Abstract
The MEK inhibitor cobimetinib (Cotellic®) is indicated for the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma, in combination with the BRAF inhibitor vemurafenib (Zelboraf®). In the pivotal coBRIM trial, previously untreated patients with BRAF V600 mutation-positive unresectable, stage IIIC or stage IV melanoma received cobimetinib 60 mg once daily for the first 21 days of each 28-day cycle plus vemurafenib 960 mg twice daily or vemurafenib alone. Compared with vemurafenib alone, cobimetinib plus vemurafenib significantly prolonged progression-free survival (primary endpoint) and was associated with a significantly higher overall response rate and significantly prolonged overall survival. Cobimetinib plus vemurafenib had a manageable tolerability profile. In conclusion, cobimetinib plus vemurafenib is a valuable option for use in BRAF V600 mutation-positive unresectable or metastatic melanoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
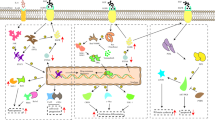
The MEK inhibitor cobimetinib and the BRAF inhibitor vemurafenib target different points on the MAPK signalling pathway. |
In the coBRIM trial in previously untreated patients with unresectable, stage IIIC or stage IV BRAF V600 mutation-positive melanoma, cobimetinib plus vemurafenib significantly prolonged progression-free survival (primary endpoint) and overall survival, compared with vemurafenib alone. |
In coBRIM, overall response rates were significantly higher with cobimetinib plus vemurafenib than with vemurafenib alone. With dual therapy, the majority of responses had occurred by the time of the first tumour assessment at 8 weeks and the median duration of response was 13 months. |
Cobimetinib plus vemurafenib had a manageable tolerability profile. |
1 Introduction
The development of molecular targeted agents and immune checkpoint inhibitors has transformed the treatment of metastatic melanoma [1]. The MAPK signalling pathway [comprising RAS (HRAS, NRAS and KRAS), RAF serine/threonine kinases (ARAF, BRAF and CRAF), MEK and ERK] plays a pivotal role in the progression of melanoma [1]. Activating BRAF mutations (most commonly BRAF V600E) are found in ≈50 % of melanomas, and lead to constitutive activation of BRAF and downstream MAPK signalling [2].
The BRAF inhibitors vemurafenib (Zelboraf®) and dabrafenib have both demonstrated efficacy in the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma, and the response to a BRAF inhibitor is enhanced by the addition of a MEK inhibitor [1]. The MEK inhibitor trametinib is approved for use in combination with dabrafenib, and the MEK inhibitor cobimetinib (Cotellic®) was recently approved in the USA [3] and the EU [4] for use in combination with vemurafenib.
This narrative review discusses the clinical efficacy and tolerability of dual therapy with cobimetinib plus vemurafenib in patients with BRAF V600 mutation-positive unresectable or metastatic melanoma, as well as discussing the pharmacological properties of these agents.
2 Pharmacological Properties of Cobimetinib and Vemurafenib
2.1 Pharmacodynamic Profile
Vemurafenib inhibits several mutated forms of BRAF, including BRAFV600E [5]. The vemurafenib concentration inhibiting kinase activity by 50 % was 10 nmol/L for BRAFV600E, 9 nmol/L for BRAFV600R, 8 nmol/L for BRAFV600G, 7 nmol/L for BRAFV600K, 7 nmol/L for BRAFV600D and 7 nmol/L for BRAFV600M [6]. In vitro, vemurafenib inhibited phosphorylation of MEK and ERK, inhibited cellular proliferation and induced apoptosis in BRAF-mutated melanoma cells [7–10]. No such anti-proliferative activity was seen in cell lines expressing wild-type BRAF [8]. Vemurafenib also demonstrated antitumour activity in animal models of BRAF V600E-mutated melanoma [8].
Increased understanding of the mechanisms underlying vemurafenib resistance (see Sect. 3.3) led to the hypothesis that targeting multiple points on the MAPK pathway may enhance antitumour activity [2]. Cobimetinib is a potent, highly selective, reversible inhibitor of MEK1 and MEK2 [3, 11, 12]. Cobimetinib potently inhibited phosphorylation of ERK in two murine xenograft models of BRAF V600-mutated melanoma (melanoma cells harboured a BRAF V600D mutation and were PTEN deficient in one xenograft model and harboured a BRAF V600E mutation in the other xenograft model) [13]. Cobimetinib also demonstrated antitumour activity in animal models of BRAF V600E-mutated melanoma [12, 13].
Preclinical studies indicated that the combination of cobimetinib and vemurafenib resulted in stronger inhibition of intracellular signalling in melanoma cells and decreased tumour cell proliferation [4].
2.2 Pharmacokinetic Profile
Cobimetinib had a mean absolute bioavailability of 46 % [14]. Dose proportional increases in the maximum plasma concentration (Cmax) and the area under the plasma concentration-time curve (AUC) were seen with oral cobimetinib ≈3.5–100 mg [15]. Following administration of oral cobimetinib 60 mg once daily to patients with cancer, the median time to the cobimetinib Cmax (tmax) was 2.4 h and steady state was reached by day 9, with a mean accumulation ratio of 2.4 [3, 4]. Food did not have a clinically significant effect on the pharmacokinetics of cobimetinib [14].
The median tmax was ≈3 h following repeated oral administration of vemurafenib [5]. Steady state was reached in ≈15–22 days; dose proportional increases in Cmax and AUC were seen at steady state with administration of vemurafenib 240–960 mg twice daily in patients with metastatic melanoma [5, 16]. Vemurafenib can be administered without regard to food [5, 6], although a high-fat meal increased vemurafenib exposure approximately threefold [17].
Both cobimetinib and vemurafenib were highly plasma protein bound (95 % for cobimetinib [3, 14] and >99 % for vemurafenib [5, 6]). The estimated apparent volume of distribution was 806 L for cobimetinib [3, 4] and 106 L for vemurafenib [5].
