people have diabetes and T1D accounts for 5–10% of all cases [1]. The treatment for T1D is exogenous insulin, delivered in the form of either multiple daily injections of insulin or continuous subcutaneous insulin infusion (CSII), also known as an insulin pump. Insulin requirements throughout the day vary depending on time, activity, carbohydrate contents of meals, menstruation, stress and illness. Achieving optimal glycaemic control in this context can be physically and psychologically challenging.
The landmark Diabetes Control and Complications Trial showed that intensive insulin therapy results in a lower glycosylated haemoglobin (HbA1c) compared with conventional treatment. It is also associated with a lower risk of diabetic microvascular complications [2]. The 30-year follow-up on Diabetes Control and Complications Trial participants also showed that intensive insulin therapy has long-term beneficial effects on the incidence of cardiovascular disease in T1D [3]. However, in achieving glycaemic control closer to target, and limiting future complications with intensive insulin therapy, the risk for hypoglycaemia is increased [4] with potential acute complications including seizures, unconsciousness and death [4, 5].
Technology aiding the self-management of T1D has advanced exponentially over the past few decades. Insulin pumps are becoming increasingly accessible worldwide for people with T1D, enabling flexibility that fits around the individual’s lifestyle. Meta-analyses of CSII in T1D have shown that CSII improves glycaemic control [6, 7], whilst at the same time is associated with significantly reduced rates of severe hypoglycaemia [6] and improved quality of life [8] compared with multiple daily injections of insulin.
Similar advances have been observed in glucose-monitoring devices since the development of blood glucose meters in the 1970s to the emergence of continuous glucose monitoring (CGM) in the 1990s. Continuous glucose monitoring provides continuous access to real-time glucose data, information on the direction and rate of change of glucose. Some CGM devices may be used without the need for calibration or confirmatory capillary blood glucose for decision making. The accuracy of CGMs in the recent years has improved compared with previous iterations [9]. Factors contributing to CGM accuracy include its calibration and software algorithm, which improve the MARD (mean absolute relative difference) by filtering and smoothing the signal [10]. A lower % MARD corresponds to better sensor performance, with a MARD of under 10% representing sufficient accuracy for CGM data to make an insulin dosing decision [11]. The MARDs of commercially available, subcutaneous CGM systems at present range between 9.0 and 13.6% [12].
Continuous glucose monitoring devices are also equipped with real-time alerts and an alarm for impending hypo- and hyperglycaemia and can therefore be beneficial in individuals with T1D at high risk of hypoglycaemia, such as those with recurrent severe hypoglycaemia and hypoglycaemia unawareness [13]. With the development of continuous glucose sensors, CSII technology has evolved. Sensor-augmented pump (SAP) therapy, which combines the technology of an insulin pump with a continuous glucose monitoring sensor, was developed, and subsequently the ability to suspend insulin delivery when glucose is low (low-glucose suspend or LGS) or when glucose is predicted to become low (predicted low-glucose suspend or PLGS) was added.
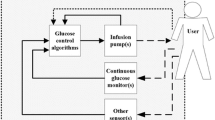
The next evolutionary step from a SAP with PLGS is the artificial pancreas (AP), also known as a closed-loop system or automated insulin delivery, which aims to mimic the endocrine function of a healthy pancreas for glucose homeostasis. The AP system incorporates a sensor for CGM, an insulin pump to deliver insulin and an algorithm connecting the two devices, which directs the pump to deliver insulin based on the real-time glucose readings from the sensor (Fig. 1). Several AP systems are currently in development at different stages [14]. Various aspects of the AP have been studied in clinical trials in the last decade, including its use in the outpatient and home setting, single vs. dual-hormone systems and its use in different cohorts. Recently, the US Food and Drug Administration approved the first hybrid AP system, the Medtronic 670G (Medtronic, Northridge, CA, USA), for use by people with T1D over 14 years of age [15]. It is also CE Mark approved for use in people with T1D over 7 years of age within Europe. The hybrid system is able to deliver and adjust basal insulin automatically without user input when used in the Auto Mode. However, the user must still manually deliver bolus insulin during meals.
This review article aims to explore the rationale behind the AP, discuss the development status of various closed-loop systems, including their benefits and limitations, report on their efficacy and tolerability in different cohorts, and explore the future outlook in the treatment of T1D. The search terms used to identify publications on PubMed included “artificial pancreas”, “closed-loop insulin” and combinations of these with “bi-hormonal”, “glucagon”, “pramlintide”, “GLP-1 agonist”, “pregnancy”, “type 2 diabetes”, “critically ill” and “psychosocial”.
2 Artificial Pancreas: How Does it Mimic a Pancreas?
The main hormones involved in the regulation of glucose homeostasis are insulin and glucagon. Insulin is synthesised and secreted by the pancreatic beta cells in response to rising glucose levels, and increases glucose uptake into skeletal muscle and fat, inhibiting gluconeogenesis, and stimulating glycogen synthesis. In contrast, glucagon is produced by the alpha cells of the islets of Langerhans and when secreted in response to low blood glucose levels, stimulates hepatic glycogenesis and activates gluconeogenesis. As with most hormonal feedback loops, the interaction between these hormones is constantly in motion and tightly regulated.
The ideal treatment for T1D would be an intervention that can mimic the glucose-regulating function of the pancreas. The development of an AP can be traced back to the 1960s when the possibility for external blood glucose regulation was established in studies in people with T1D using intravenous glucose measurement and infusions of insulin and glucose [16]. Multiple studies have since been conducted to develop a system that is portable, safe and efficient to use in the outpatient setting.
3 Control Algorithm
The control algorithm within an AP is arguably the most important piece of the system. Several control algorithms have been developed and studied, including model predictive control (MPC), proportional-integral-derivative (PID) control and fuzzy logic (FL) control. The former two approaches are more commonly used in clinical studies and the development of an AP [17].
