Abstract
Phenylalanine hydroxylase (PAH) deficiency is an inborn error of metabolism that results in elevated phenylalanine levels in blood. The classical form of the disease with phenylalanine level > 1200 µmol/L in blood is called phenylketonuria (PKU) and is associated with severe intellectual disability when untreated. In addition, phenylalanine levels above the therapeutic range in pregnant female patients lead to adverse fetal effects. Lowering the plasma phenylalanine level prevents intellectual disability, maintaining the level in the therapeutic range of 120–360 µmol/L is associated with good outcome for patients as well as their pregnancies. Patient phenotypes are on a continuous spectrum from mild hyperphenylalaninemia to mild PKU, moderate PKU, and severe classic PKU. There is a good correlation between the biochemical phenotype and the patient’s genotype. For over four decades the only available treatment was a very restrictive low phenylalanine diet. This changed in 2007 with the approval of cofactor therapy which is effective in up to 55% of patients depending on the population. Cofactor therapy typically is more effective in patients with milder forms of the disease and less effective in classical PKU. A new therapy has just been approved that can be effective in all patients with PAH deficiency regardless of their degree of enzyme deficiency or the severity of their phenotype. This article reviews the mainstay therapy, adjunct enzyme cofactor therapy, and the newly available enzyme substitution therapy for hyperphenylalaninemia. It also provides an outlook on emerging approaches for hyperphenylalaninemia treatment such as recruiting the microbiome into the therapeutic endeavor as well as therapies under development such as gene therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
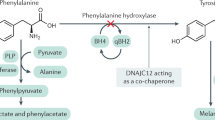
Phenylketonuria (PKU) is the most common inborn error of amino acid metabolism. It is due to either a deficiency of phenylalanine hydroxylase (PAH), the enzyme that converts phenylalanine (Phe) to tyrosine (Tyr), or a deficiency of its cofactor, tetrahydrobiopterin (BH4), which is needed for normal enzyme function [1]. PAH deficiency results in elevated blood phenylalanine levels. When untreated, classical PKU (defined as an untreated Phe level > 1200 µmol/L due to mutations in the PAH gene that result in complete enzyme deficiency [2]) leads to severe intellectual disability. PKU is the paradigm for treatment of a metabolic disease with a special medical food deficient in the offending substance, in PKU this means a medical formula devoid of phenylalanine. The low Phe diet for PKU was developed in the mid-1950s [3] and it became immediately clear that early implementation, before the onset of symptoms, would be needed to allow for the patient to have the full benefit of the treatment. This led to the development of newborn screening for PKU in the 1960s [4]. Newborn screening now leads to the diagnosis of almost all PKU patients at the time of birth, which allows institution of therapy prior to development of any intellectual compromise. Until 2007, early detection and dietary management were the only treatment modalities available for PKU. Successful treatment of young females with classical PKU led to the unexpected finding that exposure of a developing fetus in pregnant women with poorly controlled PKU caused a typical syndrome of microcephaly, cardiac defects, and intellectual deficiency in the baby, now called maternal PKU syndrome [5].
2 Pathophysiology
The pathophysiology of PKU has been the subject of much debate since discovery of the disease. Early attention was on the possible toxic effect of the phenylketones (Phe metabolites that accumulate in patients) or phenylalanine on the brain. Ultimately, IQ was found to be more significantly associated with age at treatment onset and blood Phe levels during treatment [6], while the level of urine phenylketones was only weakly associated with the clinical presentation [7]. These findings were supported by the growing recognition that lowering the blood Phe level below 600 µmol/L resulted in normal intelligence. The pathophysiology of the intellectual disability caused by elevated blood Phe levels remains controversial, with hypotheses ranging from competitive inhibition of the transport of other large neutral amino acids at the blood brain barrier by high Phe levels, to tyrosine deficiency leading to disruption of the synthesis of important neurotransmitters. Regardless of the mechanism of central nervous system damage, meta-analysis of a large body of published data has revealed that maintaining blood Phe between 120 and 360 µmol/L is associated with optimal clinical outcome. Thus, maintaining the blood Phe level in this range remains the primary object of all treatment modalities [8].