Cobimetinib was predominantly metabolized by cytochrome P450 (CYP) 3A4 and uridine diphosphate glucuronosyltransferase 2B7 in vitro [3, 4, 18]. Following oral administration of radiolabelled cobimetinib 20 mg to healthy volunteers, 77 % of the radioactivity was recovered in the faeces (6.6 % as the parent drug) and 18 % was recovered in the urine (1.6 % as the parent drug) [18]. Following administration of oral cobimetinib 60 mg once daily to patients with cancer, cobimetinib had a mean apparent clearance of 13.8 L/h and a mean elimination half-life of 44 h [3, 4].
CYP3A4 was primarily responsible for the metabolism of vemurafenib in vitro [6]. However, following oral administration of radiolabelled vemurafenib, the parent drug and its metabolites accounted for 95 and 5 % of the radioactivity in plasma over 48 h [5, 6]. Approximately 94 % of the vemurafenib dose was recovered in faeces, with ≈1 % recovered in urine [5, 6]. Vemurafenib had an estimated apparent clearance of 31 L/day, with an estimated median elimination half-life of 57 h [5].
Dosages of cobimetinib and vemurafenib do not need to be adjusted in patients with mild to moderate renal impairment [3–6]; the US prescribing information states that the appropriate dosages of cobimetinib and vemurafenib have not been established in severe renal impairment [3, 5] and the EU summary of product characteristics (SmPC) states that cobimetinib and vemurafenib should be used with caution in severe renal impairment [4, 6].
The cobimetinib dosage does not need to be adjusted in patients with mild hepatic impairment; the US prescribing information states that data are lacking in patients with moderate to severe hepatic impairment [3] and the EU SmPC states that cobimetinib should be used with caution in patients with moderate to severe hepatic impairment [4].
The US prescribing information states that the vemurafenib dosage does not need to be adjusted in patients with mild to moderate hepatic impairment; the appropriate vemurafenib dosage has not been established in severe hepatic impairment [5]. The EU SmPC states that the vemurafenib dosage does not need to be adjusted in hepatic impairment, although close monitoring is warranted in moderate to severe impairment [6].
Population pharmacokinetic analyses demonstrated that age, bodyweight, race and gender did not have a clinically relevant effect on cobimetinib pharmacokinetics [19] and age, bodyweight and gender did not have a clinically relevant effect on vemurafenib pharmacokinetics [5, 6].
2.3 Potential Drug Interactions
The pharmacokinetics of cobimetinib and vemurafenib were not altered to a clinically significant extent when these agents were coadministered in patients with advanced melanoma in the BRIM-7 trial [20]. Cobimetinib is a substrate of CYP3A and P-glycoprotein (P-gp) and may inhibit CYP3A and CYP2D6 [3, 21]. Vemurafenib is a substrate of CYP3A4, P-gp and breast cancer resistance protein (BCRP) and an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4/5, P-gp and BCRP [5].
Coadministration of cobimetinib with strong or moderate CYP3A inducers or strong CYP3A inhibitors should be avoided [3, 4]. The US prescribing information states that coadministration of cobimetinib with moderate CYP3A inhibitors should also be avoided [3], whereas the EU SmPC states that cobimetinib and moderate CYP3A inhibitors should be coadministered with caution [4].
The US prescribing information states that coadministration of vemurafenib with strong CYP3A4 inhibitors or inducers or with P-gp substrates with a narrow therapeutic window should be avoided, and coadministration of vemurafenib with drugs that have a narrow therapeutic window and are predominantly metabolized by CYP1A2 is not recommended [5]. The EU SmPC states that dosage adjustment of drugs predominantly metabolized by CYP1A2 or CYP3A4 should be considered, based on their therapeutic window, before administering concomitant vemurafenib [6]. In addition, caution is recommended when coadministering vemurafenib with P-gp substrates with a narrow therapeutic window or with potent inhibitors of CYP3A4, glucuronidation and/or transport proteins, and coadministration of vemurafenib and potent inducers of P-gp, glucuronidation or CYP3A4 should be avoided [6].
3 Therapeutic Efficacy of Cobimetinib plus Vemurafenib
A phase I study in patients with advanced solid tumours determined a vemurafenib regimen of 960 mg twice daily to be suitable for further study [22]. A phase II trial in previously treated patients with BRAF V600 mutation-positive metastatic melanoma [23] and a phase III trial in treatment-naïve patients with BRAF V600E mutation-positive unresectable, locally advanced or metastatic melanoma [24, 25] subsequently demonstrated the antitumour activity of monotherapy with vemurafenib 960 mg twice daily. This section discusses the efficacy of dual therapy with cobimetinib plus vemurafenib in patients with BRAF V600 mutation-positive unresectable, locally advanced or metastatic melanoma.
3.1 BRIM-7 Trial
BRIM-7 was a nonrandomized, open-label, multicentre, phase Ib study that was primarily designed to examine the safety of the cobimetinib plus vemurafenib combination regimen, and identify dose-limiting toxicities and the maximum tolerated dose [20]. Patients enrolled in BRIM-7 had BRAF V600 mutation-positive unresectable, stage IIIC or stage IV melanoma and had never received a BRAF inhibitor (n = 63) or had recently progressed on vemurafenib (n = 66) [20].
During the dose-escalation phase of the study, patients received oral vemurafenib 720 or 960 mg twice daily in combination with various oral cobimetinib regimens [20]. Dose-limiting toxicities were seen in four patients receiving regimens comprising cobimetinib plus vemurafenib 960 mg twice daily, and included grade 3 fatigue, fatigue/stomatitis, prolongation of the corrected QT (QTc) interval and arthralgia/myalgia. The maximum tolerated dosage was established as cobimetinib 60 mg once daily for the first 21 days of each 28-day cycle in combination with vemurafenib 960 mg twice daily; this regimen was selected for use in future studies [20].
In BRAF inhibitor-naïve patients (median follow-up duration of 13 months), the objective response rate (ORR) was 87 %, median progression-free survival (PFS) was 13.7 months and median overall survival (OS) had not been reached, with an estimated 1-year OS rate of 83 % [20]. In patients who had recently progressed on vemurafenib (median follow-up duration of 6 months), the ORR was 15 %, median PFS was 2.8 months and median OS was 8.3 months, with an estimated 1-year OS rate of 32 % [20]. With extended follow-up, median OS was 28.5 months and the 2-year OS rate was 61 % in BRAF inhibitor-naïve patients (median follow-up duration of 21 months) and 15 % in patients who had recently progressed on vemurafenib (median follow-up duration of 8 months) [analysis available as an abstract] [26].
