Abstract
Seizures are the most common neurological emergencies in the neonatal period and are associated with poor neurodevelopmental outcomes. Seizures affect up to five per 1000 term births and population-based studies suggest that they occur even more frequently in premature infants. Seizures are a sign of an underlying cerebral pathology, the most common of which is hypoxic-ischaemic encephalopathy in term infants. Due to a growing body of evidence that seizures exacerbate cerebral injury, effective diagnosis and treatment of neonatal seizures is of paramount importance to reduce long-term adverse outcomes. Electroencephalography is essential for the diagnosis of seizures in neonates due to their subtle clinical expression, non-specific neurological presentation and a high frequency of electro-clinical uncoupling in the neonatal period. Hypoxic-ischaemic encephalopathy may require neuroprotective therapeutic hypothermia, accompanying sedation with opioids, anticonvulsant drugs or a combination of all of these. The efficacy, safety, tolerability and pharmacokinetics of seven anticonvulsant drugs (phenobarbital, phenytoin, levetiracetam, lidocaine, midazolam, topiramate and bumetanide) are reviewed. This review is focused only on studies reporting electrographically confirmed seizures and highlights the knowledge gaps that exist in optimal treatment regimens for neonatal seizures. Randomised controlled trials are needed to establish a safe and effective treatment protocol for neonatal seizures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The optimal treatment protocol for neonatal seizures remains elusive. Phenobarbital remains the first-line antiepileptic of choice, despite suboptimal efficacy and altered pharmacodynamic effects in neonates. There is currently no consensus regarding second-line drug choice, which often varies between phenytoin, lidocaine, levetiracetam or benzodiazepines. |
Hypothermia is the current standard of care for neuroprotection in hypoxic-ischaemic encephalopathy and many novel neuroprotective drugs are also emerging. Drug–drug interactions as well as drug–hypothermia interactions between antiepileptic drugs, novel neuroprotectants and hypothermia need to be investigated prior to administration in neonates, due to the potential for both pharmacokinetic and pharmacodynamic interactions. |
Continuous electroencephalography monitoring is essential as a measure of antiepileptic efficacy. |
Randomised, controlled trials are required to establish a safe and effective treatment regimen for neonatal seizures. |
1 Neonatal Seizures: An Overview
Neonatal seizures affect between one and five full-term neonates per 1000 live births, and are the most common neonatal neurological emergencies [1]. Moreover, the incidence of seizures increases in very low birth weight infants [2]. Phenobarbital remains the first-line drug for treatment of neonatal seizures, despite having only around 50 % efficacy [3]. There is little consensus about the best second-line treatment for neonatal seizures and there is considerable off-label use of antiepileptic medications with sparse efficacy data in the neonatal period.
1.1 Aetiology of Neonatal Seizures
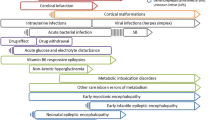
Seizures are a hallmark of neurological injury and approximately 60 % of all neonatal seizures are attributable to hypoxic-ischaemic encephalopathy (HIE) [4]. In Europe, HIE is the third most common cause of neonatal mortality, accounting for 9 % of all deaths and 21 % of term deaths, while globally it is estimated to cause approximately one million neonatal deaths each year [5, 6]. For the survivors of HIE, there is significant secondary morbidity, including cerebral palsy (29 %), cognitive delay (45 %), seizure disorders (12 %), sensorineural deafness (9 %), and visual loss (26 %) [7]. Seizures in HIE may exacerbate the underlying cerebral injury and increase the risk of detrimental neurodevelopmental consequences [8–11]. Perinatal arterial ischaemic stroke is the second most common cause of seizures in term neonates and accounts for 7.5–20 % of neonatal seizures [12, 13]. Seizures also arise from intracranial haemorrhage (7–18 %), congenital cerebral malformations (3–17 %), infection (2–14 %), metabolic causes (3–5 %), electrolyte imbalances (1–4 %) and other less common causes [14, 15]. There is little evidence on which to base recommendations for antiepileptic protocols regardless of seizure aetiology, although a prudent approach would be to treat the underlying cause and administer antiepileptic treatment according to hospital protocols.
1.2 Pathophysiological Aspects of Neonatal Seizures
Seizures are the result of excessive electrical firing of neurons in the brain [16]. The immature brain is more susceptible to seizures, primed by the early development of excitatory neurotransmitters, delayed inhibitory function of gamma-amino butyric acid (GABA) and an excess of excitatory glutamatergic neurons which are composed of more excitable subunits than the equivalent in adults [17, 18].
The binding of GABA agonists/modulators to the GABAA receptor triggers either an influx or an efflux of chloride ions, depending on the neuronal equilibrium potential for chloride [19]. It has been shown that the expression of inward sodium–potassium–chloride cotransporter (NKCC1) in human cortex is increased at birth compared to one year of age, whereas expression of outward potassium–chloride cotransporter (KCC2) increases from birth onwards [17]. This leads to an accumulation of intracellular chloride in immature neurons through NKCC1, thus the equilibrium potential for chloride becomes positive in relation to the resting membrane potential [19]. In immature neurons, activation of the GABAA receptor results in chloride efflux and neuronal depolarisation [19]. Furthermore, birth injuries such as ischaemia increases NKCC1 and decrease KCC2 expression, whereas hypoxic-ischaemic injury increases NKCC1 alone [20]. For these reasons, treatment of neonatal seizures with GABAA agonists, such as phenobarbital or benzodiazepines, may be suboptimal. Moreover, GABAA receptors are expressed at low levels in human and rodent cortex and contain less α1 subunits than their adult counterparts, decreasing their sensitivity to modulation by benzodiazepines [21]. Female rats have increased levels of KCC2, which translates to an inhibitory GABA action emerging earlier in females than in males [22]. Indeed, sex differences have been noted in mice, rats and humans with regards to susceptibility to brain injury, mechanisms of brain injury and response to treatment [23]. The increased seizure susceptibility due to developmental peculiarities of immature brain and excitatory GABA function might suggest that a class of antiepileptic drug (AED) other than GABA modulators should be considered as a first-line treatment for neonatal seizures. Furthermore, the sex differences observed in cotransporter expression raise questions regarding a differential approach to seizure treatment in male and female subjects.
1.3 Diagnosis of Neonatal Seizures
The diagnosis of neonatal seizures is challenging. Clinical seizure detection may lead to both over- and under-diagnosis [24]. Apart from classical tonic, clonic and myoclonic seizures, neonates may exhibit a wide variety of subtle seizure presentations including eye deviations, blinking, staring, chewing, sucking, cycling and boxing limb movements, apnoea and blood pressure changes [25]. Only a small portion of suspected neonatal clinical seizures are confirmed by electroencephalography (EEG) [24], while clinical signs may be absent in up to 80–90 % of electrographic seizures [26, 27]. The only randomised controlled trial comparing the effect of treatment of electrographic-only seizures to clinical-only seizures in neonates with HIE using a traditional AED protocol (phenobarbital up to 40 mg/kg, followed by fosphenytoin 20 mg/kg and third-line midazolam bolus or infusion) demonstrated significantly reduced seizure burden in neonates treated based on electrographic seizure activity [28].
The absence or cessation of clinical correlates when electrographic seizures are confirmed is called electro-clinical uncoupling [29, 30]. The subtle clinical seizure presentation in neonates and the phenomenon of electro-clinical uncoupling may be at least partly explained by the incomplete axonal dendritic and synaptic development, as well as incomplete myelination in the immature brain. Synaptic connectivity continues to increase until 2 years of age [31, 32]. Clinical seizures can become even more difficult to detect following the administration of anticonvulsants or sedative agents, during hypothermia treatment or in neonates in critical condition [30, 33]. Both phenobarbital and phenytoin produced equal rates of uncoupling, with 58 % of neonates exhibiting only or mostly electrographic evidence of seizures after drug administration [30]. Differential maturation of transporters that control intracellular chloride levels in different regions of the brain could be the mechanism underlying AED-induced uncoupling. Phenobarbital, a GABAA agonist, reduced epileptiform power in slices taken from the ventroposterior thalamus of postnatal day 9/10 rat pups, but had no such effect on slices of neocortex from the same animals, suggesting that GABA signalling is inhibitory in the ventroposterior thalamus, but may be excitatory in the neocortex at this age [29].