3 Available Treatments
To date, three different treatments are available and three more are under development to lower the Phe level and keep it in the treatment range (Fig. 1). Currently, monotherapy (low Phe diet) or, increasingly, a combination of two of these therapies (low Phe diet and chaperone therapy or low Phe diet and enzyme substitution therapy) is used to control the blood PHE level of a given patient. Infrequently does currently available drug therapy allow for the discontinuation of the low Phe diet.
3.1 Low Phenylalanine Diet
The goal of the diet is to provide enough natural protein for the patient to be healthy and grow normally with sufficient restriction to keep blood Phe in the treatment range. This goal cannot be accomplished without medical food restricted in Phe. One of the earliest and still most commonly used forms of medical food, is a phenylalanine-free amino acid mixture, usually balanced with other nutrients, taken in the form of a drink. Low protein foods that allow for more dietary variety are also available and, in combination, these dietary options serve to satisfy patient hunger, promote health and well-being, and allow normal growth. Dietary Phe tolerance in patients with classical PKU varies by age. For example, a young infant (0–6 months of age) with PKU may tolerate 20–70 mg Phe/kg/day [9] and 0.4–1.4 g natural protein per kg/day, while an older child (> 5 years of age) tolerates < 20 mg Phe/kg/day [10], 0.4 g natural protein per kg/day. A typical adult with PKU tolerates 350 − 1200 mg Phe, equivalent to 7–24 g of natural protein per day [11]. These small amounts of natural protein tolerated before the blood Phe level will rise, vary significantly from the protein content of a standard diet. The utmost self-discipline is demanded from the patient to remain adherent, and diet compliance constitutes a daily struggle for many patients. Additionally, while the diet has proven efficacy in preventing intellectual disability and the maternal PKU syndrome, most individuals with PKU gradually adhere less strictly to the low Phe diet in adolescence and adulthood, resulting in increasing Phe levels [12] and a range of neuropsychiatric problems including depression, anxiety, and poor executive functioning. These problems can be ameliorated with a return to Phe control, an extremely difficult prospect in older patients. Addition of large neutral amino acids (LNAAs) to the diet, may reduce Phe levels in brain by competitively inhibiting Phe transport at the blood brain barrier; however, this may only be a short-term effect of LNAA supplementation [13].
3.2 Sapropterin Dihydrochloride as Adjunct Therapy to a Low Phenylalanine Diet
From its discovery until 2007, a low phenylalanine diet was the only effective modality available for the treatment of classical PKU. This changed significantly in the USA with the FDA approval in 2007 of sapropterin dihydrochloride. Sapropterin is a synthetic form of tetrahydrobiopterin (BH4), the natural co-factor of phenylalanine hydroxylase. Sapropterin has since been approved in multiple other countries. Rare patients with elevated Phe levels due to a defect in the generation of the PAH cofactor BH4 had been described since the 1970s [14, 15]. Treatment of these patients with a synthetic formulation of the PAH biopterin cofactor (sapropterin) normalized Phe in these patients.
However, it took the serendipitous discovery of an accidental overdose of sapropterin during routine testing of a PKU patient for cofactor deficiency [16] to reveal that sapropterin could also function as a chaperone that stabilizes or activates an unstable mutant PAH enzyme with resultant lowering of blood Phe and the possibility of diet relaxation.
The first clinical trial of sapropterin demonstrated that 20% of PKU patients showed a 30% reduction in Phe level after 8 days on 10 mg/kg/day of sapropterin [17]. In a follow-up study, 56% of patients showed a 30% reduction in Phe level after 8 days on 20 mg/kg/day of sapropterin. [18] In patients who responded to sapropterin with a decrease of their Phe level, a low Phe diet is typically still necessary but their Phe tolerance is improved allowing them to eat more natural protein-containing foods.