Of 39 BRAF inhibitor-naïve patients who had experienced disease progression at a data cut-off of 5 September 2014 in BRIM-7, 26 continued treatment with cobimetinib plus vemurafenib [27]. The median duration of treatment was 12.5 months in patients treated beyond progression and 11.0 months in patients not treated beyond progression, with a median OS of 22.0 and 19.4 months in the corresponding treatment groups, according to the results of a retrospective analysis (available as an abstract plus poster) [27].
3.2 coBRIM Trial
The randomized, double-blind, multinational, phase III coBRIM trial compared the efficacy of cobimetinib plus vemurafenib with placebo plus vemurafenib in previously untreated patients with unresectable, stage IIIC or stage IV melanoma that was BRAF V600 mutation positive [28]. Patients were aged ≥18 years and had histologically confirmed, measurable disease and an Eastern Co-operative Oncology Group (ECOG) performance status of 0 or 1. Patients with previously treated brain metastases with stable disease for ≥3 weeks were also eligible for enrolment [28].
At baseline, 76 % of cobimetinib plus vemurafenib recipients and 67 % of placebo plus vemurafenib recipients had an ECOG performance status of 0, 24 and 33 % had an ECOG performance status of 1, and 0.4 and 0.8 % had brain metastases [28]. In terms of the mutation genotype, 69 % of cobimetinib plus vemurafenib recipients and 70 % of placebo plus vemurafenib recipients had BRAF V600E, 10 and 13 % had BRAF V600K and genotype could not evaluated in the remaining 21 and 17 % of patients [28].
Patients randomized to oral cobimetinib received 60 mg once daily for the first 21 days of each 28-day cycle and all patients received oral vemurafenib 960 mg twice daily [28]. Treatment continued until disease progression, unacceptable toxicity or withdrawal of patient consent; no crossover was permitted following progression [28].
The primary endpoint was PFS, as assessed by the investigator [28]. Efficacy was assessed in the intent-to-treat population. At the time of the final PFS analysis (data cut-off of 9 May 2014), the median duration of follow-up was 7.4 months in cobimetinib plus vemurafenib recipients and 7.0 months in vemurafenib recipients [28] and an updated analysis (data cut-off of 16 January 2015; available as an abstract) had a median duration of follow-up of 14.9 months in cobimetinib plus vemurafenib recipients and 13.6 months in vemurafenib recipients [29]. The final OS analysis (data cut-off of 28 August 2015; available as an abstract) had a median duration of follow-up of 18.5 months [30].
Cobimetinib plus vemurafenib significantly prolonged PFS, compared with vemurafenib alone, in patients with BRAF V600 mutation-positive unresectable or metastatic melanoma. At the time of the final PFS analysis (data cut-off 9 May 2014), the median PFS was 9.9 months with cobimetinib plus vemurafenib and 6.2 months with vemurafenib alone, and the risk of disease progression or death was significantly reduced by 49 % (Table 1) [28]. At the time of the updated analysis (data cut-off 16 January 2015), the median PFS was 12.3 months with cobimetinib plus vemurafenib and 7.2 months with vemurafenib alone, and the risk of disease progression or death was significantly reduced by 42 % (Table 1) [29]. Hazard ratios for PFS favoured cobimetinib plus vemurafenib over vemurafenib alone in all prespecified subgroups [28].
The ORR was significantly higher with cobimetinib plus vemurafenib than with vemurafenib alone (Table 1) [28]. At a data cut-off of 9 May 2014, a complete response was seen in 10 % of cobimetinib plus vemurafenib recipients and in 4 % of vemurafenib recipients, with a partial response seen in 57 and 40 % of patients in the corresponding treatment groups [28]. The majority of responses had occurred by the time of the first tumour assessment at week 8; the median duration of response had not yet been reached in patients receiving cobimetinib plus vemurafenib and was 7.3 months in patients receiving vemurafenib alone [28]. At a data cut-off of 16 January 2015, a complete response was seen in 16 % of cobimetinib plus vemurafenib recipients and in 11 % of vemurafenib recipients, with a partial response seen in 54 and 40 % of patients in the corresponding treatment groups; the median duration of response was 13 months with cobimetinib plus vemurafenib and 9 months with vemurafenib alone [29].
With cobimetinib plus vemurafenib, median OS had not been reached at a data cut-off of 9 May 2014 [28] or 16 January 2015 (data obtained from the EU SmPC) [4] (Table 1). At the final OS analysis (data cut-off 28 August 2015), the risk of death was significantly reduced by 30 % with cobimetinib plus vemurafenib versus vemurafenib alone, with a median OS duration of 22.3 months in patients receiving cobimetinib plus vemurafenib and 17.4 months in patients receiving vemurafenib alone (Table 1) [30]. The 12-month OS rate was 75 % in patients receiving cobimetinib plus vemurafenib and 64 % in patients receiving vemurafenib alone and the 24-month OS rate was 48 and 38 % in the corresponding treatment groups (Table 1). Hazard ratios for OS favoured cobimetinib plus vemurafenib over vemurafenib alone in all prespecified subgroups [30].
3.3 Mechanisms of Resistance
Approximately 15 % of patients have primary resistance to BRAF inhibitors [2]. Multiple mechanisms of primary resistance to BRAF inhibitors have been identified, including RAC1 P29S mutations, loss of PTEN function, dysregulation of cyclin-dependent kinase-4 and/or cyclin D1, inactivation of NF1, activation of the kinase COT1, stromal secretion of hepatocyte growth factor and mutations in HOXD8 (reviewed by Spagnolo et al. [2]).