The basic principles of the MPC approach involve a model, which is used to predict the outcome of control moves (insulin infusion) on future outputs (glucose) over a defined prediction horizon [18]. Model predictive control is a general control paradigm and is flexible, allowing it to be used in a dual-hormone AP [19].
The PID control algorithm was initially modelled on the pancreatic beta-cell response and is also referred to as physiological insulin delivery [20]. It calculates insulin delivery based on three set-points: (1) proportional: insulin delivery is adjusted in response to current measured glucose; (2) integral: insulin delivery adjusted corresponding to the area under the curve between measured and target glucose levels; (3) derivative: insulin is delivered based on the rate of change of glucose over time.
A randomised crossover study comparing personalised MPC and PID control algorithms in 30 participants was conducted for 27.5 h with an unannounced 65-g meal in a supervised outpatient suite. The results showed good overall performance in both groups. Model predictive control showed a significantly greater improvement in glucose control with a greater mean time in the range of 3.9–10.0 mmol/L (74.4 vs. 63.7%, p = 0.020), lower mean glucose during the entire trial duration (7.7 vs. 8.9 mmol/L, p = 0.012) and 5 h after the unannounced 65-g meal (10.1 vs. 12.2 mmol/L, p = 0.019). Percentage of time in hypoglycaemia (< 3.9 mmol/L) was minimal in both MPC (4.6%) and PID (2.9%) with no significant differences between the groups [19].
Other types of control algorithm used in studies of an AP include FL control and bio-inspired control algorithms. Although FL is not used as frequently as MPC or PID, its use has increased in recent years. The FL algorithm in an AP system modulates insulin delivery based on rules that attempt to replicate diabetes clinical practitioners [21]. A bio-inspired control algorithm is based on a mathematical model of pancreatic beta-cell physiology [22] but its use in AP studies has been limited thus far [23].
Most control algorithms include safety modules to constrain insulin delivery, limiting the amount of insulin on board or the maximum rate of insulin delivery, and suspending insulin delivery when glucose levels are low or decreasing [24]. Individual parameters that guide insulin delivery (such as basal rates of insulin, insulin: carbohydrate ratios and insulin sensitivity factor) are not fixed, but change over time in people with T1D. Some AP algorithms have been developed to incorporate adaptive features that enable automatic adjustment of basal insulin delivery and insulin: carbohydrate ratios/insulin sensitivity factor in response to changes seen in insulin sensitivity and post-prandial glucose responses. Different approaches to AP adaptation have been explored including the run-to-run approach [25].
4 Types of Closed-Loop Systems
The Artificial Pancreas Project launched by JDRF in 2006 developed a six-stage pathway that defines the different stages of development and types of AP, based on automation of insulin with or without glucagon delivery (Fig. 2).
4.1 Sensor-Augmented Pump with Automated Insulin Suspension
A sensor-augmented insulin pump combines the technology of CSII with a CGM sensor, which transmits the glucose readings to the insulin pump. This comprises steps 1–3 of the JDRF six-stage AP pathway, and includes LGS and PLGS systems.
An LGS system interrupts insulin delivery when the glucose level reaches a predefined threshold value (e.g. 4.0 mmol/L). Insulin delivery is suspended for 2 h if the user does not respond to the low-glucose alarm and resumes automatically after 2 h irrespective of the glucose level, although it can be restarted beforehand by the user. An example of this is the Medtronic MiniMed Paradigm VEO. In a study of 247 people with T1D comparing the use of a SAP with the threshold suspend feature with a standard sensor-augmented insulin-pump therapy over 3 months, the results showed a reduction in nocturnal hypoglycaemic events (by 31.8%) in the threshold-suspend group (1.5 ± 1.0 vs. 2.2 ± 1.3 per patient-week, p < 0.001) without an increase in HbA1c [26].
Subsequently, insulin pumps are available with a PLGS function that reduces or suspends insulin delivery when the glucose reading is predicted to be low. As well as reducing or suspending insulin delivery, PLGS also automatically resumes basal insulin infusion after up to 2 h in the absence of intervention. Insulin delivery can automatically restart after 30 min if the glucose level rises above a predefined threshold value. Randomised studies using PLGS in children, adolescents and adults compared to a SAP have demonstrated a reduction in hypoglycaemia without an increase in hyperglycaemia [27, 28] and no difference in HbA1c at 6 months [29].
Later generation systems in steps 4 and 5 comprise automated insulin-only delivery systems. Step 6 is a fully automated multi-hormone closed loop system, using glucagon in addition to insulin.
4.2 Insulin-Only Artificial Pancreas
An insulin-only system controls the glucose level by increasing or decreasing the amount of insulin infused based on CGM data. This includes hybrid systems that automatically adjust basal insulin with manual bolus insulin delivery at meal times managed by the user, and fully closed-loop systems that automatically adjust basal and prandial insulin.
Most of the AP systems being developed and investigated use a single-hormone (insulin-only) system. Insulin-only AP has been trialled in various settings including research facilities, diabetes camps and home conditions across different age populations from children to adults with type 1 diabetes.
Meta-analyses and systematic reviews on the use of AP in the adult and paediatric populations have shown a better mean glucose level [30], a higher percentage of time in the target range [31, 32] and reduced time in hypoglycaemia and hyperglycaemia [31]. A meta-analysis and systematic review on the use of an AP in 2018 included eight studies and 354 participants of which seven studies (318 participants) were based in the home setting. The AP significantly maintained a better mean glucose level (weighted mean difference [WMD] − 1.03, 95% confidence interval [CI] − 1.32 to − 0.75; p = 0.00001) compared with the control group over 24 h. Time spent in the hypoglycaemia state was also significantly lower (WMD − 1.23, 95% CI − 1.56 to − 0.91; p = 0.00001) [30].