A simplified and compressed version of multichannel EEG called amplitude-integrated EEG (aEEG) uses fewer channels than traditional EEG and requires less expertise for interpretation. It is often used for diagnosis of neonatal seizures in the neonatal intensive care unit (NICU) [1]. However, some seizures may be missed using this technology, as it struggles to identify low amplitude, short duration (<1 min) and infrequent seizures [27, 34, 35]. In addition, neonatal seizures often remain focal and do not generalise [27]. Therefore, focal seizures in the regions beyond aEEG electrode placement sites may remain undetected. Furthermore, artefacts that mimic seizure activity on aEEG may cause additional complications and lead to false-positive readings [36]. Experience is required for reliable interpretation of both clinical and electrographic seizures, and studies have shown that non-expert users perform poorly in aEEG seizure detection [37]. There has been considerable effort in recent years to develop an automated neonatal seizure detection system to aid in clinical decision support in the NICU and one such algorithm is currently undergoing a clinical trial across Europe (NCT02160171) [38, 39].
Reliable diagnosis of neonatal seizures can only be performed using continuous EEG (cEEG) monitoring, which is considered the gold standard for the diagnosis of all neonatal seizures and for the assessment of anticonvulsant efficacy [24]. The role of cEEG monitoring extends to the differential diagnosis of seizure aetiology, particularly for HIE, stroke, infantile encephalopathy and congenital metabolic diseases [40, 41]. Multichannel cEEG monitoring of neonates at risk of seizures or suspected clinical seizures should be implemented rapidly to confirm diagnosis and optimise outcomes [42]. Laboratory tests and magnetic resonance imaging are also required to determine the underlying seizure pathology [43]. A protocol for laboratory workup in seizures is detailed in a previously published review [25].
2 Treatment Strategies
Once neonatal seizures are suspected, the neonate should be rapidly assessed for treatable underlying causes, such as hypoglycaemia or electrolyte disturbances [44]. AEDs are then administered according to clinical preference, independent of seizure cause. AEDs should only be initiated once seizure activity is confirmed, due to a lack of evidence for any positive outcomes if they are administered in the absence of seizures [3, 45].
As HIE is responsible for the majority of neonatal seizures and seizures are treated with the same AEDs regardless of underlying injury, the various treatments available for HIE-induced seizures are reviewed here. Neuro-protective strategies, currently led by therapeutic hypothermia, are initiated during the latent phase of HIE and may interact with AEDs that are administered during the secondary phase of HIE, and are therefore briefly mentioned in this context (Sect. 3).
2.1 Drug Treatment for Neonatal Seizures
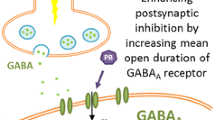
Neonatal seizures are neurological emergencies and must be treated promptly since seizures, particularly high seizure burden, may exacerbate neuronal injury in the immature brain and contribute to pathogenesis of later cerebral palsy and epilepsy [10, 46, 47]. In neonates with HIE who do not receive therapeutic hypothermia, there is a peak in seizure burden shortly after seizure onset (within 6 h) [48]. AEDs should ideally be administered within the time period prior to the peak seizure burden. However, current AEDs are sub-optimal in terms of effectiveness, safety and long-term outcomes [3, 49] and a systematic review has shown that the use of AEDs following perinatal asphyxia in the absence of confirmed seizures are of little benefit with no improvement in survival or neurodevelopmental outcome [45]. AEDs used in neonates act through a variety of mechanisms to reduce excitability in the brain, thereby suppressing the seizure. The mode of action of neonatal AEDs is illustrated in Fig. 1.
Mode of action of neonatal antiepileptic drugs (AEDs). Many drugs act by reducing excitatory neurotransmission (glutamatergic synapse). Phenytoin, lidocaine and topiramate prevent depolarisation by inhibiting voltage-gated sodium channels [50, 51]. Levetiracetam prevents calcium influx through N-type calcium channels which in turn reduces exocytosis and reduces the release of glutamate from intracellular vesicles by modulating synaptic vesicle protein 2A (SV2A) [51, 52]. On the postsynaptic terminal, phenobarbital and topiramate reduce excitatory neurotransmission via the AMPA/kainate glutamate receptor [51, 53]. Anticonvulsants, including phenobarbital, benzodiazepines and topiramate, work by enhancing inhibitory neurotransmission via the GABAA receptor (GABAergic synapse) [51]. Bumetanide can alter the action of GABAergic agents by preventing intracellular accumulation of chloride through NKCC1 [17]. AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, NMDA N-methyl-D-aspartate, GABA γ-amino butyric acid, GAD glutamic acid decarboxylase, SV2A synaptic vesicle protein 2A
2.2 Antiepileptic Drugs: Efficacy, Safety and Tolerability
The most frequently used AEDs in both term and preterm babies include phenobarbital, phenytoin, midazolam, lorazepam, clonazepam, and lidocaine [54]. Current recommendations suggest initiating anticonvulsant therapies in neonates with phenobarbital, adding either a benzodiazepine, phenytoin or lidocaine as a second-line agent if seizures continue [3] (Table 1). In a treatment protocol designed by Slaughter et al., a similar treatment regimen is proposed starting with phenobarbital, followed by levetiracetam, phenytoin or lidocaine and finally the addition of a benzodiazepine as a third-line agent [55]. In other studies, if seizures were not controlled by phenobarbital and/or phenytoin, drugs such as midazolam, clonazepam, lidocaine, levetiracetam and topiramate have been used [42, 55–57]. A survey of clinicians in the USA found that a majority (73 %) would use levetiracetam and/or topiramate despite limited knowledge about the pharmacokinetics of these drugs in new-born infants [58]. However, topiramate was shown to exacerbate cell apoptosis caused by phenytoin in rat pups, despite the absence of neurodegenerative properties when administered as monotherapy [59]. Thus, certain AED combinations may be detrimental to neurodevelopment. While the use of other AEDs (carbamazepine, paraldehyde, sodium valproate, vigabatrin, lamotrigine) in the treatment of neonatal seizures has been described in case reports [60, 61], and recent animal studies have shown a beneficial anti-seizure effect of potassium channel-opener flupirtine in a hypoxiamodel of neonatal seizures [62], we will focus on AEDs that have been recommended in neonatal treatment protocols and that have been studied in conjunction with EEG monitoring. AED efficacy is defined differently in many of the studies cited in Sects. 2.2, 2.3 and Table 1, but the vast majority state that efficacy is an 80 % reduction in seizure severity or complete seizure cessation, with one notable exception that defined 50 % seizure reduction as efficacious [33]. However, further work is required to define AED efficacy optimally using EEG criteria in view of the well described natural evolution of acute seizures in neonates, particularly those with HIE [48, 63, 64].
2.2.1 Phenobarbital and Phenytoin
Phenobarbital remains the first choice of AED in neonatal seizures, due to an extensive history of its use in this population [3]. Phenobarbital acts by increasing GABAA-mediated inhibition [51]. Neonates with persistent seizures are likely to have more severe brain damage and poor neurodevelopmental outcomes; thus, half of the babies on two AEDs and a staggering 95 % of babies on three AEDs were reported to have poor outcomes [47, 65]. Phenytoin, an antiepileptic that reduces excitatory neurotransmission by blocking a voltage-gated sodium channel, is often administered second-line to phenobarbital [51]. A Cochrane review found that there was very little supportive evidence for the main AEDs currently used in the neonatal period, as even with a combination treatment with phenobarbital and phenytoin, seizures remained in up to 50 % of babies, as confirmed by cEEG [42, 49, 66, 67].