Baseline genomic heterogeneity in tumours did not appear to have a detrimental effect on clinical outcome in patients receiving cobimetinib plus vemurafenib. For example, in coBRIM, PFS outcomes were similar in cobimetinib plus vemurafenib recipients with higher versus lower allelic frequencies of BRAF V600 mutations at baseline (analysis available as an abstract) [31]. In addition, the presence of co-existing activating mutations in RAS or receptor tyrosine kinases and loss of PTEN function at baseline did not affect PFS outcomes in patients receiving cobimetinib plus vemurafenib [29, 31]. In coBRIM, loss of PTEN function and high Ki67 levels had a detrimental effect on OS in patients receiving vemurafenib alone, but did not affect OS in patients receiving cobimetinib plus vemurafenib [30]. The BRAF V600 allele frequency and pathway activation (assessed by levels of phosphorylated ERK and phosphorylated S6) did not affect OS in either treatment arm [30].
An exploratory, retrospective analysis (available as an abstract) of pretreatment tumour samples from four vemurafenib trials (including BRIM7 and coBRIM) demonstrated that although baseline expression of cell cycle genes appeared to have a detrimental effect on PFS in patients receiving vemurafenib alone, no such effect was seen in patients receiving cobimetinib plus vemurafenib [32].
Most patients treated with BRAF inhibitors will eventually develop secondary resistance [2]. The most common mechanisms of acquired vemurafenib resistance involve reactivation of the MAPK pathway as a result of events upstream of BRAF (e.g. activating mutations in NRAS), downstream of BRAF (e.g. activating mutations in MEK) or at the level of BRAF (e.g. BRAF V600E copy number amplification and alternative splicing of BRAF V600E) [2, 33]. Activation of other pathways, such as the PI3K/AKT/mTOR pathway, may also result in acquired resistance [2]. Adaptive resistance mechanisms have also been identified, including upregulation of receptor tyrosine kinases and changes in metabolic pathways [2].
Cross resistance between BRAF inhibitors and MEK inhibitors has been observed in vitro [34, 35]. Acquired resistance to dual therapy with a BRAF inhibitor and a MEK inhibitor appears to involve the augmentation or combining of the mechanisms of resistance associated with BRAF inhibitor monotherapy [36].
4 Tolerability and Safety of Cobimetinib plus Vemurafenib
4.1 General Adverse Event Profile
The tolerability profile of oral cobimetinib plus oral vemurafenib was manageable in patients with unresectable or metastatic melanoma. In coBRIM, the most commonly reported adverse events in patients receiving cobimetinib plus vemurafenib included diarrhoea, nausea, rash, arthralgia, fatigue, photosensitivity reactions, pyrexia, vomiting, increased alanine aminotransferase (ALT) levels, increased aspartate aminotransferase (AST) levels and increased creatine kinase levels [28]. Some adverse events (diarrhoea, nausea, vomiting, photosensitivity reactions, increased creatine kinase levels, chorioretinopathy) occurred with a numerically higher incidence in patients receiving cobimetinib plus vemurafenib than in patients receiving vemurafenib alone, although these adverse events were mostly of grade 1 or 2 severity. For example, diarrhoea of grade 1, 2 or 3 severity occurred in 39, 11 and 6 % of cobimetinib plus vemurafenib recipients, respectively, and in 21, 7 and 0 % of vemurafenib recipients, respectively, and photosensitivity reactions of grade 1, 2 or 3 severity occurred in 19, 7 and 2 % of cobimetinib plus vemurafenib recipients, respectively, and in 10, 5 and 0 % of vemurafenib recipients, respectively. Rash (any grade) was reported in 39 % of cobimetinib plus vemurafenib recipients versus 36 % of vemurafenib recipients, fatigue (any grade) was reported in 32 versus 31 % and pyrexia (any grade) was reported in 26 versus 22 %. Some adverse events [e.g. cutaneous squamous cell carcinoma (SCC), keratoacanthoma, alopecia, arthralgia] had a numerically lower incidence in patients receiving cobimetinib plus vemurafenib than in those receiving vemurafenib alone [28].
In coBRIM, the median time to onset of diarrhoea was 0.33 months with cobimetinib plus vemurafenib versus 1.84 months with vemurafenib alone, the median time to onset of photosensitivity was 1.38 versus 0.59 months and the median time to onset of rash was 0.43 versus 0.39 months (analysis available as an abstract plus poster) [37]. Diarrhoea was mostly managed with antidiarrhoeals, with treatment interruption, dose reduction or discontinuation required in 6, 2 and 0 % of patients receiving cobimetinib plus vemurafenib, respectively. Photosensitivity was usually managed conservatively, with treatment interruption required in 1 % of patients receiving cobimetinib plus vemurafenib; no patient in this treatment arm required dose reduction or discontinuation because of photosensitivity. Treatment interruption, dose reduction or discontinuation because of rash occurred in 5, 4 and 3 % of patients receiving cobimetinib plus vemurafenib, respectively [37].
The most commonly reported grade 3 adverse events in patients receiving dual therapy included increased ALT levels (11 % of cobimetinib plus vemurafenib recipients vs. 6 % of vemurafenib recipients), increased AST levels (8 vs. 2 %), increased creatine kinase levels (7 vs. 0 %), diarrhoea (6 vs. 0 %), rash (5 vs. 5 %) and fatigue (4 vs. 3 %) [28]. Grade 4 adverse events reported in patients receiving cobimetinib plus vemurafenib included increased creatine kinase levels (4 % of patients), rash (1 %), increased ALT levels (0.4 %) and retinal detachment (0.4 %), with grade 4 increased ALT and AST levels each reported in 0.4 % of patients receiving vemurafenib alone [28]. The majority of first adverse events of at least grade 3 severity occurred early in treatment; among patients who experienced adverse events of at least grade 3 severity, the median time to onset was 0.53 months with cobimetinib plus vemurafenib and 0.79 months with vemurafenib alone [37]. The median time to resolution of adverse events of at least grade 3 severity occurring within the first 28 days of treatment was 0.5 months in both treatment arms [37].
Death was attributed to adverse events in six recipients of cobimetinib plus vemurafenib and three recipients of vemurafenib alone [28].