These findings were supported by another meta-analysis on AP treatment for outpatients with T1D [31]. Forty randomised controlled trials of any AP systems (single and bi-hormonal) compared to any manual insulin treatment in non-pregnant outpatients with T1D were included. The use of both single and bi-hormonal APs is associated with a modest but significantly higher proportion of time in the target range (3.9–10.0 mmol/L) over 24 h. In particular, the single-hormone system favoured a higher percentage of time in target both overnight and over 24 h (WMD 12.77, 95% CI 9.82–15.71 and WMD 8.53, 95% CI 6.34–10.72, respectively). Time in hypoglycaemia (glucose < 3.9 mmol/L, WMD − 1.28, 95% CI − 1.65 to − 0.92) and hyperglycaemia (glucose > 10.0 mmol/L, WMD − 7.52, 95% CI − 10.38 to − 4.66) are also reduced.
In the paediatric population, a recent systematic review and meta-analysis examined 25 studies comparing AP and open-loop interventions for children with T1D. Twenty-one out of 25 studies were conducted in the outpatient setting. In a total of 305 paediatric participants with T1D, the percentage time in the target range was increased by approximately 12% in the AP group compared with SAP therapy [32]. The findings also showed that the closed-loop system was associated with significantly reduced percentage times in the hypoglycaemic and hyperglycaemic range (− 0.67% and − 3.01%, respectively).
4.2.1 Approved Hybrid Artificial Pancreas for Clinical Use
In 2017, the US Food and Drug Administration approved the use of the Medtronic MiniMed 670G system (Medtronic), the first commercially available, hybrid AP system in people aged 14 years and above with T1D. This approval was expanded in August 2018 to be used within an older paediatric group aged 7–13 years with T1D. More recently, it received CE Mark approval for use within the same age group in Europe. A hybrid AP is partially automated in that it only delivers basal insulin automatically and requires the user to manually input carbohydrate content into the bolus calculator for the insulin pump to deliver insulin at meal times.
The pivotal study evaluating the safety of a hybrid AP system in T1D included 123 people aged 14–75 years in ten investigational sites [15]. Each subject wore the system for 3.5 months in three study phases. Although there were no statistically powered endpoints in the study, it did demonstrate a reduction in mean HbA1c from 7.4% ± 0.9 to 6.9% ± 0.6 with an increase in the mean percentage of time in range (3.9–10.0 mmol/L) from 66.7% ± 12.2 to 72.2% ± 8.8. There were 24 severe hyperglycaemic events reported (defined in the protocol as a glucose level of > 16.7 mmol/L with blood ketones > 0.6 mmol/L or accompanied by symptoms of nausea, vomiting or abdominal pain). However, there were no reports of diabetic ketoacidosis or severe hypoglycaemic events.
The latest published study evaluating the safety of the Medtronic MiniMed 670G system in 105 children (ages 7–13 years) with T1D over 3 months showed that in-home use of this system was safe and associated with reduced HbA1c levels (from 7.9 ± 0.8 to 7.5 ± 0.6%, p < 0.001) and increased time in the target glucose range (from 56.2 ± 11.4 to 65.0 ± 7.7%, p < 0.001) compared with baseline [33].
Although hybrid AP systems are a significant advance in the development of an AP, they are not a fully closed-loop system. Barriers remaining to full automation include the slow pharmacokinetics of subcutaneous insulin, sensor accuracy and the impact of other factors such as activity. Further developments including the addition of glucagon, better accuracy of CGM and the availability of insulins with more rapid onsets of action have the potential to improve current AP systems [34].
4.3 Dual-Hormone Artificial Pancreas (Glucagon)
Glucagon is secreted from alpha cells of a healthy pancreas, and acts as a counter-regulatory hormone to insulin by elevating glucose levels through promotion of gluconeogenesis and glycogenolysis. Alpha-cell function and glucagon secretion are impaired in long-standing T1D. The use of glucagon in an AP is therefore logical with the aim to reduce the risk of hypoglycaemia.
Dual-hormone closed-loop systems have been extensively investigated in trials and have been shown to have better outcomes than a SAP, but have not clearly been demonstrated to be superior to an insulin-alone system. A randomised crossover trial involving 39 participants aged 18 years and above, assigned to glycaemic regulation with a bi-hormonal bionic pancreas or usual care (conventional or sensor-augmented insulin pump therapy) showed that bi-hormonal AP can be safely used at home. It also demonstrated a significantly lower mean CGM glucose level in the AP period (7.8 mmol/L, standard deviation [SD] 0.6) compared with the usual care period (9.0 mmol/L, SD 1.6) [difference 1.3 mmol/L, 95% CI 0.8–1.8; p < 0.0001] [35]. Additionally, as meal announcement was optional, therefore not requiring carbohydrate counting, this bi-hormonal AP could reduce part of the user burden associated with the management of diabetes.
A randomised crossover study in 19 children aged 6–11 years investigated the use of a bi-hormonal closed loop system vs. conventional insulin pump therapy in a diabetes camp setting. The study showed a better mean CGM-measured glucose level (7.6 mmol/L [SD 0.6] vs. 9.3 mmol/L [SD 1.7], p = 0.00037) and a lower proportion of time with CGM-measured hypoglycaemia (1.2% [SD 1.1] vs. 2.8% [SD 1.2], p < 0.0001) with the bi-hormonal AP system relative to insulin pump therapy [36]. In a recent randomised crossover study, better glucose control (mean percentage of time spent in the plasma glucose target range over 24 h: 63% [SD 18] vs. 62% [SD 18] in single-hormone AP vs. 51% [SD 19] in CSII) and a significant reduction of time spent in hypoglycaemia (episodes of hypoglycaemic events: 9 vs. 13 vs. 52, respectively) were observed with a dual-hormone closed loop system compared with a single-hormone closed-loop system and CSII [37]. The clinical significance of the time in the range difference is small and is achieved with a greater insulin infusion.
