Abstract
Nivolumab (Opdivo®; Nivolumab BMS™) was the first programmed death (PD)-1 immune checkpoint inhibitor to be approved for use in advanced, squamous non-small cell lung cancer (NSCLC) following prior chemotherapy. In the pivotal CheckMate 017 trial, intravenous nivolumab 3 mg/kg every 2 weeks was associated with significantly better overall survival and progression-free survival and a significantly higher overall response rate than intravenous docetaxel in the second-line treatment of advanced, squamous NSCLC. Nivolumab was also better tolerated than docetaxel in CheckMate 017, and its adverse event profile (which included immune-mediated adverse events) was manageable. In conclusion, nivolumab represents an important advance in previously-treated, advanced, squamous NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First PD-1 immune checkpoint inhibitor approved for the treatment of advanced, squamous NSCLC following prior chemotherapy |
Significantly better overall survival and progression-free survival seen with nivolumab versus docetaxel in the second-line treatment setting |
Durable responses seen with nivolumab, with a significantly higher objective response rate than docetaxel |
Manageable adverse event profile (including immune-mediated adverse events) |
Better tolerated than docetaxel |
1 Introduction
Lung cancer is often diagnosed at a late stage and is the leading cause of cancer death worldwide [1, 2]. Non-small cell lung cancer (NSCLC) accounts for >85 % of lung cancer cases, with the nonsquamous histological subtype (including adenocarcinoma, large-cell carcinoma and other cell types) seen in ≈70 % of NSCLC cases and the squamous histological subtype seen in the remaining 30 % of cases [2, 3].
The programmed death (PD)-1 receptor is expressed on activated T cells and the key ligands for this receptor are PD-L1 and PD-L2 [4, 5]. PD-L1 is upregulated in many tumours, including NSCLC, and this overexpression is thought to help tumours evade immune responses [6, 7]. Binding of PD-L1 or PD-L2 to PD-1 receptors inhibits T-cell activation and dampens antitumour immune responses [7]. Thus, the PD-1 receptor represents a rational target for cancer immunotherapy.
Nivolumab (Opdivo®; Nivolumab BMS™) was the first PD-1 immune checkpoint inhibitor to be approved for use in advanced, squamous NSCLC following prior chemotherapy. Specifically, nivolumab is approved in the USA for the treatment of patients with metastatic squamous NSCLC with progression on or after platinum-based chemotherapy [8] and in the EU for the treatment of adults with locally advanced or metastatic squamous NSCLC after prior chemotherapy [9]. This narrative review discusses the clinical efficacy and tolerability of nivolumab in advanced, squamous NSCLC, as well as discussing its pharmacology. Nivolumab is also approved in the USA [8] and the EU [10] for the treatment of advanced melanoma and in the USA for use in previously-treated patients with advanced, nonsquamous NSCLC [11]; however, discussion of these indications is beyond the scope of this review.
2 Pharmacodynamic Properties of Nivolumab
Nivolumab is a fully human, IgG4 monoclonal antibody [4, 7, 12]. It binds to the PD-1 receptor and blocks the interaction of the receptor with PD-L1 and PD-L2, thereby preventing T-cell inhibition and restoring antitumour immune responses [4, 7, 12].
Nivolumab has high affinity and specificity for the PD-1 receptor [4, 13]. The binding affinity of nivolumab for the human PD-1 receptor was 2.6 nmol/L [7], with a nivolumab concentration of 0.04 µg/mL being sufficient to occupy >70 % of PD-1 molecules on T cells in vitro [13]. Thus, even if serum concentrations of nivolumab are undetectable (<1.2 µg/mL), sufficient nivolumab should be present to maintain plateau PD-1 receptor occupancy [13]. PD-1 receptor occupancy was independent of dose in patients with solid tumours receiving a single dose of intravenous nivolumab 0.3–10 mg/kg; mean peak PD-1 receptor occupancy was 85 % and mean plateau PD-1 receptor occupancy was 72 % at 4–24 h and ≥57 days post-infusion [13]. In patients with advanced melanoma receiving intravenous nivolumab 0.1–10.0 mg/kg every 2 weeks for 8 weeks, median PD-1 receptor occupancy was 64–70 % [14]. Nivolumab may continue to occupy PD-1 receptors for up to 3 months after treatment [13, 15].