Sapropterin was FDA approved in 2007 for adjunct treatment in addition to diet in patients with hyperphenylalaninemia due to tetrahydrobiopterin-responsive PKU. Patients with milder forms of PKU, i.e. with some amount of residual enzyme activity, are more likely to respond to sapropterin treatment because they have mutant enzyme that can be stabilized. Thus, the percentage of patients who respond to sapropterin will differ depending on the population and its specific complement of PAH gene mutations. A population where a pathogenic variant that abolishes enzyme activity is common, will have fewer tetrahydrobiopterin responders because patients with those mutations have no enzyme to stabilize [19]. In very heterogeneous populations such as in the USA [20], one can expect a higher percentage of tetrahydrobiopterin responders. Response to sapropterin is not always predicted by the genotype and generally should be assessed by formal testing.
The most common side effects of sapropterin (differing from placebo and in at least 4% of patients) at doses of 10–20 mg/kg/day consist of rhinorrhea, pharyngolaryngeal pain, diarrhea, cough, nasal congestion (first documented during double-blind, placebo-controlled clinical trials). In addition to these side effects, 25% of PKU patients aged 1 month to 6 years receiving 20 mg/kg/day of sapropterin had Phe levels below the normal range [17].
Sapropterin is a pregnancy ‘Category C’ drug [21]. In rabbits, administration of high-dose sapropterin during embryogenesis was associated with a non-statistically significant increase in the incidence of holoprosencephaly in two high-dose-treated litters (four fetuses), compared to one control-treated litter (one fetus) using a higher dose than that administered to humans [17]. The safety of the use of sapropterin in humans during pregnancy is under investigation through a patient registry (Maternal Phenylketonuria Observational Program (PKU MOMS) sub-registry [21]. By the middle of 2013, the registry had collected data from 16 pregnancies where the mother took sapropterin during pregnancy and 5 pregnancies where the mother had taken sapropterin before pregnancy. Two spontaneous abortions occurred in each of these groups. All 4 spontaneous abortions occurred between 8 and 10 weeks’ gestation. Three of the four women had also other risk factors. One spontaneous abortion and one case of premature labor were judged as possibly related to sapropterin. In the offspring, one case of feeding difficulties was judged as possibly related. On balance, because the severe embryo- and fetopathy caused by elevated Phe levels is well established, the authors recommended sapropterin use should be considered during pregnancy in women previously demonstrated to be sapropterin responders who showed poor metabolic control on diet alone.
3.3 Phenylalanine Ammonia Lyase (PAL)
The recognition of the ability of phenylalanine ammonia lyase (PAL) to metabolize phenylalanine (to trans-cinnamic acid and ammonia) and its potential utility in the treatment of PKU was first reported in 1980 [22] and ultimately led to proof of concept experiments in PAH-deficient mice [23]. PAL represents a new category of enzyme replacement therapy in which the deficient enzyme (PAH) is substituted by another enzyme that can replace some of its function. PAL was an elegant solution to overcome many of the functional hurdles faced in the use of PAH itself. PAH is a very unstable enzyme that is difficult to express and purify in vitro and requires the presence of its biopterin cofactor for function. PAL, in turn, was much more amenable to drug development as it proved more stable and did not require a cofactor for activity. Following initial efforts at developing an orally administered PAL, it was demonstrated that an intraperitoneal injection of PAL in the PKU mouse model decreased the phenylalanine level in a dose-responsive fashion, and that the effect lasted for 24 hours [23]. When developed for human use, the enzyme was modified by addition of polyethylene glycol (PEGylation) for protection from the host immune response and protease resistance. Subcutaneous injection led to a significant decrease in plasma and brain Phe levels [24]. The PEGylated form of PAL was called PEG-PAL and eventually pegvaliase. The safety and efficacy of pegvaliase has been assessed in four Phase 2 clinical trials, and two Phase 3 trials (PRISM-1 and -2 [25,26,27]. PRISM-2 included an open-label, long-term extension study.
Patients treated over a 12-month period showed a 51% decrease and patients treated 24 months showed a 69% decrease in Phe level from baseline [26]. Over a 24-month period, 68% of patients reached plasma Phe levels of ≤ 600 μmol/L, 61% levels of ≤ 360 μmol/L, and 51% of patients’ levels ≤ 120 μmol/L with 360 μmol/L being the upper limit and 120 μmol/L the lower limit of the recommended treatment range [26]. In the long-term extension study, 80% of patients reached Phe levels ≤ 360 μmol/L, and 82.5% ≤ 600 μmol/L, while 79% had plasma Phe levels ≤ 120 μmol/L [27]. There was an improvement in investigator-rated ADHD scores associated with decrease in Phe levels – the significance of this finding was not reported.