The use of single- and dual-hormone AP in exercise has also been investigated. Using wearable sensors to detect exercise, a dual-hormone AP was shown to have a lower mean time in hypoglycaemia during the exercise period (3.4% [SD 4.5]) compared with a single-hormone AP (8.3% [SD 12.6]; p = 0.009) and PLGS (7.6% [SD 8.0]; p < 0.001) [38].
Including glucagon within the AP system is aimed to theoretically reduce the incidence of hypoglycaemia. This is especially important in high-risk groups such as individuals with impaired awareness of hypoglycaemia, those who exercise frequently and very young children However, it comes with added complexity and with an additional cannula site. Currently available formulations of glucagon are also unstable and further development of a stable glucagon analogue is required. Table 1 summarises 24-h (day-and-night) AP studies performed in the home setting from 2016 onwards.
4.4 Intraperitoneal Delivery of Insulin in Artificial Pancreas Systems
To overcome the challenges associated with the pharmacokinetics of insulin delivered in the interstitial space, as well as the constraints of wearing an external device, an implantable AP system (based on intraperitoneal insulin delivery, a PID controller and a venous glucose sensor) was developed by Renard et al. [39]. In 2010, the same study group conducted a 2-day semi-automatic (pre-meal boluses of insulin given) closed-loop trial (n = 8) using a simpler system with a subcutaneous sensor, intraperitoneal insulin delivery and a PID algorithm, which showed that a higher percentage of time was spent in the study glucose target (4.4–6.6 mmol/L) during closed-loop vs. open-loop systems (39.1% vs. 27.7%, p = 0.05) [40]. More recently, a non-randomized 24-h sequential AP study (n = 10) comparing a subcutaneous AP system (using a fast-acting insulin analogue) with an intraperitoneal AP system (using regular insulin via the Diaport system) using an MPC algorithm was conducted. Percentage of time spent within the primary endpoint glucose target range (4.4–7.8 mmol/L) was significantly higher for intraperitoneal delivery than for subcutaneous delivery: 39.8 ± 7.6 vs. 25.6 ± 13.1 (p = 0.03) [41]. The evaluation of the first AP system integrating the Eversense implantable subcutaneous glucose sensor is planned as part of the International Diabetes Closed Loop trial, but outcome data have not yet been published.
5 Adjunctive Therapy in an Artificial Pancreas
Postprandial hyperglycaemia following an unannounced meal remains an issue with a single-hormone AP. This was demonstrated in a study involving ten adults and adolescents investigating the safety and performance of an AP system using a probabilistic estimation of meals to allow for automated meal detection [42]. Participants underwent daily exercise and meal challenges (announced and unannounced meals). The results showed that post-prandial hyperglycaemia was significantly more pronounced for unannounced meals compared with announced meals (4-h post-meal CGM: 11.0 mmol/L ± 2.5 mmol/L vs. 7.8 mmol/L ± 1.9 mmol/L, p < 0.001). This difference in post-prandial glucose with unannounced meals arises from the delay in subcutaneous insulin administration with a meal that is not announced to the controller, and the pharmacokinetics of subcutaneous insulin, which may not be well matched to the absorption of macronutrients.
5.1 Amylin Analogues
Amylin is a peptide produced in pancreatic beta cells and co-secreted with insulin. It affects glucose control by slowing gastric emptying, regulating postprandial glucagon, and reducing food intake [43] and is deficient in T1D [44]. Pramlintide is an amylin analogue that can be administered subcutaneously at meal times.
In a 52-week randomised study evaluating the use of pramlintide vs. placebo as an adjunct to insulin therapy in T1D, treatment with pramlintide led to a mean significant reduction in HbA1c from baseline to week 13 (0.67% vs. 0.16%, p < 0.001) without inducing weight gain or increasing the overall risk of severe hypoglycaemia [45].
Because of its effect in lowering post-prandial glucose excursions, and potentially eliminating the need for meal announcement, the use of pramlintide has been evaluated in AP clinical trials. One of the earlier studies showed that the use of pramlintide (30-µg pre-meal injections) in addition to AP was associated with an overall delayed time to peak blood glucose and a reduced magnitude of glycaemic excursion compared with a closed-loop system only [46].
A more recent clinical trial investigated the effects of adjunctive pramlintide with AP in ten participants over a 24-h period [47]. Pramlintide was shown to delay the time to peak plasma glucose excursion (AP 1.6 ± 0.5 hour vs. AP + pramlintide 2.6 ± 0.9 hour, p < 0.001). Pramlintide with AP was also associated with blunting of peak post-prandial increments in plasma glucose (p < 0.001) and reductions in post-meal incremental plasma glucose area under the curve (p = 0.0002).
Even though pramlintide is associated with side effects such as nausea, vomiting and abdominal bloating, the major barrier to its use with AP is the requirement for manual subcutaneous administration at meal times, which may increase the treatment burden of the AP. The effect of co-administration of insulin and pramlintide within an AP in T1D is currently being investigated.
5.2 Glucagon-Like Peptide-1 Receptor Agonists
Glucagon-like peptide-1 is an endogenous hormone that regulates secretion of insulin and glucagon in response to meals. It also slows gastric emptying, inhibits inappropriate post-meal glucagon release and reduces food intake. Glucagon-like peptide-1 receptor agonists are established treatment options in the type 2 diabetes management pathway [48]. Evidence for its use in T1D is limited. In general, HbA1c lowering with glucagon-like peptide-1 receptor agonists in T1D has been modest, with a relative decrease in HbA1c of 0.1–0.2% when tested against a control group [49]. However, it is associated with weight loss in all trials and may be considered in people with T1D who are overweight or obese.
The use of glucagon-like peptide-1 receptor agonists in an AP has been investigated. A randomised crossover trial comparing insulin monotherapy with adjuvant subcutaneous liraglutide 1.2 mg and insulin in an AP system was conducted in 15 participants. The liraglutide arm was associated with overall significantly decreased mean blood glucose levels and better 2-h post-breakfast and post-lunch glucose profiles [50]. There was no difference in hypoglycaemic episodes between the groups.