2.2.2 Lidocaine
Lidocaine acts by inhibiting voltage-gated sodium channels, thereby preventing depolarisation [50]. Lidocaine is a promising AED in neonatal seizures administered either second-line or third-line with efficacy rates as high as 78 %, based on aEEG assessment [68–70]. A very recent retrospective study of aEEG data has found that lidocaine as a second- or third-line AED had a good (seizure control for at least 4 h) or intermediate (seizure control for at least 2 h) antiepileptic effect in 71.4 % of neonates, both term and preterm [70]. An earlier study demonstrated the lower efficacy rate of 60 % with lidocaine, supported by cEEG [42]. One of the main challenges of using lidocaine in neonates is the risk of adverse events, particularly with plasma concentrations >9 mg/L, including both bradycardia and ventricular tachycardia [68, 71]. As with many AEDs, a tailored neonatal dosage regimen is needed, as cardio-toxic levels were found in the majority of neonates treated with a standard lidocaine infusion [68]. A neonate-specific regimen was designed using pharmacokinetic modelling, and optimal lidocaine plasma levels were achieved in the majority of treated full-term neonates [68]. Furthermore, lidocaine dosing was studied in both term and preterm neonates, and it was found that both cohorts of neonates should receive approximately 50 % of the previously recommended dose, i.e. a 1 kg neonate should receive 52 mg as opposed to 110 mg [72]. However, lidocaine demonstrated a good safety profile in neonates [73].
2.2.3 Benzodiazepines
Benzodiazepines have had varied success as second- and third-line agents in the treatment of neonatal seizures. Benzodiazepines allosterically modulate the chloride channel in the GABAA receptor to increase inhibitory neurotransmission [51]. Midazolam response rates vary from 0–100 %, with both 0 and 100 % efficacy being observed using cEEG monitoring (see Table 1) [42, 67]. Efficacy rates measured by aEEG are reported as 50 % when midazolam is used as a second-line AED, increasing to 73–100 % when administered as a third-line AED [69, 74]. Midazolam appears to be less effective than lidocaine at treating persistent seizures, particularly those caused by the most severe form of HIE [69, 75].
The evidence for the effect of other benzodiazepines used in neonatal seizures is less convincing [55]. Clonazepam did not abolish any seizures as a second-line AED in three neonates monitored by cEEG [42]. The support for lorazepam as an AED is sparse, with less than half of the studied neonates monitored by cEEG [76, 77]. Seizure control rates were as high as 86 and 100 % in two studies, but these results are unreliable due to the absence of cEEG monitoring [76, 77].
2.2.4 Levetiracetam
Levetiracetam is a relatively new AED which is proposed to act through synaptic vesicle glycoprotein 2A (SV2A), which is a protein thought to be involved in the release of neurotransmitters [78]. Levetiracetam is efficacious in treating various seizures in both adults and children. In addition, levetiracetam has a very favourable pharmacokinetic and safety profile in neonates [79, 80]. Levetiracetam has demonstrated some efficacy as a neonatal and paediatric AED, according to cEEG findings which show 35–64 % efficacy within 24 h, rising to improvements in 52–100 % of patients in 72 h [33, 56]. Levetiracetam was initiated as a second- or third- line AED in the majority of recorded cases [33]. Evidence from randomised-controlled trials is needed to endorse levetiracetam as a safe and effective AED. A trial is ongoing in the USA looking at the safety, efficacy and pharmacokinetic profile of levetiracetam in neonates (NCT01720667), with more efficacy/safety trials planned in France (NCT02229123) and China (NCT02550028) [38].
2.2.5 Topiramate
Topiramate reduces the frequency of action potential firing by altering GABA neurotransmission, blocking voltage-gated sodium channels and by weakly blocking AMPA glutamate receptors [81]. Similar to levetiracetam, pharmacokinetic and safety profiles are favourable, but little is known about the safety, efficacy or pharmacokinetics in a critically ill new-born population [57]. In a small, retrospective study, topiramate was considered an effective add-on agent in neonatal seizures in four out of six neonates, and no major safety concerns were highlighted [57]. However, this study was limited by the lack of EEG monitoring [57].
2.3 Potential Adjunct Antiepileptics
2.3.1 Bumetanide
Bumetanide is a potential adjunct to AED treatments for neonatal seizures [82]. A number of years ago, bumetanide was observed to have antiepileptic effects in kainic acid-induced seizures in vivo [83]. This was believed to be due to its ability to block ion cotransporters in neurons and glia of the central nervous system (CNS), which in turn affected GABA signalling [83]. Bumetanide blocks NKCC co-transporters, NKCC1 and NKCC2, which both move chloride into cells [84]. Bumetanide was originally developed as a loop diuretic, which reduces oedema by inhibiting the reabsorption of sodium, potassium and chloride through NKCC2 in the thick ascending loop of Henle of the kidney [84]. Bumetanide also inhibits NKCC1, an isoform of the NKCC cotransporter that is widely expressed, including on neurons in the brain [84]. GABA is excitatory in immature neurons due to the accumulation of chloride through NKCC1 [85]. By preventing intracellular chloride build-up, bumetanide is thought to decrease or even reverse the excitatory action of GABA, thus presenting a potentially useful combination therapy with GABAergic anticonvulsants [64, 82]. There are gaps in our knowledge of this potential adjunct to AEDs for the treatment of neonatal seizures, namely the dose at which it acts in the brain, the human blood-brain barrier permeability/transport of bumetanide as well as its effect on development of the CNS. Two clinical trials were initiated to establish the safety and efficacy of bumetanide in neonatal seizures, one in Europe (NCT01434225) and one in the USA (NCT00830531) [38]. In the European dose-finding clinical study, bumetanide was administered according to a bivariate Bayesian sequential dose-escalation design, in which participants were treated with four doses of bumetanide (0.05–0.3 mg/kg) each given 12 h apart, with the first dose given in conjunction with phenobarbital [64]. However, the trial was concluded early as the benefit:risk ratio was no longer favourable and the efficacy endpoint was not achieved in any of the trial participants [64, 86]. It has been suggested that this is partially due to a poor CNS effect of bumetanide at the doses used and evidence to corroborate this have come from animal studies that indicate a poor brain permeability of bumetanide [87]. Many studies are examining novel ways to enhance brain levels of bumetanide in an effort to overcome the pharmacokinetic issues hindering its therapeutic success [87–90].
2.4 Pharmacokinetic Properties of AEDs
There are a variety of physiological differences between neonates and adults. These variations in physiology affect all pharmacokinetic processes in the neonate, including absorption, distribution, metabolism and elimination [60]. These variations are detailed in a review by Alcorn et al [98] but the salient changes are noted in Table 2. Key pharmacokinetic parameters, including volume of distribution (V d), fraction unbound in plasma (f u), clearance (Cl) and elimination half-life (t 1/2), are different in neonates compared to adults. Moreover, there is wide variability in these pharmacokinetic parameters within the neonatal population, as can be seen by the ranges reported (Table 3).