4.2 Specific Adverse Events of Interest
New primary cutaneous malignancies (including cutaneous SCC, basal cell carcinoma, keratoacanthoma and melanoma) have been reported in patients receiving cobimetinib plus vemurafenib [28]. In coBRIM, grade 3 cutaneous SCC was reported in 2 % of cobimetinib plus vemurafenib recipients and 11 % of vemurafenib recipients, with grade 3 keratoacanthoma reported in 1 and 8 % of patients in the corresponding treatment groups [28]. Vemurafenib may also promote the growth of noncutaneous malignancies, including SCC of the head and neck and malignancies associated with the activation of RAS [5].
MEK inhibitors, including cobimetinib, are associated with serous retinopathy [3, 4, 38]. In coBRIM, serous retinopathy events (most commonly chorioretinopathy and retinal detachment) occurred in 26 % of cobimetinib plus vemurafenib recipients and in 3 % of vemurafenib recipients (analysis available as an abstract plus poster) [38]. The median time to the first onset of serous retinopathy was 1 month. The majority of patients were asymptomatic or had mild symptoms and were managed by close observation without modification of the cobimetinib or vemurafenib dosage [38]. Vemurafenib may also be associated with uveitis, blurry vision and photophobia [5].
Vemurafenib is associated with concentration-dependent QTc interval prolongation [5, 6], although no further prolongation of the QTc interval was seen with the coadministration of cobimetinib [3]. In coBRIM, QT-interval prolongation of grade 1, 2 or 3 severity was reported in 2, 1 and 0.4 % of cobimetinib plus vemurafenib recipients, respectively, and in 3, 1 and 1 % of vemurafenib recipients, respectively [28]. In an open-label, multinational study primarily designed to examine the safety of vemurafenib in patients (n = 3222) with BRAF V600 mutation-positive stage IIIC or stage IV melanoma, Fridericia-corrected QT-interval prolongation of >500 ms was reported in 54 (2 %) patients; cardiac arrhythmias were also seen in two of these patients, both of whom had predisposing cardiac risk factors (hypertension with or without ischaemic heart disease) [39].
Cardiomyopathy has been reported in cobimetinib recipients, with a grade 2 or 3 reduction in left ventricular ejection fraction occurring in 26 % of cobimetinib plus vemurafenib recipients and 19% of vemurafenib recipients in coBRIM [3]. Haemorrhage has also been reported in cobimetinib recipients. In coBRIM, haemorrhage (any grade) occurred in 13 % of cobimetinib plus vemurafenib recipients and 7 % of vemurafenib recipients, with grade 3 or 4 haemorrhage reported in 1.2 and 0.8 % of patients in the corresponding treatment arms [3].
5 Dosage and Administration of Cobimetinib plus Vemurafenib
Cobimetinib is approved for use in combination with vemurafenib in patients with BRAF V600E or BRAF V600K mutation-positive unresectable or metastatic melanoma in the USA [3] and with BRAF V600 mutation-positive unresectable or metastatic melanoma in the EU [4]. The presence of a BRAF V600 mutation should be confirmed using a validated test. The recommended treatment regimen comprises oral cobimetinib 60 mg once daily for the first 21 days of each 28-day cycle and oral vemurafenib 960 mg every 12 h, without regard to food [3–6]. Patients should be treated until disease progression or unacceptable toxicity occurs [3–6].
Both cobimetinib and vemurafenib can cause fetal harm if administered to a pregnant woman [3, 5]. Local prescribing information for cobimetinib and vemurafenib should be consulted for more information pertaining to warnings, precautions and dose modification for adverse reactions.
6 Place of Cobimetinib plus Vemurafenib in the Management of BRAF V600 Mutation-Positive Unresectable or Metastatic Melanoma
An earlier phase III study showed a survival benefit for vemurafenib monotherapy over dacarbazine in previously untreated patients with BRAF V600E mutation-positive unresectable, stage IIIC or stage IV melanoma, with significantly (p < 0.001) prolonged OS and PFS [24, 25]. However, disease progression typically occurs within 5–7 months when BRAF inhibitors are administered as monotherapy, reflecting acquired resistance [1]. Given this, there is a strong rationale for blocking the MAPK pathway at two different points (e.g. with a BRAF inhibitor and further downstream with a MEK inhibitor) [1]. Indeed, the addition of cobimetinib to vemurafenib significantly prolonged PFS and OS in patients with BRAF V600 mutation-positive unresectable, stage IIIC or stage IV melanoma in coBRIM (Sect. 3.2). ORR rates were also significantly higher with cobimetinib plus vemurafenib than with vemurafenib alone; the majority of responses had occurred by the time of the first tumour assessment at week 8 and the median duration of response was 13 months.
Dual therapy with the MEK inhibitor trametinib and the BRAF inhibitor dabrafenib has also shown greater efficacy than monotherapy with dabrafenib [40] or vemurafenib [41] in patients with BRAF V600 mutation-positive unresectable, stage IIIC or stage IV melanoma and is available in the USA [42, 43] and the EU [44, 45]. The BRAF inhibitor encorafenib and the MEK inhibitor binimetinib are also under development in unresectable or metastatic melanoma.
Despite the enhanced antitumour activity, most patients receiving dual therapy with a MEK inhibitor and a BRAF inhibitor will eventually develop resistance [36]. In terms of strategies to combat resistance, results of a preclinical study suggest that intermittent dosing may delay the onset of vemurafenib resistance [46]. Phase II trials examining the ability of intermittent regimens of cobimetinib plus vemurafenib (NCT02583516) and trametinib plus dabrafenib (NCT02199730) to delay the emergence of acquired resistance are currently underway. It has also been suggested that triple therapy with a BRAF inhibitor plus a MEK inhibitor and a third molecular targeted agent (e.g. a pan-RAF inhibitor, a receptor tyrosine kinase inhibitor or an inhibitor of the PI3K/AKT/mTOR pathway) may provide additional benefit [36, 47].
Three patients with previously treated brain metastases were included in coBRIM [28]. A pilot study demonstrated that vemurafenib had antitumour activity and an acceptable tolerability profile in patients (n = 24) with BRAF V600 mutation-positive metastatic melanoma and unresectable, previously treated brain metastases [48]. A phase II study (coBRIM-B) is underway examining the efficacy of dual therapy with cobimetinib plus vemurafenib in patients with active melanoma brain metastases [49].