Similar results were seen in another study evaluating the use of adjunctive liraglutide with an AP [47]. Liraglutide with an AP was associated with marginal reductions in peak glucose excursions (p = 0.05) and incremental peak glucose area under the curve (p = 0.004). There was also a 26% reduction in total daily insulin dose (p = 0.05) and weight loss of 3.2 ± 1.8 kg (p = 0.003) in the liraglutide arm.
6 Artificial Pancreas Use in Specific Groups
6.1 Pregnancy
Type 1 diabetes in pregnancy is associated with an increased risk of foetal and maternal adverse outcomes including congenital malformations, miscarriage, preterm delivery, preeclampsia, macrosomia and perinatal mortality [51]. Maintaining tight glycaemic control during pregnancy minimises risk but can be challenging as insulin requirements increase during the later trimesters [52]. Hypoglycaemia can also occur more frequently during pregnancy [53].
In a randomised crossover study comparing an overnight AP with a SAP, 16 pregnant women with T1D were recruited. This was followed by a continuation phase in which an AP was used day and night. An overnight AP was shown to improve glucose control compared with a SAP (74.7% vs. 59.5% in the target range; p = 0.002). There were no significant differences in time spent in hypoglycaemia, insulin doses or in adverse-event rates [54]. Fourteen out of the 16 women also chose to continue using the AP up to an additional 14.6 weeks, including time during labour and delivery. During this period, glucose levels were in the target range 68.7% of the time with a mean glucose level of 7.0 mmol/L [54].
A more recent study by the same group looked at the longer term feasibility of day-and-night AP use. In this randomised crossover trial, 16 pregnant women completed 28 days of AP and SAP insulin delivery and were given the option to continue using the AP up to 6 weeks post-partum. An AP was associated with comparable glucose control and fewer hypoglycaemic episodes than SAP therapy (median 8.0 [range 1–17] vs. 12.5 [range 1–53] over 28 days, p = 0.04) [55].
6.2 Critical Care
Hyperglycaemia and insulin resistance are common in critically ill people, with or without diabetes. Intensive insulin therapy (glucose maintenance of between 4.4 and 6.1 mmol/L) in critical illness, even without previous diabetes, may reduce mortality during intensive care compared with conventional treatment [56]. However, subsequent studies have shown conflicting outcomes with intensive insulin therapy in the critical care setting, mostly reflecting an increased risk of hypoglycaemia [57]. The use of an AP to optimise glucose without hypoglycaemia has therefore been explored.
The effect of an AP device (STG-22; Nikkiso Co. Ltd, Tokyo, Japan) on the maintenance of blood glucose levels was investigated in 280 intensive care participants. The STG-22 system, which monitors blood glucose levels using a dual-lumen intravenous catheter and delivers insulin intravenously, was associated with maintenance of blood glucose between 3.9 and 10 mmol/L for 87.9% of the study period (33.9 ± 42.4 h), with no hypoglycaemic events [58].
The use of an AP, the majority of which use subcutaneous insulin delivery and interstitial glucose sampling, is currently limited in the critical care setting. This is owing to various limitations that may cause inaccuracies in glucose readings and affect insulin delivery in this cohort (e.g. oedema, vasoconstriction) [59].
The feasibility of an automated closed-loop therapy based on a subcutaneous CGM system compared to intravenous sliding-scale insulin in critically ill adults was evaluated [60]. The authors concluded that the AP system is safe and efficacious, and may improve glucose levels without increasing the risk of hypoglycaemia in this cohort.
6.3 Type 2 Diabetes
The efficacy and safety of an automated AP without meal-time boluses compared with conventional subcutaneous insulin therapy were assessed in 40 participants with type 2 diabetes in a non-critical care inpatient setting for a maximum of 72 h. The use of an AP in this setting was associated with a larger proportion of time spent in the target glucose range compared with the control (59.8% vs. 38.1%, p = 0.0004), with no episodes of severe hypoglycaemia or hyperglycaemia in either group [61].
Another study evaluated the feasibility of AP in insulin-naïve people with type 2 diabetes compared with conventional therapy with oral hypoglycaemic agents [62]. Their results showed a greater period of time spent in the target glucose range of 3.9–8.0 mmol/L (median 78 vs. 35%; p = 0.041) and a smaller period of time in hyperglycaemia (22 vs. 65%; p = 0.041) overnight.
This outcome was replicated in a recent two-centre randomised study that investigated the use of an AP vs. conventional subcutaneous insulin therapy in 136 adults with type 2 diabetes in general inpatient wards. The AP was shown to result in significantly better glycaemic control than conventional subcutaneous insulin therapy, without a higher risk of hypoglycaemia [63], clearly demonstrating the feasibility and potential effectiveness of AP in in-patients with diabetes. However, education and resource allocation need to be overcome to implement AP in this environment. The impact on clinical outcomes in this cohort has also not been demonstrated.
7 Psychosocial Aspects of Artificial Pancreas
It is recognised that psychosocial factors, encompassing environmental, social, behavioural and emotional factors, may affect people with diabetes and their outcomes. The American Diabetes Association recommends that psychosocial care should be integrated in patient-centred care and provided to all individuals with diabetes to optimise health outcomes and health-related quality of life [64].
As technology implementation in diabetes care continues to grow, so should assessments on the impact of these technologies on the psychosocial aspect of these individuals. A review reported that despite its association with body image and self-consciousness, insulin pump therapy is also associated with a high level of satisfaction, improved or similar levels of depression, reduced anxiety, and improved self-efficacy, family functioning and quality of life [65]. The use of CGM shows generally high levels of satisfaction and reduced fear of hypoglycaemia amongst its users [66]. However, poorer sleep and increased anxiety have also been reported in parents of children with T1D using CGM [67].