3 Combining Therapeutic Strategies
3.1 Adjunct Therapies in HIE with Potential for Interaction with Antiepileptics
3.1.1 Hypothermia
Hypothermia has demonstrated neuro-protective properties in neonates with moderate to severe HIE [107–109]. Since the introduction of therapeutic hypothermia, the composite risk of death and major disability has been reduced by approximately 25 % [108]. Neurological outcomes in cooled neonates with HIE improved at both 18 months and 6–7 years of age [108, 109]. Hypothermia significantly reduces seizure burden, as measured by cEEG, in neonates with HIE [110, 111]. Seizure burden during hypothermia is characterised by a more even distribution over time (as opposed to the accumulation seen at seizure-onset in normothermia) and de-novo seizures may occur after re-warming [10, 48, 63]. It has been proposed that hypothermia should also be tested as a therapeutic strategy in late premature neonates with HIE and neonatal stroke, both of which can also result in seizures [112].
3.1.2 Emerging Neuro-Protective Treatments
Additional neuro-protective strategies that are emerging include xenon, erythropoietin, melatonin, allopurinol and sevoflurane [113–119]. Thus far, the authors have found no reports of combination treatment with AEDs and emerging neuro-protective drugs. However, the combination of these novel neuro-protective agents, hypothermia and AEDs are a definite possibility in the future. Briefly, xenon protects the brain from excitatory injury by antagonising the N-methyl-d-aspartate (NMDA) glutamate receptor reducing total neurotransmission, and is currently under investigation in a Phase 2 trial [120]. Erythropoietin has anti-inflammatory properties and is also anti-apoptotic [121, 122]. It has been shown to reduce detrimental neurodevelopmental outcomes in neonates with moderate-severe HIE [123]. Melatonin reduces oxidative stress through a variety of mechanisms, such as scavenging oxygen free radicals and has been shown to augment neuroprotection by hypothermia in a piglet model of HIE [117]. Sevoflurane reduced hippocampal apoptosis in a rat model of intrauterine perinatal asphyxia and thus may be neuroprotective [113]. Allopurinol was found to have anti-oxidant properties [116].
3.1.3 Sedation
Intravenous morphine is commonly used as a sedative during hypothermia, as it reduces pain and stress, allows the patient to tolerate hypothermia and can be titrated to optimal response [124]. In a preclinical model of HIE, hypothermia without sedation lacked neuro-protective properties [125]. In a small group of term and preterm neonates without underlying brain injury, morphine infusion at a rate of 10–20 μg/kg/h was found to be associated with excessive epileptiform activity on cEEG [126].
It is known that morphine clearance is decreased during hypothermia, resulting in an increased concentration of morphine in both cerebrospinal fluid and plasma [127, 128]. In terms of pharmacodynamic considerations, the affinity of morphine for its receptor appears reduced in hypothermia, but the incidence of hypotension is increased [127, 129]. Neonates with HIE who are sedated with opioids show less brain injury and display better outcomes [130]. Little is known about drug–drug interactions with AEDs, but it is advised that barbiturates such as phenobarbital may increase the sedating effect of opioids [131].
3.2 Antiepileptics and Hypothermia
It is thought that synergistic therapy including a traditional AED and hypothermia may augment neuro-protective properties of either treatment given alone [132]. Combination treatments need to be explored further to complement this claim. However, caution needs to be exercised as hypothermia may alter pharmacokinetics of AEDs in neonates by decreasing absorption, distribution or metabolism/clearance [100, 133–135]. Moreover, as multi-organ dysfunction is frequently a characteristic of HIE, the combination of therapeutic hypothermia and organ impairment, particularly renal and hepatic, may have additive detrimental effects on fundamental pharmacokinetic processes [136]. The rewarming phase following hypothermia is another period of pharmacokinetic and pharmacodynamic uncertainty and is likely to be a window of time in which serious toxicity and adverse reactions could occur, due to a lag time between the return of normal metabolic enzyme and transporter function [135]. There have been reports of seizures re-occurring during the rewarming phase, but the affected infants were not receiving regular AEDs [10, 137]. Thus, combination treatment with hypothermia and AEDs may be useful, but must be approached with caution due to uncertainties regarding the effect of hypothermia on efficacy, safety and pharmacokinetics of such medications. It is important to identify AEDs, doses and dosage intervals that are suitable for neonates during and after hypothermia.
3.2.1 Phenobarbital and Hypothermia
Positive synergism of first-line AED phenobarbital and hypothermia was observed in a rodent model of HIE, with both early and late assessment of neuropathology and sensorimotor performance demonstrating improvements [138]. However, current evidence suggests that this combination has not translated to a reduced risk of death or brain damage in neonates [139, 140]. Seizures were detected using aEEG, and a 66 % reduction in seizures was demonstrated for neonates treated with hypothermia and with plasma concentrations of phenobarbital above 20 mg/L [140].
The pharmacokinetic profile of phenobarbital was examined in hypothermic critically ill neonates [100, 101]. It was found that minimum, maximum and average plasma concentrations were all larger in cooled neonates versus normothermia [100]. However, V d and clearance remained unchanged [101]. It was concluded the alterations in pharmacokinetics of phenobarbital during hypothermia in neonates were not clinically significant, and that a total maximum dose of 40 mg/kg can be safely administered in hypothermia prior to initiation of second-line AED [140]. In contrast, metabolism of phenobarbital via cytochrome P450 (CYP)2C19 was significantly reduced when it was administered to critically injured children who were cooled under more severe hypothermic conditions to 30–31 °C [135, 141]. Therapeutic drug monitoring of phenobarbital allows for tight control of AED concentrations, which may be particularly important during hypothermia.
3.2.2 Lidocaine and Hypothermia
Lidocaine was administered as a third-line anticonvulsant to neonates undergoing hypothermia treatment for asphyxia-induced seizures with aEEG monitoring. An impressive 91 % of these patients responded to lidocaine [134]. This is a similar response rate to that observed in normothermic babies [42].
The pharmacokinetics of lidocaine are altered by hypothermia. Clearance of lidocaine is reduced by 24 % as hepatic blood flow is reduced during hypothermia [134]. Despite these changes, no cardiotoxicity was observed in hypothermic neonates when an altered dosing regimen, equating to 70 % of the total lidocaine dose given to normothermic neonates, was administered [134].
3.2.3 Topiramate and Hypothermia
Animal studies suggested that the combination of topiramate and hypothermia improved motor and brain tissue damage in a model of HIE, where neither drug alone conferred any neuroprotection [142]. In neonates, there were no statistically significant changes in survival rate or brain damage observed when topiramate was given in combination with hypothermia when compared to hypothermia alone [97]. A randomised-controlled trial of topiramate and hypothermia in combination is underway, which will examine efficacy of seizure control with this treatment strategy (NCT01765218) [38, 81].
The pharmacokinetic profile of topiramate is altered when administered during hypothermia treatment: maximum, minimum and average concentrations, t 1/2 and area under the concentration-time curve are significantly higher in hypothermia [96]. However, these pharmacokinetic variations are not clinically significant, and the majority of neonates are observed to have topiramate concentrations within the safe, effective concentration range [96].
3.2.4 Midazolam and Hypothermia
The efficacy of midazolam as a second-line AED in seizing neonates undergoing hypothermia treatment is modest, achieving seizure control in only 23 % of neonates, confirmed using aEEG monitoring [75].
The pharmacokinetic profile of midazolam in neonates was not significantly changed by hypothermia [75, 143]. However, the incidence of midazolam-induced hypotension increased in neonates undergoing therapeutic hypothermia [75]. Midazolam levels in the serum of asphyxiated infants (both normothermic and hypothermic) were found to be highly variable and unpredictable, due to renal/hepatic impairment caused by the initial injury [143]. Furthermore, it is worth noting that the combination of midazolam and hypothermia in adults with disorders of the CNS gave rise to significant decreases in clearance and increases in V d of midazolam compared to midazolam treatment alone [144].