Dual therapy with cobimetinib plus vemurafenib had a manageable tolerability profile in patients with unresectable or metastatic melanoma (Sect. 4). Importantly, although some adverse events occurred with a numerically higher incidence with dual therapy than with vemurafenib alone, the majority of these adverse events were of grade 1 or 2 severity, and the proportion of patients discontinuing treatment because of adverse events was similar in both treatment arms. In addition, some adverse events (including cutaneous SCC and keratoacanthoma) occurred with a numerically lower incidence in patients receiving cobimetinib plus vemurafenib than in those receiving vemurafenib alone. Cutaneous SCC and keratoacanthoma occur with BRAF inhibitor monotherapy because of paradoxical activation of the MAPK pathway in keratinocytes [50]. This can be blocked by the addition of a MEK inhibitor, thereby explaining the numerically lower incidence of these adverse events in patients receiving dual therapy (Sect. 4.2) Patients receiving cobimetinib plus vemurafenib should be regularly monitored for new cutaneous malignancies, as well as for signs and symptoms of noncutaneous malignancies [3, 5, 6]. Various other cutaneous adverse events have been reported in patients receiving dual therapy with a MEK inhibitor and a BRAF inhibitor including acneiform eruptions, plantar hyperkeratosis and actinic keratosis [51]. Patients experienced the onset of new cutaneous adverse events even after 52 weeks of continuous therapy with a BRAF inhibitor (with or without a MEK inhibitor), meaning that dermatological follow-up should continue, regardless of the duration of therapy [51].
ECG recordings and electrolytes should be regularly monitored in patients receiving vemurafenib [5]. Vemurafenib therapy should not be started in patients with uncorrectable electrolyte abnormalities, a QTc interval of >500 ms or long QT syndrome, or who are receiving drugs known to prolong the QT interval [5, 6]. Interruption of vemurafenib treatment, dose reduction or treatment discontinuation may be required in patients who develop a QTc interval of >500 ms [5, 6].
It should be noted that there are some differences between vemurafenib and dabrafenib in terms of their adverse event profiles. For example, pyrexia appears more common with dabrafenib than with vemurafenib, and photosensitivity reactions appear more frequent with vemurafenib than with dabrafenib [41].
MEK inhibitors, including cobimetinib, are associated with serous retinopathy [28]. Most cases of serous retinopathy reported in the coBRIM trial were asymptomatic or mild and were managed by close observation (Sect. 4.2). However, interruption of cobimetinib treatment, dose reduction or treatment discontinuation may be required [3]. Patients receiving cobimetinib should undergo regular ophthalmological evaluation [3, 4].
Treatment interruption, dose reduction or treatment discontinuation may be required to manage certain other adverse reactions associated with cobimetinib (including haemorrhage, cardiomyopathy, dermatological reactions, liver function test abnormalities or hepatotoxicity, rhabdomyolysis or creatine kinase elevations, photosensitivity reactions, diarrhoea) or vemurafenib (including hypersensitivity reactions, severe dermatological reactions, hepatotoxicity, photosensitivity reactions, diarrhoea) [3–6]; local prescribing information should be consulted for further details.
Vemurafenib plasma concentrations have been linked to the risk of disease progression [52–54] and/or toxicity (e.g. rash of at least grade 2 severity) [53]. It has been suggested that therapeutic drug monitoring in the early stages of vemurafenib treatment may help identify patients at risk of nonresponse and toxicity [52, 53], although more data are needed.
Guidelines from the European Society for Medical Oncology (ESMO) recommend first-line treatment with a programmed cell death (PD)-1 immune checkpoint inhibitor (nivolumab or pembrolizumab) or dual therapy with a MEK inhibitor plus a BRAF inhibitor for BRAF V600 mutation-positive metastatic melanoma [55]. US National Comprehensive Cancer Network (NCCN) guidelines include several options for the first-line treatment of BRAF V600 mutation-positive unresectable or metastatic melanoma, including cobimetinib plus vemurafenib, trametinib plus dabrafenib, vemurafenib, dabrafenib, nivolumab, pembrolizumab, and nivolumab plus ipilimumab [56]. Second-line agents should not have been used in first-line treatment, and should be of a different class [56].
The optimal sequence and timing of therapies in BRAF V600 mutation-positive unresectable or metastatic melanoma remains unclear [1]. Nivolumab [57, 58] and pembrolizumab [59, 60] are approved in the USA and the EU for use in advanced melanoma. BRAF V600 status did not appear to affect the efficacy of these PD-1 immune checkpoint inhibitors; 18–36 % of patients included in trials of nivolumab or pembrolizumab were BRAF V600 mutation-positive [61–64]. Nivolumab and pembrolizumab have manageable tolerability profiles (characterized by immune-mediated adverse reactions), and although the response rates achieved with these agents tend to be lower than those seen with molecular targeted agents, the responses are often more durable [55]. However, immune checkpoint inhibitors take time to maximize their antitumour activity, whereas molecular targeted agents are associated with a more rapid symptom-modifying response [55, 65]. Given this, NCCN [56] and ESMO [55] guidelines state that in patients with BRAF V600 mutation-positive unresectable or metastatic melanoma, first-line treatment with molecular targeted agents is preferred if an early response is needed (e.g. in patients with symptomatic, bulky or rapidly growing disease) [65]. Given that dual therapy with a MEK inhibitor plus a BRAF inhibitor has consistently shown greater efficacy than a BRAF inhibitor alone, it is suggested that monotherapy with a BRAF inhibitor be reserved for patients who cannot receive MEK inhibitors because of issues such as retinopathy or severe heart failure [1]. Sequential treatment with a MEK inhibitor after failure of a BRAF inhibitor has not been shown to be a successful approach [1].