Users’ perception of an AP is generally positive, with perceived advantages of stable glucose regulation, less need for self-monitoring, relief of daily concerns and time saving [68]. In a study of overnight AP use within the home setting, adolescents with T1D reported a positive impact on their sleep, improved blood glucose control, and reduced parental fear of hypoglycaemia and anxiety. There are also negatives associated with an AP; these include practical difficulties with carrying and using several devices and feeling that the device controls one’s life [69]. Alarm fatigue has been shown to be an important negative factor that decreases adherence to AP systems [70]. Levels of reported trust in the AP also vary across studies [68]. This may in turn affect the level of anxiety of the user. Future longer term research exploring the use of an AP and its psychosocial impacts in different cohorts should be considered.
8 Challenges, Limitations and the Future of an Artificial Pancreas
The AP is regarded as cutting-edge technology in the management of T1D. Although the development of the AP system is progressing, there are challenges and limitations to current systems that need to be overcome before a fully automated AP can be achieved.
A long-standing concern in the development of an AP has been sensor performance. Continuous glucose monitoring works by measuring glucose levels in the interstitial fluid within the subcutaneous tissue. There is a physiological lag of glucose transport from the intravascular to interstitial fluid compartments, and therefore in CGM measurements. The lag time is at least 6–7 min but may be up to 10 min in people with type 1 diabetes [71, 72].
The pharmacokinetics of currently available, rapid-acting insulin analogues is relatively slow with onset within 10–15 min, and a prolonged duration of action, with time to maximal glucose excursion of 40–60 min and duration of action of 4–6 h [73]. This may limit control of rising glucose and avoidance of hypoglycaemia at times of rapidly changing glucose.
Faster acting insulin aspart (Fiasp; Novo Nordisk) is a newer formulation of insulin aspart with two additional formulation excipients, l-arginine and niacinamide [74]. Pharmacokinetic and pharmacodynamic studies comparing Fiasp and insulin aspart have shown a 5-min earlier onset of first appearance of insulin (4 vs. 9 min), approximately two times higher early insulin exposure and a 74% increased early glucose-lowering effect [75]. Data assessing Fiasp compared to insulin aspart in the closed-loop system (e.g. NCT03554486, NCT03579615, NCT03212950) are pending.
Newer oral anti-glycaemic agents such as sodium-glucose co-transporter inhibitors, which act by increasing renal glucose excretion, may have a potential adjunctive role in future AP trials aiming for improved post-prandial glucose control. The practicality of wearing and carrying several devices of a closed-loop system may be a hindrance to future use of the system. Dual-chamber pumps for the use of the dual-hormone closed-loop system are currently in development and may reduce the burden of wear for the users.
A recent critical review investigated potential ethically problematic situations arising through artificial pancreas use. These include confidentiality and data safety, cost coverage and insurability of care, patient selection, patient coaching and support, and personal identity and agency [76]. More consideration is needed to validate the ethical issues raised to improve our understanding of the implementation of the technology.
Despite these challenges, the future of the AP seems promising. Iterations of the hybrid closed-loop system are commercially available and are being improved. A randomised study using an enhanced performance version of the Medtronic hybrid algorithm including insulin bolus correction and improved automode parameters [77] reported improved time in the target glucose sensor range (3.9–10 mmol/L) with intervention compared with baseline values (74.32% ± 8.41% during study vs. 52.15% ± 9.55% at baseline, relative change 42%) and other systems are imminently available (Tandem Control-IQ).
9 Conclusions
In the future, further evaluation of faster acting insulin in the AP, increased accuracy and reduced lag time of CGM as well as self-learning adapting algorithms will improve the level of automation and effectiveness. Longer term home-setting studies using an AP, single or dual hormone, need to be conducted and extended into more targeted groups of people with T1D for us to understand its overall benefits and, importantly, cost effectiveness in the general population.
References
International Diabetes Federation. Diabetes facts and figures. 2017. https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed 8 June 2019.
Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686–93.
The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46(2):271–86.
Cryer PE. Hypoglycaemia: pathophysiology, diagnosis and treatment. Oxford: Oxford University Press; 1997.
Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765–74.
Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51(6):941–51.
Group RS. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ. 2017;356:j1285.
Facchinetti A. Continuous glucose monitoring sensors: past, present and future algorithmic challenges. Sensors (Basel). 2016;16:E2093.
Bailey TS. Clinical implications of accuracy measurements of continuous glucose sensors. Diabetes Technol Ther. 2017;19(S2):S51–4.
Kovatchev BP, Patek SD, Ortiz EA, Breton MD. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther. 2015;17(3):177–86.
Avari P, Reddy M, Oliver N. Is it possible to constantly and accurately monitor blood sugar levels, in people with type 1 diabetes, with a discrete device (non-invasive or invasive)? Diabet Med. 2019. https://doi.org/10.1111/dme.13942(Epub ahead of print).
van Beers CA, DeVries JH, Kleijer SJ, Smits MM, Geelhoed-Duijvestijn PH, Kramer MH, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893–902.
Trevitt S, Simpson S, Wood A. Artificial pancreas device systems for the closed-loop control of type 1 diabetes: what systems are in development? J Diabetes Sci Technol. 2016;10(3):714–23.
US Food and Drug Administration. Summary of safety and effectiveness data (SSED) of the Medtronic MiniMed 670G system. 2016. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160017b.pdf. Accessed 8 June 2019.
Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60(11):2672–82.
Doyle FJ 3rd, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37(5):1191–7.
Bequette BW. Algorithms for a closed-loop artificial pancreas: the case for model predictive control. J Diabetes Sci Technol. 2013;7(6):1632–43.
Pinsker JE, Lee JB, Dassau E, Seborg DE, Bradley PK, Gondhalekar R, et al. Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care. 2016;39(7):1135–42.
Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery: the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56(2):125–44.
Nimri R, Bratina N, Kordonouri O, Avbelj Stefanija M, Fath M, Biester T, et al. MD-Logic overnight type 1 diabetes control in home settings: a multicentre, multinational, single blind randomized trial. Diabetes Obes Metab. 2017;19(4):553–61.