3.2.5 Bumetanide and Hypothermia
Bumetanide pharmacokinetics including clearance and V d were calculated in a neonatal population [64]. These patients were also receiving hypothermia treatment and phenobarbital. Clearance values appear to generally be in agreement with values previously reported in a neonatal population [145, 146]. The combination of phenobarbital, bumetanide and hypothermia in a neonatal population with HIE-induced seizures was not effective, as none of the neonates achieved the requisite 80 % seizure reduction without the need for rescue AEDs [64, 86].
3.2.6 Phenytoin and Hypothermia
There are no data from neonates with seizures on the efficacy or safety of phenytoin and hypothermia together. However, there are reports from trials on the use of phenytoin and hypothermia in older children aged 2–16 years, as well as adult patients, for the treatment of traumatic brain injury [147, 148]. In these populations, decreases in metabolism by CYP2C9 and CYP2C19 resulted in reduced clearance compared to values obtained after rewarming [135, 147, 148]. In children, it was also found that increased phenytoin levels are present both during and after rewarming, which increased the risk of drug toxicity even after hypothermia had finished [147]. Moreover, a case report has described an additive bradycardic effect of therapeutic hypothermia and phenytoin [149]. Hypothermia in this case occurred during surgery and was not controlled. The authors hypothesise that the cardiac depressant effects of both treatments acted synergistically and that extreme caution should be exercised when co-administration is necessary [149].
4 Knowledge Gaps
There is an urgent need for more randomised controlled trials in neonates to validate a treatment algorithm for seizures, especially when used in combination with hypothermia. Due to a lack of evidence from clinical studies, seizure treatments consist of older generation drugs that have more side-effects than newer drugs [3]. In general, efficacy rates of treatments are underwhelming (Table 1). Furthermore, there is a need to observe long-term neurodevelopmental outcomes following each of the proposed treatments, and to define the optimal length of time to continue with AED therapy given the concern regarding their effect on long-term brain development [16, 150].
There is a paucity of data on the pharmacokinetics and efficacy of many AEDs used in neonates, including levetiracetam, lidocaine and topiramate [151]. There are also major gaps in our knowledge about the efficacy and safety of most anticonvulsant drugs, particularly in preterm neonates.
In HIE, it is imperative that the pharmacokinetics of AEDs during both active therapeutic hypothermia and the rewarming phase in neonates with seizures are elucidated, particularly with regards to phenytoin, a popular second-line AED [135]. Furthermore, drug–drug interactions are significantly under-investigated, especially co-administration of AEDs with novel neuro-protective drugs such as xenon, allopurinol, melatonin and erythropoietin.
Multichannel EEG is essential to accurately measure the efficacy of AEDs [24]. The optimal algorithm for detecting seizures remains to be developed, to enhance bedside recognition of seizures by non-EEG experts.
In the last decade there has been an increased interest in developing safe and effective drugs for neonates. The European Commission through the FP7 Framework promoted research on the safe and effective use of medicine in children by specifically supporting applications for the neonatal age group [152]. More recently, the International Neonatal Consortium was launched in May 2015. This is a consortium of stakeholders focused on the development of effective medicines for neonates, including the Food and Drug Administration (FDA), European Medicines Agency (EMA), the pharmaceutical industry, academia, patient research groups, and family advocates. The consortium aims to align priorities and initiate collaborations to accelerate the development of safe and effective treatments for neonates. One of the first topics that this consortium has prioritised for further development is the treatment of neonatal seizures.
5 Conclusion
The treatment of neonatal seizures remains sub-optimal. Treatment algorithms are based on minimal trial data on older generation drugs. Phenobarbital remains the first-line antiepileptic of choice, despite suboptimal efficacy and altered underlying pharmacodynamics in the immature brain. However, there is no consensus on a replacement first-line drug or even on the most efficacious and suitable second-line AED. A lack of randomised controlled trials to guide treatment regimens in neonatal seizures is halting progress in the field. There are a multitude of drug–drug and drug–hypothermia interactions that remain to be elucidated, including the efficacy/safety of antiepileptic polypharmacy in neonates. These knowledge gaps have been identified and urgently need to be bridged by designing and conducting high quality clinical trials in neonates. Until the pharmacokinetic/pharmacodynamic profiles of antiepileptic medications in hypothermia are sufficiently investigated, therapeutic drug monitoring of serum antiepileptic levels is encouraged. The efficacy of antiepileptic treatment protocols should always be measured using cEEG monitoring.
References
Glass HC. Neonatal seizures: advances in mechanisms and management. Clin Perinatol. 2014;41(1):177–90.
Lanska MJ, Lanska DJ. Neonatal seizures in the United States: results of the National Hospital Discharge Survey, 1980–1991. Neuroepidemiology. 1996;15(3):117–25.
World Health Organisation. Guideline on Neonatal Seizures. 2011. http://apps.who.int/mental_health/publications/guidelines_neonatal_seizures/en/. Accessed 17 Apr 2015.
Volpe J. Neonatal Seizures. In: Volpe J, editor. Neurology of the Newborn. 5th ed. Philadelphia: WB Saunders; 2008. p. 203–44.
Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, Niermeyer S, Ellis M, Robertson NJ, Cousens S, Lawn JE. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(Suppl 1):50–72.
Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900.
Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379(9814):445–52.
Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, Newton N, Partridge JC, Glidden DV, Vigneron DB, Barkovich AJ. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58(4):542–8.
Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155(3):318–23.
Shah DK, Wusthoff CJ, Clarke P, Wyatt JS, Ramaiah SM, Dias RJ, Becher JC, Kapellou O, Boardman JP. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99(3):F219–24.
McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55(4):506–13.
Wu YW, Lynch JK, Nelson KB. Perinatal arterial stroke: understanding mechanisms and outcomes. Semin Neurol. 2005;25(4):424–34.
Kirton A, Armstrong-Wells J, Chang T, Deveber G, Rivkin MJ, Hernandez M, Carpenter J, Yager JY, Lynch JK, Ferriero DM. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011;128(6):e1402–10.
Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18(4):185–91.
Levene MI, Trounce JQ. Cause of neonatal convulsions. Towards more precise diagnosis. Arch Dis Child. 1986;61(1):78–9.
Beaulieu MJ. Levetiracetam. Neonatal Netw. 2013;32(4):285–8.
Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11(11):1205–13.
D’Souza SW, Slater P. Excitatory amino acids in neonatal brain: contributions to pathology and therapeutic strategies. Arch Dis Child Fetal Neonatal Ed. 1995;72(3):F147–50.
Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18(4):175–84.
Kang S, Kadam S. Pre-clinical models of acquired neonatal seizures: differential effects of injury on function of chloride co-transporters. Austin J Cerebrovasc Dis Stroke. 2014;1(6):1026.
Silverstein FS, Jensen FE, Inder T, Hellstrom-Westas L, Hirtz D, Ferriero DM. Improving the treatment of neonatal seizures: National Institute of Neurological Disorders and Stroke workshop report. J Pediatr. 2008;153(1):12–5.
McGoldrick MK, Galanopoulou AS. Developmental pharmacology of benzodiazepines under normal and pathological conditions. Epileptic Disord. 2014;16(Suppl 1):59–68.
Fatemi A, Wilson MA, Johnston MV. Hypoxic ischemic encephalopathy in the term infant. Clin Perinatol. 2009;36(4):835–58.
Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93(3):F187–91.
Hallberg B, Blennow M. Investigations for neonatal seizures. Semin Fetal Neonatal Med. 2013;18(4):196–201.
Abend NS, Wusthoff CJ, Goldberg EM, Dlugos DJ. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013;12(12):1170–9.
Bye AM, Flanagan D. Spatial and temporal characteristics of neonatal seizures. Epilepsia. 1995;36(10):1009–16.
Srinivasakumar P, Zempel J, Trivedi S, Wallendorf M, Rao R, Smith B, Inder T, Mathur AM. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136(5):e1302–9.