The safety profile of pegvaliase is not without concern. PAL metabolizes Phe to trans-cinnamic acid and a small amount of ammonia. In animal experiments trans-cinnamic acid did not result in birth defects or other damage in embryos or fetuses. It is metabolized to benzoate, conjugated with glycine, which facilitates its excretion in the urine as hippurate [28]. However, in the clinical trials, every participant had an adverse event (AE), most frequent of which were arthralgia (65.0%), injection-site erythema (67.5%), headache (67.5%), and injection-site reaction (72.5%); 76.3% of AEs were moderately severe and 11.3% severe [27]. Severe AEs were anaphylactic or acute systemic hypersensitivity reactions (3.8% and 4.6%, respectively [26]). The frequency of adverse reactions decreased over time, being more frequent in the first 6 months on drug and the frequency was similar for different dosages used.
Pegvaliase (trade name Palynziq®) was approved in the USA by the FDA in May of 2018 for treatment of adult patients with uncontrolled blood Phe concentrations on current treatment, and the prescribing information [29] notes that “anaphylaxis has been reported after administration of pegvaliase and may occur at any time during treatment”. Minor allergic reactions can be mitigated by pretreatment with antihistamines, and acute, serious anaphylaxis reactions can be managed with steroid therapy. Hypersensitivity reactions and anaphylaxis have been observed and may be worse in patients who concomitantly receive other PEGylated products [26]. Use of pegvaliase in pregnancy has not been studied. When starting a woman of childbearing age on pegvaliase, the treating physician needs to consider that a woman receiving pegvaliase will be on a very liberalized diet. Because the safety of pegvaliase during pregnancy is unknown, treatment of the drug may need to cease, and dietary treatment be resumed in pregnant women. In addition to the significant difficulty with compliance that this creates, it would require significant time to bring about, which increases the risk of high blood Phe during the critical first weeks of pregnancy.
4 Emerging Approaches
As the most common inborn error of metabolism identified by newborn screening, PKU is attracting multiple new options for treatment. Most of these programs are either still in preclinical phases with only animal data published, and/or only online information is available at websites such as clinicaltrials.gov. Gene therapy, which has long been the holy grail for treatment of inborn errors of metabolism, has proven successful in mouse models [30]. At least two gene therapy trials are in development according to pharmaceutical company websites, but to date, none have, as yet dosed patients. A variation on gene therapy uses powerful gene editing techniques (Crispr/Cas9 or TALENS) to repair common mutations or more accurately insert an active PAH gene into “safe” areas of the genome. Use of an expressible synthetic RNA for PAH given intravenously or subcutaneously and targeted to the liver, is in development, but again, not with human subjects. Manipulation of the microbiome through a probiotic organism programmed to digest Phe in the gut has been tested in healthy volunteers, but not yet given to PKU patients.
5 Conclusions
Identification of PKU through newborn screening and treatment from birth remains a major public health success story and has spawned an expanding menu of diseases detected at birth. While early dietary treatment with or without the addition of sapropterin has overcome the most devastating problems associated with classical PKU, the clinical impact of the disease is far from eliminated. Rising Phe levels in older children and adults related to the difficulty of adhering to a strict diet, create additional problems in these patients. While enzyme substitution therapy with pegvaliase represents a breakthrough therapy, significant problems with its use make development of other therapies a high priority. Nucleic acid therapy (therapeutic mRNA or gene therapy) is likely to provide longer term solutions with few side effects. However, the cost of such therapies represents a new challenge that healthcare systems will need to address. These issues notwithstanding, the future is bright for continued improvement in PKU therapies.
References
Kaufman S. Phenylketonuria: biochemical mechanisms, 1–32. In: Agranoff BW, Aprison MH (eds): Adv Neurochem, 1976, vol 2. Plenum Press, New York.