Herrero P, Georgiou P, Oliver N, Johnston DG, Toumazou C. A bio-inspired glucose controller based on pancreatic beta-cell physiology. J Diabetes Sci Technol. 2012;6(3):606–16.
Reddy M, Herrero P, Sharkawy ME, Pesl P, Jugnee N, Pavitt D, et al. Metabolic control with the bio-inspired artificial pancreas in adults with type 1 diabetes: a 24-hour randomized controlled crossover study. J Diabetes Sci Technol. 2015;10(2):405–13.
Bally L, Thabit H, Hovorka R. Glucose-responsive insulin delivery for type 1 diabetes: the artificial pancreas story. Int J Pharm. 2018;544(2):309–18.
Toffanin C, Visentin R, Messori M, Palma FD, Magni L, Cobelli C. Toward a run-to-run adaptive artificial pancreas: in silico results. IEEE Trans Biomed Eng. 2018;65(3):479–88.
Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–32.
Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41(10):2155–61.
Calhoun PM, Buckingham BA, Maahs DM, Hramiak I, Wilson DM, Aye T, et al. Efficacy of an overnight predictive low-glucose suspend system in relation to hypoglycemia risk factors in youth and adults with type 1 diabetes. J Diabetes Sci Technol. 2016;10(6):1216–21.
Abraham MB, Nicholas JA, Smith GJ, Fairchild JM, King BR, Ambler GR, et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care. 2018;41(2):303–10.
Dai X, Luo ZC, Zhai L, Zhao WP, Huang F. Artificial pancreas as an effective and safe alternative in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2018;9(3):1269–77.
Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310.
Karageorgiou V, Papaioannou TG, Bellos I, Alexandraki K, Tentolouris N, Stefanadis C, et al. Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. Metabolism. 2019;90:20–30.
Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, et al. Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):11–9.
Stone JY, Haviland N, Bailey TS. Review of a commercially available hybrid closed-loop insulin-delivery system in the treatment of type 1 diabetes. Ther Deliv. 2018;9(2):77–87.
El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389(10067):369–80.
Russell SJ, Hillard MA, Balliro C, Magyar KL, Selagamsetty R, Sinha M, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233–43.
Haidar A, Legault L, Matteau-Pelletier L, Messier V, Dallaire M, Ladouceur M, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(8):595–604.
Castle JR, El Youssef J, Wilson LM, Reddy R, Resalat N, Branigan D, et al. Randomized outpatient trial of single- and dual-hormone closed-loop systems that adapt to exercise using wearable sensors. Diabetes Care. 2018;41(7):1471–7.
Renard E, Costalat G, Chevassus H, Bringer J. Closed loop insulin delivery using implanted insulin pumps and sensors in type 1 diabetic patients. Diabetes Res Clin Pract. 2006;74:S173–7.
Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010;33(1):121–7.
Dassau E, Renard E, Place J, Farret A, Pelletier MJ, Lee J, et al. Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study. Diabetes Obes Metab. 2017;19(12):1698–705.
Forlenza GP, Cameron FM, Ly TT, Lam D, Howsmon DP, Baysal N, et al. Fully closed-loop multiple model probabilistic predictive controller artificial pancreas performance in adolescents and adults in a supervised hotel setting. Diabetes Technol Ther. 2018;20(5):335–43.
Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev. 2015;67(3):564–600.
Hieronymus L, Griffin S. Role of amylin in type 1 and type 2 diabetes. Diabetes Educ. 2015;41(1 Suppl.):47s–56s.
Whitehouse F, Kruger DF, Fineman M, Shen L, Ruggles JA, Maggs DG, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25(4):724–30.
Weinzimer SA, Sherr JL, Cengiz E, Kim G, Ruiz JL, Carria L, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35(10):1994–9.
Sherr JL, Patel NS, Michaud CI, Palau-Collazo MM, Van Name MA, Tamborlane WV, et al. Mitigating meal-related glycemic excursions in an insulin-sparing manner during closed-loop insulin delivery: the beneficial effects of adjunctive pramlintide and liraglutide. Diabetes Care. 2016;39(7):1127–34.
Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;10:CD006423.
Janzen KM, Steuber TD, Nisly SA. GLP-1 agonists in type 1 diabetes mellitus. Ann Pharmacother. 2016;50(8):656–65.
Ilkowitz JT, Katikaneni R, Cantwell M, Ramchandani N, Heptulla RA. Adjuvant liraglutide and insulin versus insulin monotherapy in the closed-loop system in type 1 diabetes: a randomized open-labeled crossover design trial. J Diabetes Sci Technol. 2016;10(5):1108–14.
Casson IF, Clarke CA, Howard CV, McKendrick O, Pennycook S, Pharoah PO, et al. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ. 1997;315(7103):275–8.
Garcia-Patterson A, Gich I, Amini SB, Catalano PM, de Leiva A, Corcoy R. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia. 2010;53(3):446–51.
Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Damm P, Mathiesen ER. Hypoglycaemia during pregnancy in women with Type 1 diabetes. Diabet Med. 2012;29(5):558–66.
Stewart ZA, Wilinska ME, Hartnell S, Temple RC, Rayman G, Stanley KP, et al. Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med. 2016;375(7):644–54.
Stewart ZA, Wilinska ME, Hartnell S, O’Neil LK, Rayman G, Scott EM, et al. Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care. 2018;41(7):1391–9.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67.
Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–44.
Yatabe T, Yamazaki R, Kitagawa H, Okabayashi T, Yamashita K, Hanazaki K, et al. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med. 2011;39(3):575–8.
Salinas PD, Mendez CE. Glucose management technologies for the critically ill. J Diabetes Sci Technol. 2019. https://doi.org/10.1177/1932296818822838.
Leelarathna L, English SW, Thabit H, Caldwell K, Allen JM, Kumareswaran K, et al. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Crit Care. 2013;17(4):R159.