Glykys J, Dzhala VI, Kuchibhotla KV, Feng G, Kuner T, Augustine G, Bacskai BJ, Staley KJ. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009;63(5):657–72.
Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28(4):277–80.
Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484(2):156–67.
van den Heuvel MP, Kersbergen KJ, de Reus MA, Keunen K, Kahn RS, Groenendaal F, de Vries LS, Benders MJNL. The neonatal connectome during preterm brain development. Cereb Cortex (New York, NY). 2015;25(9):3000–13.
Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. J Child Neurol. 2011;26(4):465–70.
Shah DK, Boylan GB, Rennie JM. Monitoring of seizures in the newborn. Arch Dis Child Fetal Neonatal Ed. 2012;97(1):F65–9.
Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120(4):770–7.
Shah DK, Mackay MT, Lavery S, Watson S, Harvey AS, Zempel J, Mathur A, Inder TE. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121(6):1146–54.
Rennie JM, Chorley G, Boylan GB, Pressler R, Nguyen Y, Hooper R. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch Dis Child Fetal Neonatal Ed. 2004;89(1):F37–40.
US National Institutes of Health. ClinicalTrials.gov. 2015. http://clinicaltrials.gov. Accessed 13 July 2015.
Mathieson SR, Stevenson NJ, Low E, Marnane WP, Rennie JM, Temko A, Lightbody G, Boylan GB. Validation of an automated seizure detection algorithm for term neonates. Clin Neurophysiol. 2016;127(1):156–8.
Low E, Mathieson SR, Stevenson NJ, Livingstone V, Ryan CA, Bogue CO, Rennie JM, Boylan GB. Early postnatal EEG features of perinatal arterial ischaemic stroke with seizures. PLoS One. 2014;9(7):e100973.
Pressler R, Binnie CD, Cooper R, Robinson R, editors. Neonatal and paediatric clinical neurophysiology. London: Churchill Livingstone; 2007.
Boylan GB, Rennie JM, Chorley G, Pressler RM, Fox GF, Farrer K, Morton M, Binnie CD. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology. 2004;62(3):486–8.
Weeke LC, Groenendaal F, Toet MC, Benders MJ, Nievelstein RA, van Rooij LG, de Vries LS. The aetiology of neonatal seizures and the diagnostic contribution of neonatal cerebral magnetic resonance imaging. Dev Med Child Neurol. 2015;57(3):248–56.
Glass HC, Sullivan JE. Neonatal seizures. Curr Treat Options Neurol. 2009;11(6):405–13.
Evans DJ, Levene MI, Tsakmakis M. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database Syst Rev. 2007;(3):CD001240.
van Rooij LG, Toet MC, van Huffelen AC, Groenendaal F, Laan W, Zecic A, de Haan T, van Straaten IL, Vrancken S, van Wezel G, van der Sluijs J, Ter Horst H, Gavilanes D, Laroche S, Naulaers G, de Vries LS. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125(2):e358–66.
Glass HC, Nash KB, Bonifacio SL, Barkovich AJ, Ferriero DM, Sullivan JE, Cilio MR. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159(5):731–5.
Lynch NE, Stevenson NJ, Livingstone V, Murphy BP, Rennie JM, Boylan GB. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53(3):549–57.
Booth D, Evans DJ. Anticonvulsants for neonates with seizures. Cochrane Database Syst Rev. 2004;(4):CD004218.
Borowicz KK, Banach M. Antiarrhythmic drugs and epilepsy. Pharmacol Rep. 2014;66(4):545–51.
Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9(1):68–82.
Irish Medicines Board. Summaries of product characteristics-levetiracetam [Online]. 2013. http://www.medicines.ie. Accessed 5 January 2016.
Nardou R, Yamamoto S, Bhar A, Burnashev N, Ben-Ari Y, Khalilov I. Phenobarbital but not diazepam reduces AMPA/kainate receptor mediated currents and exerts opposite actions on initial seizures in the neonatal rat hippocampus. Front Cell Neurosci. 2011;5:16.
van Rooij LG, Hellstrom-Westas L, de Vries LS. Treatment of neonatal seizures. Semin Fetal Neonatal Med. 2013;18(4):209–15.
Slaughter LA, Patel AD, Slaughter JL. Pharmacological treatment of neonatal seizures: a systematic review. J Child Neurol. 2013;28(3):351–64.
Khan O, Chang E, Cipriani C, Wright C, Crisp E, Kirmani B. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44(4):265–9.
Glass HC, Poulin C, Shevell MI. Topiramate for the treatment of neonatal seizures. Pediatr Neurol. 2011;44(6):439–42.
Silverstein FS, Ferriero DM. Off-label use of antiepileptic drugs for the treatment of neonatal seizures. Pediatr Neurol. 2008;39(2):77–9.
Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J Pharmacol Exp Ther. 2007;323(1):165–73.
Tulloch JK, Carr RR, Ensom MH. A systematic review of the pharmacokinetics of antiepileptic drugs in neonates with refractory seizures. J Pediatr Pharmacol Ther. 2012;17(1):31–44.
Mikati MA, Fayad M, Koleilat M, Mounla N, Hussein R, Kazma A, Yunis K. Efficacy, tolerability, and kinetics of lamotrigine in infants. J Pediatr. 2002;141(1):31–5.
Sampath D, Shmueli D, White AM, Raol YH. Flupirtine effectively prevents development of acute neonatal seizures in an animal model of global hypoxia. Neurosci Lett. 2015;607:46–51.
Lynch NE, Stevenson NJ, Livingstone V, Mathieson S, Murphy BP, Rennie JM, Boylan GB. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
Pressler RM, Boylan GB, Marlow N, Blennow M, Chiron C, Cross JH, de Vries LS, Hallberg B, Hellstrom-Westas L, Jullien V, Livingstone V, Mangum B, Murphy B, Murray D, Pons G, Rennie J, Swarte R, Toet MC, Vanhatalo S, Zohar S. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015;14(5):469–77.
Maartens IA, Wassenberg T, Buijs J, Bok L, de Kleine MJ, Katgert T, Andriessen P. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101(4):e173–8.
Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, Paneth N, Minnigh B, Alvin J. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341(7):485–9.
Castro Conde JR, Hernandez Borges AA, Domenech Martinez E, Gonzalez Campo C, Perera Soler R. Midazolam in neonatal seizures with no response to phenobarbital. Neurology. 2005;64(5):876–9.
Malingre MM, Van Rooij LG, Rademaker CM, Toet MC, Ververs TF, van Kesteren C, de Vries LS. Development of an optimal lidocaine infusion strategy for neonatal seizures. Eur J Pediatr. 2006;165(9):598–604.
Shany E, Benzaqen O, Watemberg N. Comparison of continuous drip of midazolam or lidocaine in the treatment of intractable neonatal seizures. J Child Neurol. 2007;22(3):255–9.
Weeke LC, Toet MC, van Rooij LG, Groenendaal F, Boylan GB, Pressler RM, Hellstrom-Westas L, van den Broek MP, de Vries LS. Lidocaine response rate in aEEG-confirmed neonatal seizures: retrospective study of 413 full-term and preterm infants. Epilepsia. 2015 [Epub ahead of print].
van Rooij LG, Toet MC, Rademaker KM, Groenendaal F, de Vries LS. Cardiac arrhythmias in neonates receiving lidocaine as anticonvulsive treatment. Eur J Pediatr. 2004;163(11):637–41.
van den Broek MP, Huitema AD, van Hasselt JG, Groenendaal F, Toet MC, Egberts TC, de Vries LS, Rademaker CM. Lidocaine (lignocaine) dosing regimen based upon a population pharmacokinetic model for preterm and term neonates with seizures. Clin Pharmacokinet. 2011;50(7):461–9.