Camp KM, Parisi MA, Acosta PB, Berry GT, Bilder DA, Blau N, Bodamer OA, Brosco JP, Brown CS, Burlina AB, Burton BK, Chang CS, Coates PM, Cunningham AC, Dobrowolski SF, Ferguson JH, Franklin TD, Frazier DM, Grange DK, Greene CL, Groft SC, Harding CO, Howell RR, Huntington KL, Hyatt-Knorr HD, Jevaji IP, Levy HL, Lichter-Konecki U, Lindegren ML, Lloyd-Puryear MA, Matalon K, MacDonald A, McPheeters ML, Mitchell JJ, Mofidi S, Moseley KD, Mueller CM, Mulberg AE, Nerurkar LS, Ogata BN, Pariser AR, Prasad S, Pridjian G, Rasmussen SA, Reddy UM, Rohr FJ, Singh RH, Sirrs SM, Stremer SE, Tagle DA, Thompson SM, Urv TK, Utz JR, van Spronsen F, Vockley J, Waisbren SE, Weglicki LS, White DA, Whitley CB, Wilfond BS, Yannicelli S, Young JM. Phenylketonuria Scientific Review Conference: state of the science and future research needs. Mol Genet Metab. 2014;112:87–122 (PMID: 24667081).
Bickel H, Gerrard J, Hickmans EM. Influence of phenylalanine intake on phenylketonuria. Lancet. 1953;265(6790):812–3.
Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43.
Mabry CC, Denniston JC, Nelson TL, Son CD. Maternal phenylketonuria. A cause of mental retardation in children without the metabolic defect. N Engl J Med. 1963;269:1404–8.
Williamson ML, Koch R, Azen C, Chang C. Correlates of intelligence test results in treated phenylketonuric children. Pediatrics. 1981;68(2):161–7.
Kaufman S. An evaluation of the possible neurotoxicity of metabolites of phenylalanine. J Pediatr. 1989;114:895–900.
Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, Mitchell J, Smith WE, Thompson BH, Berry SA, American College of Medical Genetics and Genomics Therapeutics Committee. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188–200 (Erratum in: Genet Med. 2014;16(4):356.).
Acosta P, Yannicelli S. in The Ross Metabolic Formula System Nutrition Support Protocols. ed 4. Columbus: Ross Products Division/Abbot Laboratories; 2001. Protocol 1 – phenylketonuria (PKU).
Güttler F, Wamberg E. On indications for treatment of the hyperphenylalaninemic neonate. Acta Paediatr Scand. 1977;66(3):339–44.
MacLeod EL, Gleason ST, van Calcar SC, Ney DM. Reassessment of phenylalanine tolerance in adults with phenylketonuria is needed as body mass changes. Mol Genet Metab. 2009;98(4):331–7.
Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health. 2004;16(1):41–5 (PMID: 15148857).
Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest. 1999;103(8):1169–78.
Bartholomé K, Byrd DJ, Kaufman S, Milstien S. Atypical phenylketonuria with normal phenylalanine hydroxylase and dihydropteridine reductase activity in vitro. Pediatrics. 1977;59(5):757–61.
Schaub J, Däumling S, Curtius HC, Niederwieser A, Bartholomé K, Viscontini M, Schircks B, Bieri JH. Tetrahydrobiopterin therapy of atypical phenylketonuria due to defective dihydrobiopterin biosynthesis. Arch Dis Child. 1978;53(8):674–6.
Trefz FK, Aulela-Scholz C, Blau N. Successful treatment of phenylketonuria with tetrahydrobiopterin. Eur J Pediatr. 2001;160(5):315.
Kuvan™ (Sapropterin Dihydrochloride) prescribing information, BioMarin Pharmaceutical Inc., Novato, CA, 2013. (v5/2015, revised: 07/2015).
Trefz FK, Burton BK, Longo N, Casanova MM, Gruskin DJ, Dorenbaum A, Kakkis ED, Crombez EA, Grange DK, Harmatz P, Lipson MH, Milanowski A, Randolph LM, Vockley J, Whitley CB, Wolff JA, Bebchuk J, Christ-Schmidt H, Hennermann JB, Sapropterin Study G. Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J Pediatr. 2009;154:700–7.