In vitro, nivolumab enhanced T-cell responses and cytokine production in the mixed lymphocyte reaction and superantigen or cytomegalovirus stimulation assays [4]. Nivolumab did not induce antibody-dependent cell-mediated cytotoxicity or complement-dependent cytotoxicity in activated T cells in vitro [4]. Nivolumab did not have a significant effect on memory T-cell immune responses to Candida albicans, tetanus toxoid or viral antigens [13].
Negligible changes in serum levels of the cytokines interleukin (IL)-6, IL-10 and IL-2 soluble receptor-α occurred in patients with metastatic clear-cell renal cell carcinoma who received intravenous nivolumab 0.3, 2 or 10 mg/kg every 3 weeks [16].
Nivolumab 0.3, 2 or 10 mg/kg administered every 3 weeks did not have a clinically significant effect on the corrected QT interval in patients with advanced metastatic clear-cell renal cell carcinoma [17].
3 Pharmacokinetic Properties of Nivolumab
Intravenous nivolumab had linear pharmacokinetics [13, 14, 18, 19]. Nivolumab exposure increased dose-proportionally with administration of nivolumab 0.1–10 mg/kg every 2 weeks [14]. Steady state was reached by 12 weeks with administration of nivolumab 3 mg/kg every 2 weeks, with approximately threefold systemic accumulation [8].
Analysis of data from patients with advanced squamous NSCLC who received nivolumab 3 mg/kg every 2 weeks indicated that the trough serum nivolumab concentration after the first dose did not significantly predict the probability of achieving an objective response [20]. In addition, in patients with advanced solid tumours receiving nivolumab 0.1–10 mg/kg every 2 weeks, time-averaged steady-state nivolumab concentrations did not significantly predict the probability of experiencing treatment-related grade ≥3 adverse events or adverse events leading to treatment discontinuation [20].
The geometric mean steady-state volume of distribution for nivolumab was 8.04 L, according to population pharmacokinetic analysis [18].
Similar to other therapeutic proteins, it is expected that nivolumab would be eliminated by nonspecific catabolism via enzymes in the reticuloendothelial system [16]. Population pharmacokinetic analysis revealed a geometric mean nivolumab clearance of 9.5 mL/h [18]. Nivolumab had a serum half-life of ≈12 days at doses of 0.3–3 mg/kg and ≈20 days at a dose of 10 mg/kg [13].
Based on results of population pharmacokinetic analysis, the nivolumab dosage does not need to be adjusted in patients with mild hepatic impairment, although data are limited in patients with moderate to severe hepatic impairment [8, 9] and the EU summary of product characteristics (SmPC) recommends caution in this population [9]. The US prescribing information states that no nivolumab dosage adjustment is needed in patients with renal impairment [8], and the EU SmPC states that no dosage adjustment is needed in patients with mild or moderate renal impairment, although data in patients with severe renal impairment are limited [9]. Age, gender and race did not affect the clearance of nivolumab to a clinically relevant extent [18]. Use of a weight-based dosage regimen is supported by the finding that nivolumab clearance increased with increasing bodyweight [8, 18]. Anti-drug antibodies did not appear to affect the clearance of nivolumab to a clinically significant extent [21].
Given that enzymes in the reticuloendothelial system are not thought to be inhibited or induced by drugs, it is considered unlikely that the pharmacokinetics of nivolumab would be affected to a clinically significant extent by other drugs [9, 16].
Cytokines may affect the activity of some cytochrome P450 (CYP) enzymes, meaning that the pharmacokinetics of CYP substrates may be affected by therapeutic proteins with the potential to modulate cytokines [16]. However, given that no meaningful changes in serum levels of IL-6, IL-10 or IL-2 soluble receptor-α (which are known to affect CYP enzymes) were seen in patients with metastatic clear-cell renal cell carcinoma who received intravenous nivolumab 0.3–10 mg/kg every 3 weeks (Sect. 2), there is considered to be a low risk of drug interactions between nivolumab and CYP substrates [16].