Lundqvist M, Agren J, Hellstrom-Westas L, Flink R, Wickstrom R. Efficacy and safety of lidocaine for treatment of neonatal seizures. Acta Paediatr. 2013;102(9):863–7.
van Leuven K, Groenendaal F, Toet MC, Schobben AF, Bos SA, de Vries LS, Rademaker CM. Midazolam and amplitude-integrated EEG in asphyxiated full-term neonates. Acta Paediatr. 2004;93(9):1221–7.
van den Broek MP, van Straaten HL, Huitema AD, Egberts T, Toet MC, de Vries LS, Rademaker K, Groenendaal F. Anticonvulsant effectiveness and hemodynamic safety of midazolam in full-term infants treated with hypothermia. Neonatology. 2015;107(2):150–6.
Deshmukh A, Wittert W, Schnitzler E, Mangurten HH. Lorazepam in the treatment of refractory neonatal seizures. A pilot study. Am J Dis Child. 1986;140(10):1042–4.
Maytal J, Novak GP, King KC. Lorazepam in the treatment of refractory neonatal seizures. J Child Neurol. 1991;6(4):319–23.
Talos DM, Chang M, Kosaras B, Fitzgerald E, Murphy A, Folkerth RD, Jensen FE. Antiepileptic effects of levetiracetam in a rodent neonatal seizure model. Pediatr Res. 2013;73(1):24–30.
Merhar SL, Schibler KR, Sherwin CM, Meinzen-Derr J, Shi J, Balmakund T, Vinks AA. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159(1):152–4.e3.
Briggs DE, French JA. Levetiracetam safety profiles and tolerability in epilepsy patients. Expert Opin Drug Saf. 2004;3(5):415–24.
Filippi L, Fiorini P, Daniotti M, Catarzi S, Savelli S, Fonda C, Bartalena L, Boldrini A, Giampietri M, Scaramuzzo R, Papoff P, Del Balzo F, Spalice A, la Marca G, Malvagia S, Della Bona ML, Donzelli G, Tinelli F, Cioni G, Pisano T, Falchi M, Guerrini R. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI). BMC Pediatr. 2012;12:144.
Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63(2):222–35.
Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Res. 1998;32(1–2):275–85.
Haas M, Forbush B 3rd. The Na–K–Cl cotransporters. J Bioenerg Biomembr. 1998;30(2):161–72.
Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–39.
Pressler RM, Boylan GB, Marlow N, de Vries LS, Blennow M, Chiron C, Cross JH, Hallberg B, Hellstrom-Westas L, Jullien V, Mangum B, Murphy B, Murray D, Pons G, Rennie J, Toet MC, Zohar S. Bumetanide for neonatal seizures-back from the cotside. Nat Rev Neurol. 2015;11(24):724.
Donovan MD, O’Brien FE, Boylan GB, Cryan JF, Griffin BT. The effect of organic anion transporter 3 inhibitor probenecid on bumetanide levels in the brain: an integrated in vivo microdialysis study in the rat. J Pharm Pharmacol. 2015;67(4):501–10.
Topfer M, Tollner K, Brandt C, Twele F, Broer S, Loscher W. Consequences of inhibition of bumetanide metabolism in rodents on brain penetration and effects of bumetanide in chronic models of epilepsy. Eur J Neurosci. 2014;39(4):673–87.
Tollner K, Brandt C, Topfer M, Brunhofer G, Erker T, Gabriel M, Feit PW, Lindfors J, Kaila K, Loscher W. A novel prodrug-based strategy to increase effects of bumetanide in epilepsy. Ann Neurol. 2014;75(4):550–62.
Tollner K, Brandt C, Romermann K, Loscher W. The organic anion transport inhibitor probenecid increases brain concentrations of the NKCC1 inhibitor bumetanide. Eur J Pharmacol. 2014;746c:167–73.
Brodie MJ, Kwan P. Current position of phenobarbital in epilepsy and its future. Epilepsia. 2012;53(Suppl 8):40–6.
Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993:103–14.
Loiacono G, Masci M, Zaccara G, Verrotti A. The treatment of neonatal seizures: focus on Levetiracetam. J Matern Fetal Neonatal Med. 2016;29(1):69–74.
Khan O, Cipriani C, Wright C, Crisp E, Kirmani B. Role of intravenous levetiracetam for acute seizure management in preterm neonates. Pediatr Neurol. 2013;49(5):340–3.
Sharpe CM, Capparelli EV, Mower A, Farrell MJ, Soldin SJ, Haas RH. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72(1):43–9.
Filippi L, la Marca G, Fiorini P, Poggi C, Cavallaro G, Malvagia S, Pellegrini-Giampietro DE, Guerrini R. Topiramate concentrations in neonates treated with prolonged whole body hypothermia for hypoxic ischemic encephalopathy. Epilepsia. 2009;50(11):2355–61.
Filippi L, Poggi C, la Marca G, Furlanetto S, Fiorini P, Cavallaro G, Plantulli A, Donzelli G, Guerrini R. Oral topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia: a safety study. J Pediatr. 2010;157(3):361–6.
Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55(5):667–86.
Marsot A, Brevaut-Malaty V, Vialet R, Boulamery A, Bruguerolle B, Simon N. Pharmacokinetics and absolute bioavailability of phenobarbital in neonates and young infants, a population pharmacokinetic modelling approach. Fundam Clin Pharmacol. 2014;28(4):465–71.
Filippi L, la Marca G, Cavallaro G, Fiorini P, Favelli F, Malvagia S, Donzelli G, Guerrini R. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: a pharmacokinetic study during whole body hypothermia. Epilepsia. 2011;52(4):794–801.
Shellhaas RA, Ng CM, Dillon CH, Barks JD, Bhatt-Mehta V. Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therapeutic hypothermia. Pediatr Crit Care Med. 2013;14(2):194–202.
Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, Leppik IE, Tomson T, Perucca E. Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239–76.
Loughnan PM, Greenwald A, Purton WW, Aranda JV, Watters G, Neims AH. Pharmacokinetic observations of phenytoin disposition in the newborn and young infant. Arch Dis Child. 1977;52(4):302–9.
Al Za’abi M, Lanner A, Xiaonian X, Donovan T, Charles B. Application of routine monitoring data for determination of the population pharmacokinetics and enteral bioavailability of phenytoin in neonates and infants with seizures. Ther Drug Monit. 2006;28(6):793–9.
Bjorkman S. Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol. 2005;59(6):691–704.
Jullien V, Pressler RM, Boylan G, Blennow M, Marlow N, Chiron C, Pons G. Pilot evaluation of the population pharmacokinetics of bumetanide in term newborn infants with seizures. J Clin Pharmacol. 2015 [Epub ahead of print].
Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58.
Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–66.
Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140–9.
Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013;163(2):465–70.
Low E, Boylan GB, Mathieson SR, Murray DM, Korotchikova I, Stevenson NJ, Livingstone V, Rennie JM. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97(4):F267–72.
Gancia P, Pomero G. Therapeutic hypothermia in the prevention of hypoxic-ischaemic encephalopathy: new categories to be enrolled. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):94–6.
Yang T, Zhuang L, Rei Fidalgo AM, Petrides E, Terrando N, Wu X, Sanders RD, Robertson NJ, Johnson MR, Maze M, Ma D. Xenon and sevoflurane provide analgesia during labor and fetal brain protection in a perinatal rat model of hypoxia-ischemia. PLoS One. 2012;7(5):e37020.
Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J. Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29(4):707–14.
Palmer C, Towfighi J, Roberts RL, Heitjan DF. Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr Res. 1993;33(4 Pt 1):405–11.