Dual therapy with a molecular targeted agent and an immune checkpoint inhibitor would seem a rational treatment strategy [66]. A phase I study in patients with BRAF V600 mutation-positive metastatic melanoma was terminated when dual therapy with vemurafenib and the immune checkpoint inhibitor ipilimumab (a monoclonal antibody targeting cytotoxic T-lymphocyte-associated antigen 4) was associated with asymptomatic, reversible hepatotoxicity [67]. However, a recent phase Ib study in treatment-naïve patients (n = 19) with BRAF V600 mutation-positive unresectable or metastatic melanoma found that dual therapy with vemurafenib and the investigational immune checkpoint inhibitor atezolizumab (a monoclonal antibody targeting PD-L1, a key ligand of the PD-1 receptor) demonstrated promising antitumour activity and a manageable tolerability profile [68]. The ORR was 76 % with a median duration of response of 20.9 months and a median PFS of 12.2 months [68]. Triple therapy with vemurafenib plus cobimetinib and atezolizumab is also under investigation (NCT01656642).
In conclusion, cobimetinib plus vemurafenib is a valuable option for use in BRAF V600 mutation-positive unresectable or metastatic melanoma. Dual therapy with cobimetinib plus vemurafenib is more effective than vemurafenib alone and has a manageable tolerability profile.
Data selection sources:
Relevant medical literature (including published and unpublished data) on cobimetinib and vemurafenib was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 29 February 2016], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Cobimetinib, Cotellic, GDC-0973, vemurafenib, Zelboraf, PLX-4032, RG-7204, coBRIM, BRIM-3, melanoma, V600.
Study selection: Studies in patients with BRAF V600 mutation-positive unresectable or metastatic melanoma who received cobimetinib plus vemurafenib. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Dossett LA, Kudchadkar RR, Zager JS. BRAF and MEK inhibition in melanoma. Expert Opin Drug Saf. 2015;14(4):559–70.
Spagnolo F, Ghiorzo P, Queirolo P. Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget. 2014;5(21):10206–21.
Genentech USA Inc. Cotellic (cobimetinib) tablets, for oral use: US prescribing information. 2015. http://www.gene.com. Accessed 2 Feb 2016.
European Medicines Agency. Cotellic (cobimetinib): EU summary of product characteristics. 2015. http://ec.europa.eu. Accessed 2 Feb 2016.
Genentech USA Inc. Zelboraf® (vemurafenib) tablet for oral use: US prescribing information. 2015. http://www.gene.com. Accessed 2 Feb 2016.
European Medicines Agency. Zelboraf (vemurafenib) film-coated tablets: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 2 Feb 2016.
Beck D, Niessner H, Smalley KSM, et al. Vemurafenib potently induces endoplasmic reticulum stress-mediated apoptosis in BRAFV600E melanoma cells. Sci Signal. 2013;6(260):ra7.
Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70(13):5518–27.
Søndergaard JN, Nazarian R, Wang Q, et al. Differential sensitivity of melanoma cell lines with BRAF V600E mutation to the specific Raf inhibitor PLX4032. J Transl Med. 2010;8:39.
Sala E, Mologni L, Truffa S, et al. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res. 2008;6(5):751–9.
Rice KD, Aay N, Anand NK, et al. Novel carboxamide-based allosteric MEK inhibitors: discovery and optimization efforts toward XL518 (GDC-0973). ACS Med Chem Lett. 2012;3(5):416–21.
Hoeflich KP, Merchant M, Orr C, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72(1):210–9.
Wong H, Vernillet L, Peterson A, et al. Bridging the gap between preclinical and clinical studies using pharmacokinetic-pharmacodynamic modeling: an analysis of GDC-0973, a MEK inhibitor. Clin Cancer Res. 2012;18(11):3090–9.
Musib L, Choo E, Deng Y, et al. Absolute bioavailability and effect of formulation change, food, or elevated pH with rabeprazole on cobimetinib absorption in healthy subjects. Mol Pharm. 2013;10(11):4046–54.
Musib L, Eppler S, Choo E, et al. Clinical pharmacokinetics of GDC-0973, an oral MEK inhibitor, in cancer patients: data from a phase 1 study [abstract no. 1304]. Cancer Res. 2011;71(8 Suppl).
Grippo JF, Zhang W, Heinzmann D, et al. A phase I, randomized, open-label study of the multiple-dose pharmacokinetics of vemurafenib in patients with BRAFV600E mutation-positive metastatic melanoma. Cancer Chemother Pharmacol. 2014;73(1):103–11.
Ribas A, Zhang W, Chang I, et al. The effects of a high-fat meal on single-dose vemurafenib pharmacokinetics. J Clin Pharmacol. 2014;54(4):368–74.
Takahashi RH, Choo EF, Ma S, et al. Absorption, metabolism, excretion, and the contribution of intestinal metabolism to the oral disposition of [14C]cobimetinib, a MEK inhibitor, in humans. Drug Metab Dispos. 2016;44(1):28–39.
Han K, Jin J, Marchand M, et al. Population pharmacokinetics and dosing implications for cobimetinib in patients with solid tumors. Cancer Chemother Pharmacol. 2015;76(5):917–24.
Ribas A, Gonzalez R, Pavlick A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAFV600-mutated melanoma: a phase 1b study. Lancet Oncol. 2014;15(9):954–65.
Choo EF, Ly J, Chan J, et al. Role of P-glycoprotein on the brain penetration and brain pharmacodynamic activity of the MEK inhibitor cobimetinib. Mol Pharm. 2014;11(11):4199–207.
Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19.
Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAFV600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–14.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16.
McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF V600E and BRAF V600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;5(3):323–32.
Pavlick AC, Ribas A, Gonzalez R, et al. Extended follow-up results of phase Ib study (BRIM7) of vemurafenib (VEM) with cobimetinib (COBI) in BRAF-mutant melanoma [abstract no. 9020]. J Clin Oncol. 2015;33(Suppl).
Lewis K, Ribas A, Gonzalez R, et al. Treatment beyond progression in advanced BRAF-mutated melanoma with vemurafenib and cobimetinib: results from the BRIM7 trial [abstract no. 3340 plus poster]. In: European Cancer Congress. 2015.
Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76.
Larkin JMG, Yan Y, McArthur GA, et al. Update of progression-free survival (PFS) and correlative biomarker analysis from coBRIM: phase III study of cobimetinib (cobi) plus vemurafenib (vem) in advanced BRAF-mutated melanoma. [abstract no. 9006]. J Clin Oncol. 2015;33(15 Suppl 1).