Thabit H, Hartnell S, Allen JM, Lake A, Wilinska ME, Ruan Y, et al. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 2017;5(2):117–24.
Kumareswaran K, Thabit H, Leelarathna L, Caldwell K, Elleri D, Allen JM, et al. Feasibility of closed-loop insulin delivery in type 2 diabetes: a randomized controlled study. Diabetes Care. 2014;37(5):1198–203.
Bally L, Thabit H, Hartnell S, Andereggen E, Ruan Y, Wilinska ME, et al. Closed-loop insulin delivery for glycemic control in noncritical care. N Engl J Med. 2018;379(6):547–56.
Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126–40.
Franklin V. Influences on technology use and efficacy in type 1 diabetes. J Diabetes Sci Technol. 2016;10(3):647–55.
Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabet Med. 2018;35(4):436–49.
Patton SR, Clements MA. Psychological reactions associated with continuous glucose monitoring in youth. J Diabetes Sci Technol. 2016;10(3):656–61.
van Bon AC, Kohinor MJ, Hoekstra JB, von Basum G, deVries JH. Patients’ perception and future acceptance of an artificial pancreas. J Diabetes Sci Technol. 2010;4(3):596–602.
Barnard KD, Wysocki T, Allen JM, Elleri D, Thabit H, Leelarathna L, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care. 2014;2(1):e000025.
Barnard KD, Wysocki T, Thabit H, Evans ML, Amiel S, Heller S, et al. Psychosocial aspects of closed- and open-loop insulin delivery: closing the loop in adults with type 1 diabetes in the home setting. Diabet Med. 2015;32(5):601–8.
Christiansen SC, Fougner AL, Stavdahl O, Kolle K, Ellingsen R, Carlsen SM. A review of the current challenges associated with the development of an artificial pancreas by a double subcutaneous approach. Diabetes Ther. 2017;8(3):489–506.
Basu A, Dube S, Veettil S, Slama M, Kudva YC, Peyser T, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9(1):63–8.
Home PD. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14(9):780–8.
Akturk HK, Rewers A, Joseph H, Schneider N, Garg SK. Possible ways to improve postprandial glucose control in type 1 diabetes. Diabetes Technol Ther. 2018;20(S2):S224–32.
Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56(5):551–9.
Quintal A, Messier V, Rabasa-Lhoret R, Racine E. A critical review and analysis of ethical issues associated with the artificial pancreas. Diabetes Metab. 2019;45(1):1–10.
de Bock M, Dart J, Hancock M, Smith G, Davis EA, Jones TW. Performance of Medtronic hybrid closed-loop iterations: results from a randomized trial in adolescents with type 1 diabetes. Diabetes Technol Ther. 2018;20(10):693–7.
Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, et al. Home use of day-and-night hybrid closed-loop insulin delivery in very young children: a multicenter, 3-week, randomized trial. Diabetes Care. 2019;42(4):594–600.
Deshpande S, Pinsker JE, Zavitsanou S, Shi D, Tompot R, Church MM, et al. Design and clinical evaluation of the Interoperable Artificial Pancreas System (iAPS) Smartphone App: interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol Ther. 2019;21(1):35–43.
Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321–9.
Benhamou PY, Huneker E, Franc S, Doron M, Charpentier G. Customization of home closed-loop insulin delivery in adult patients with type 1 diabetes, assisted with structured remote monitoring: the pilot WP7 Diabeloop study. Acta Diabetol. 2018;55(6):549–56.
Biester T, Nir J, Remus K, Farfel A, Muller I, Biester S, et al. DREAM5: an open-label, randomized, cross-over study to evaluate the safety and efficacy of day and night closed-loop control by comparing the MD-Logic automated insulin delivery system to sensor augmented pump therapy in patients with type 1 diabetes at home. Diabetes Obes Metab. 2018. https://doi.org/10.1111/dom.13585(Epub ahead of print).
Bally L, Thabit H, Kojzar H, Mader JK, Qerimi-Hyseni J, Hartnell S, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017;5(4):261–70.
Forlenza GP, Deshpande S, Ly TT, Howsmon DP, Cameron F, Baysal N, et al. Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: a randomized crossover-controlled home-use trial. Diabetes Care. 2017;40(8):1096–102.
DeBoer MD, Breton MD, Wakeman C, Schertz EM, Emory EG, Robic JL, et al. Performance of an artificial pancreas system for young children with type 1 diabetes. DiabetesTechnol Ther. 2017;19(5):293–8.
Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–63.
Haidar A, Messier V, Legault L, Ladouceur M, Rabasa-Lhoret R. Outpatient 60-hour day-and-night glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or sensor-augmented pump therapy in adults with type 1 diabetes: an open-label, randomised, crossover, controlled trial. Diabetes Obes Metab. 2017;19(5):713–20.
Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2016;39(7):1168–74.
Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, et al. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: a 3-week, free-living, randomized crossover trial. Diabetes Care. 2016;39(11):2019–25.
Renard E, Farret A, Kropff J, Bruttomesso D, Messori M, Place J, et al. Day-and-night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: results of a single-arm 1-month experience compared with a previously reported feasibility study of evening and night at home. Diabetes Care. 2016;39(7):1151–60.
Acknowledgements
Infrastructure support is provided by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre and the NIHR Imperial Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Rozana Ramli has no conflicts of interest that are directly relevant to the content of this article. Monika Reddy has received research funding towards an investigator-initiated study from Dexcom, and has participated in advisory boards for Roche Diabetes. Nick Oliver has received research funding towards investigator-initiated studies from Dexcom, and has participated in advisory boards for Roche Diabetes, Dexcom and Medtronic Diabetes.
Rights and permissions
About this article
Cite this article
Ramli, R., Reddy, M. & Oliver, N. Artificial Pancreas: Current Progress and Future Outlook in the Treatment of Type 1 Diabetes. Drugs 79, 1089–1101 (2019). https://doi.org/10.1007/s40265-019-01149-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01149-2