Kaandorp JJ, Benders MJ, Rademaker CM, Torrance HL, Oudijk MA, de Haan TR, Bloemenkamp KW, Rijken M, van Pampus MG, Bos AF, Porath MM, Oetomo SB, Willekes C, Gavilanes AW, Wouters MG, van Elburg RM, Huisjes AJ, Bakker SC, van Meir CA, von Lindern J, Boon J, de Boer IP, Rijnders RJ, Jacobs CJ, Uiterwaal CS, Mol BW, Visser GH, van Bel F, Derks JB. Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-Trial); a randomized double blind placebo controlled multicenter study. BMC Pregnancy Childbirth. 2010;10:8.
Robertson NJ, Faulkner S, Fleiss B, Bainbridge A, Andorka C, Price D, Powell E, Lecky-Thompson L, Thei L, Chandrasekaran M, Hristova M, Cady EB, Gressens P, Golay X, Raivich G. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136(Pt 1):90–105.
Mazur M, Miller RH, Robinson S. Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. J Neurosurg Pediatr. 2010;6(3):206–21.
Rangarajan V, Juul SE. Erythropoietin: emerging role of erythropoietin in neonatal neuroprotection. Pediatr Neurol. 2014;51(4):481–8.
Dingley J, Tooley J, Liu X, Scull-Brown E, Elstad M, Chakkarapani E, Sabir H, Thoresen M. Xenon ventilation during therapeutic hypothermia in neonatal encephalopathy: a feasibility study. Pediatrics. 2014;133(5):809–18.
Traudt CM, McPherson RJ, Bauer LA, Richards TL, Burbacher TM, McAdams RM, Juul SE. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev Neurosci. 2013;35(6):491–503.
Traudt CM, Juul SE. Erythropoietin as a neuroprotectant for neonatal brain injury: animal models. Methods Mol Biol. 2013;982:113–26.
Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, Ji L, Guo X, Xiong H, Simbruner G, Blomgren K, Wang X. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124(2):e218–26.
Gill H, Thoresen M, Smit E, Davis J, Liu X, Dingley J, Elstad M. Sedation management during therapeutic hypothermia for neonatal encephalopathy: atropine premedication for endotracheal intubation causes a prolonged increase in heart rate. Resuscitation. 2014;85(10):1394–8.
Thoresen M, Satas S, Loberg EM, Whitelaw A, Acolet D, Lindgren C, Penrice J, Robertson N, Haug E, Steen PA. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res. 2001;50(3):405–11.
Young GB, da Silva OP. Effects of morphine on the electroencephalograms of neonates: a prospective, observational study. Clin Neurophysiol. 2000;111(11):1955–60.
Bansinath M, Turndorf H, Puig MM. Influence of hypo and hyperthermia on disposition of morphine. J Clin Pharmacol. 1988;28(9):860–4.
Roka A, Melinda KT, Vasarhelyi B, Machay T, Azzopardi D, Szabo M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121(4):e844–9.
Puig MM, Warner W, Tang CK, Laorden ML, Turndorf H. Effects of temperature on the interaction of morphine with opioid receptors. Br J Anaesth. 1987;59(11):1459–64.
Angeles DM, Ashwal S, Wycliffe ND, Ebner C, Fayard E, Sowers L, Holshouser BA. Relationship between opioid therapy, tissue-damaging procedures, and brain metabolites as measured by proton MRS in asphyxiated term neonates. Pediatr Res. 2007;61(5 Pt 1):614–21.
Stockley’s Drug Interactions. In: Medicines complete database. 2009. http://www.medicinescomplete.com. Accessed: 23 July 2015.
Cilio MR, Ferriero DM. Synergistic neuroprotective therapies with hypothermia. Semin Fetal Neonatal Med. 2010;15(5):293–8.
Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35(9):2196–204.
van den Broek MP, Rademaker CM, van Straaten HL, Huitema AD, Toet MC, de Vries LS, Egberts AC, Groenendaal F. Anticonvulsant treatment of asphyxiated newborns under hypothermia with lidocaine: efficacy, safety and dosing. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F341–5.
Zhou J, Poloyac SM. The effect of therapeutic hypothermia on drug metabolism and response: cellular mechanisms to organ function. Expert Opin Drug Metab Toxicol. 2011;7(7):803–16.
Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F152–5.
Kendall GS, Mathieson S, Meek J, Rennie JM. Recooling for rebound seizures after rewarming in neonatal encephalopathy. Pediatrics. 2012;130(2):e451–5.
Barks JD, Liu YQ, Shangguan Y, Silverstein FS. Phenobarbital augments hypothermic neuroprotection. Pediatr Res. 2010;67(5):532–7.
Meyn DF Jr, Ness J, Ambalavanan N, Carlo WA. Prophylactic phenobarbital and whole-body cooling for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2010;157(2):334–6.
van den Broek MP, Groenendaal F, Toet MC, van Straaten HL, van Hasselt JG, Huitema AD, de Vries LS, Egberts AC, Rademaker CM. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin Pharmacokinet. 2012;51(10):671–9.
Kadar D, Tang BK, Conn AW. The fate of phenobarbitone in children in hypothermia and at normal body temperature. Can Anaesth Soc J. 1982;29(1):16–23.
Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35(6):1460–5.
Welzing L, Junghaenel S, Weiss V, Roth B, Mueller C, Wiesen MH. Disposition of midazolam in asphyxiated neonates receiving therapeutic hypothermia–a pilot study. Klin Padiatr. 2013;225(7):398–404.
Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60(2):225–30.
Lopez-Samblas AM, Adams JA, Goldberg RN, Modi MW. The pharmacokinetics of bumetanide in the newborn infant. Biol Neonate. 1997;72(5):265–72.
Pacifici GM. Clinical pharmacology of the loop diuretics furosemide and bumetanide in neonates and infants. Paediatr Drugs. 2012;14(4):233–46.
Empey PE, de Mendizabal NV, Bell MJ, Bies RR, Anderson KB, Kochanek PM, Adelson PD, Poloyac SM. Therapeutic hypothermia decreases phenytoin elimination in children with traumatic brain injury. Crit Care Med. 2013;41(10):2379–87.
Iida Y, Nishi S, Asada A. Effect of mild therapeutic hypothermia on phenytoin pharmacokinetics. Ther Drug Monit. 2001;23(3):192–7.
Bhagat H, Bithal PK, Chouhan RS, Arora R. Is phenytoin administration safe in a hypothermic child? J Clin Neurosci. 2006;13(9):953–5.
Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36(4):881–900.
Kanhere S. Recent advances in neonatal seizures. Indian J Pediatr. 2014;81(9):917–25.
Donnelly F. EU initiatives for research involving children. Eur J Pediatr. 2008;167(7):837–8.
Acknowledgments
The authors would like to extend their gratitude to Dr. Roman Stilling for providing expert help with artwork.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
MDD is in receipt of an Irish Research Council for Science Engineering and Technology scholarship. GBB was supported under European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no 241479 and by Science Foundation Ireland in the form of a centre grant (INFANT SFI/12/RC/2272). JFC is supported in part by Science Foundation Ireland in the form of a centre grant (Alimentary Pharmabiotic Centre Grant Number SFI/12/RC/2273), by the Health Research Board of Ireland (Grant Numbers HRA_POR/2011/23 and HRA_POR/2012/32) and by the European Community’s Seventh Framework Programme, Grant no. FP7/2007-2013, Grant Agreement number 278948 (TACTICS—Translational Adolescent and Childhood Therapeutic Interventions in Compulsive Syndrome). No funding was specifically received for the publication of this article.
Conflict of interest
Maria Donovan, Brendan Griffin, Liudmila Kharoshankaya, John Cryan and Geraldine Boylan declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Donovan, M.D., Griffin, B.T., Kharoshankaya, L. et al. Pharmacotherapy for Neonatal Seizures: Current Knowledge and Future Perspectives. Drugs 76, 647–661 (2016). https://doi.org/10.1007/s40265-016-0554-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0554-7