Atkinson V, Larkin J, McArthur G, et al. Improved overall survival (OS) with cobimetinib (COBI) + vemurafenib (V) in advanced BRAF-mutated melanoma and biomarker correlates of efficacy [abstract]. In: Society for Melanoma Research 2015 Congress. 2015.
McArthur G, Larkin J, Dréno B, et al. Impact of baseline genetic heterogeneities on progression-free survival (PFS) in patients (pts) with advanced BRAFV600-mutated melanoma treated with cobimetinib (COBI) + vemurafenib (VEM) in the phase 3 coBRIM study [abstract no. 25LBA]. In: European Cancer Congress. 2015.
Wongchenko M, McArthur GA, Andries L, et al. Gene expression profiling reveals distinct subpopulations of BRAF-mutant melanoma patients each with different outcomes when treated with vemurafenib or combined vemurafenib and cobimetinib [abstract]. Pigment Cell Melanoma Res. 2015;28(6):823.
Trunzer K, Pavlick AC, Schuchter L, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol. 2013;31(14):1767–74.
Atefi M, von Euw E, Attar N, et al. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS ONE. 2011;6(12):e28973.
Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20(7):1965–77.
Moriceau G, Hugo W, Hong A, et al. Tunable-combinatorial mechanisms of acquired resistance limit the efficacy of BRAF/MEK cotargeting but result in melanoma drug addiction. Cancer Cell. 2015;27(2):240–56.
Dréno B, Ribas A, Larkin J, et al. Incidence, course, and management of toxicities associated with vemurafenib and cobimetinib in the coBRIM study [abstract plus poster]. In: Society for Melanoma Research 2014 Congress. 2014.
De La Cruz-Merino L, Di Guardo L, Grob J-J, et al. Clinical features of cobimetinib (COBI)-associated serous retinopathy (SR) in BRAF-mutated melanoma patients (pts) treated in the coBRIM study [abstract no. 9033 plus poster]. J Clin Oncol. 2015;33(15 Suppl 1).
Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF V600 mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15(4):436–44.
Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–88.
Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–9.
GlaxoSmithKline. Tafinlar (dabrafenib) capsules, for oral use: US prescribing information. 2015. http://www.pharma.us.novartis.com. Accessed 2 Feb 2016.
GlaxoSmithKline. Mekinist (trametinib) tablets, for oral use: US prescribing information. 2015. http://www.pharma.us.novartis.com. Accessed 2 Feb 2016.
European Medicines Agency. Tafinlar (dabrafenib): EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 2 Feb 2016.
European Medicines Agency. Mekinist (trametinib): EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 2 Feb 2016.
Das Thakur M, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494(7436):251–5.
Sullivan R, LoRusso P, Boerner S, et al. Achievements and challenges of molecular targeted therapy in melanoma. Am Soc Clin Oncol Educ Book. 2015:177–86.
Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF V600 mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611–21.
Yee MK, Lin Y, Gorantla VC, et al. Phase 2 study of cobimetinib in combination with vemurafenib in active melanoma brain metastases (coBRIM-B) [abstract no. TPS9088 plus poster]. J Clin Oncol. 2015;33(15 Suppl 1).
Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–15.
Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks. Br J Dermatol. 2015;172(1):239–43.
Goldwirt L, Chami I, Feugeas JP, et al. Low vemurafenib plasma exposure as a short-term predictive parameter of progression disease in metastatic BRAFV600 mut melanoma [abstract no. 9072 plus poster]. J Clin Oncol. 2015;33(15 Suppl 1).
Kramkimel N, Thomas-Schoemann A, Sakji L, et al. Vemurafenib pharmacokinetics and its correlation with efficacy and safety in outpatients with advanced BRAF-mutated melanoma. Target Oncol. 2015;11(1):59–69.
Funck-Brentano E, Alvarez JC, Longvert C, et al. Plasma vemurafenib concentrations in advanced BRAFV600mut melanoma patients: impact on tumour response and tolerance. Ann Oncol. 2015;26(7):1470–5.
Dummer R, Hauschild A, Lindenblatt N, et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v126–32.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma (version 2.2016). 2015. http://www.nccn.org. Accessed 2 Feb 2016.
Bristol-Myers Squibb Company. Nivolumab (Opdivo) injection, for intravenous use: US prescribing information. 2016. http://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed 2 Feb 2016.
European Medicines Agency. Opdivo (nivolumab): EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 2 Feb 2016.
Merck & Co Inc. Keytruada® (pembrolizumab): US prescribing information. 2015. http://www.keytruda.com. Accessed 2 Feb 2016.
European Medicines Agency. Keytruda (pembrolizumab): EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 2 Feb 2016.
Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17.
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18.
Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–84.
Olszanski AJ. Current and future roles of targeted therapy and immunotherapy in advanced melanoma. J Manag Care Pharm. 2014;20(4):346–56.
Hu-Lieskovan S, Robert L, Homet Moreno B, et al. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol. 2014;32(21):2248–54.
Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–6.
Hamid O, Patel M, Hodi S, et al. Preliminary clinical safety, tolerability and activity of atezolizumab (anti-PDL1) combined with vemurafenib in BRAFV600 metastatic melanoma [abstract]. Pigment Cell Melanoma Res. 2015;28(6):778.
Acknowledgments
During the peer review process, the manufacturer of cobimetinib and vemurafenib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Gillian Keating is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: L. de la Cruz Merino, Clinical Oncology Department, Virgen Macarena University Hospital, Seville, Spain; I. Krajsová, Department of Dermato-oncology, University Hospital Prague, and Charles University First Medical Faculty, Prague, Czech Republic; M. Mandalà, Unit of Medical Oncology, Department of Oncology and Hematology, Papa Giovanni XXIII Hospital, Bergamo, Italy; P. Queirolo, Medical Oncology 2, IRCCS San Martino - IST, Genova, Italy.
Rights and permissions
About this article
Cite this article
Keating, G.M. Cobimetinib Plus Vemurafenib: A Review in BRAF V600 Mutation-Positive Unresectable or Metastatic Melanoma. Drugs 76, 605–615 (2016). https://doi.org/10.1007/s40265-016-0562-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0562-7