Blau N, Hennermann JB, Langenbeck U, Lichter-Konecki U. Diagnosis, classification, and genetics of phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol Genet Metab. 2011;104:S2–9 (PMID: 21937252).
Guldberg P, Levy HL, Hanley WB, Koch R, Matalon R, Rouse BM, Trefz F, de la Cruz F, Henriksen KF, Güttler F. Phenylalanine hydroxylase gene mutations in the United States: report from the Maternal PKU Collaborative Study. Am J Hum Genet. 1996;59(1):84–94.
Grange DK, Hillman RE, Burton BK, Yano S, Vockley J, Fong CT, Hunt J, Mahoney JJ, Phenylketonuria Demographics Outcomes and Safety (PKUDOS) registry; Maternal Phenylketonuria Observational Program (PKU MOMS) sub-registry. Cohen-Pfeffer JL Sapropterin dihydrochloride use in pregnant women with phenylketonuria: an interim report of the PKU MOMS sub-registry. Mol Genet Metab. 2014;112(1):9–16. https://doi.org/10.1016/j.ymgme.2014.02.016 (Epub 2014 Mar 12).
Hoskins JA, Jack G, Wade HE, Peiris RJ, Wright EC, Starr DJ, Stern J. Enzymatic control of phenylalanine intake in phenylketonuria. Lancet. 1980;1(8165):392–4.
Sarkissian CN, Shao Z, Blain F, Peevers R, Su H, Heft R, Chang TM, Scriver CR. A different approach to treatment of phenylketonuria: phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc Natl Acad Sci USA. 1999;96(5):2339–44 (PMID: 10051643).
Sarkissian CN, Gámez A. Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now? Mol Genet Metab. 2005;86(Suppl 1):S22-6 (Epub 2005 Sep 13. Review).
Zori R, Thomas JA, Shur N, Rizzo WB, Decker C, Rosen O, Li M, Schweighardt B, Larimore K, Longo N. Induction, titration, and maintenance dosing regimen in a phase 2 study of pegvaliase for control of blood phenylalanine in adults with phenylketonuria. Mol Genet Metab. 2018;125:217–27 (PMID: 30146451).
Thomas J, Levy H, Amato S, Vockley J, Zori R, Dimmock D, Harding CO, Bilder DA, Weng HH, Olbertz J, Merilainen M, Jiang J, Larimore K, Gupta S, Gu Z, Northrup H, PRISM investigators. Pegvaliase for the treatment of phenylketonuria: Results of a long-term phase 3 clinical trial program (PRISM). Mol Genet Metab. 2018;124(1):27–38 (PMID: 29653686).
Longo N, Zori R, Wasserstein MP, Vockley J, Burton BK, Decker C, Li M, Lau K, Jiang J, Larimore K, Thomas JA. Long-term safety and efficacy of pegvaliase for the treatment of phenylketonuria in adults: combined phase 2 outcomes through PAL-003 extension study. Orphanet J Rare Dis. 2018;13:108 (PMID: 29973227).
Sarkissian CN, Gámez A, Wang L, Charbonneau M, Fitzpatrick P, Lemontt JF, Zhao B, Vellard M, Bell SM, Henschell C, Lambert A, Tsuruda L, Stevens RC, Scriver CR. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc Natl Acad Sci USA. 2008;105(52):20894–9. https://doi.org/10.1073/pnas.0808421105.
Palynziq prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761079s000lbl.pdf. Accessed 9 April 2018.
Harding CO, Gillingham MB, Hamman K, Clark H, Goebel-Daghighi E, Bird A, Koeberl DD. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006;13:457–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No financial assistance was obtained to prepare this publication.
Conflict of interest
ULK and JV have participated in clinical trials sponsored by BioMarin Pharmaceuticals, manufacturer of sapropterin and pegvaliase. JV is funded for research on PKU by the National Institutes of Health. JV has served as a consultant for Homology Pharmaceuticals, Moderna Pharmaceuticals, Synlogic Pharmaceuticals, ATG gene therapies, and Kaleido Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Lichter-Konecki, U., Vockley, J. Phenylketonuria: Current Treatments and Future Developments. Drugs 79, 495–500 (2019). https://doi.org/10.1007/s40265-019-01079-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01079-z