4 Therapeutic Efficacy of Nivolumab
In phase I trials, single doses of intravenous nivolumab 0.3–10 mg/kg [13] and multiple doses of intravenous nivolumab 0.1–10 mg/kg every 2 weeks [14] demonstrated antitumour activity in patients with advanced solid tumours (advanced melanoma, NSCLC, castrate-resistant prostate cancer, renal cell carcinoma or colorectal cancer) (n = 39 [13] and n = 296 [14]); the maximum tolerated nivolumab dose was not reached in either trial. An objective response rate (ORR) of 17 % was seen in patients with heavily-treated, advanced NSCLC who received nivolumab 1–10 mg/kg every 2 weeks (n = 129), according to a subgroup analysis [22] of the multiple-dose trial [14]. Objective responses were seen in 1 of 33 (3 %), 9 of 37 (24 %) and 12 of 59 (20 %) patients receiving nivolumab dosages of 1, 3 and 10 mg/kg, respectively, and in 9 of 54 patients (17 %) with squamous NSCLC and 13 of 74 patients (18 %) with nonsquamous NSCLC. Overall, the median duration of response was 17.0 months, the median overall survival (OS) was 9.9 months and the OS rate at 1, 2 and 3 years was 42, 24 and 18 %, respectively [22]. Taking into account pharmacodynamic, pharmacokinetic, efficacy and tolerability data from these earlier analyses [14, 22], a nivolumab dosage of 3 mg/kg every 2 weeks was selected for further study. This section discusses the results of phase II [23, 24] and III [25] trials in previously-treated patients with advanced, squamous NSCLC who received nivolumab 3 mg/kg every 2 weeks.
4.1 Phase II Trials
The efficacy of nivolumab in previously-treated patients with advanced, squamous NSCLC was examined in two noncomparative phase II trials [23, 24]. CheckMate 063 was a multinational trial that enrolled patients aged ≥18 years with measurable, histologically or cytologically documented stage IIIB or IV squamous NSCLC, disease recurrence or progression after platinum-based chemotherapy and at least one additional systemic treatment, and an Eastern Co-operative Oncology Group (ECOG) performance status of 0 or 1 [23]. Patients with treated, stable brain metastases were eligible for enrolment [23]. The second trial (study ONO-4538-05; available as an abstract and poster) was conducted in Japan and enrolled patients aged ≥20 years with stage IIIB or IV or recurrent squamous NSCLC who had received at least one prior platinum-based chemotherapy regimen and had an ECOG performance status of 0 or 1 [24].
At baseline in CheckMate 063, median patient age was 65 years, 73 % of patients were male, 92 % of patients had a history of smoking, 17 and 83 % of patients had stage IIIB and IV disease, 22 and 78 % of patients had an ECOG performance status of 0 or 1, and 2 % of patients had brain metastases [23]. Two, three or at least four previous systemic treatments had been administered to 35, 44 and 21 % of patients, respectively [23]. At baseline in study ONO-4538-05, median patient age was 65 years, 91 % of patients were male, 17 and 69 % of patients had stage IIIB and IV disease and 14 % had recurrent disease, and 51 and 49 % of patients had an ECOG performance status of 0 or 1 [24].
Patients in both trials received intravenous nivolumab 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity [23, 24]. The primary endpoint was the ORR, as assessed by an independent radiology review committee (IRC) [23, 24]. In CheckMate 063, the median duration of nivolumab therapy was 2.3 months and the minimum duration of follow-up for IRC-assessed response was 11.0 months [23]. The median duration of follow-up in study ONO-4538-05 was 10.4 months [24].
In CheckMate 063, the IRC-assessed ORR was 14.5 % (Table 1); all of the responses were partial responses [23]. The median duration of response had not been reached at the time of analysis. Stable disease was seen in 25.6 % of patients, with a median duration of stable disease of 6.0 months. The median progression-free survival (PFS) and OS was 1.9 and 8.2 months (Table 1). PFS rates at 6 months and 1 year and OS rates at 1 year and 18 months are shown in Table 1 [23, 26].
In study ONO-4538-05, the IRC-assessed ORR was 25.7 % and median duration of response had not been reached (Table 1) [24]. All of the responses were partial responses. Stable disease was seen in 28.6 % of patients. The median PFS was 4.2 months and median OS had not been reached (Table 1). PFS and OS rates at 6 months are shown in Table 1 [24].
4.2 Phase III Trial: CheckMate 017
CheckMate 017 was a randomized, open-label, multinational, phase III trial comparing the efficacy and safety of nivolumab with that of docetaxel in patients with advanced, squamous NSCLC who had disease recurrence after one prior platinum-based chemotherapy regimen [25]. Patients were aged ≥18 years, had stage IIIB or IV squamous NSCLC and an ECOG performance status of 0 or 1; patients with treated, stable brain metastases were eligible for enrolment. In addition, pretreatment specimens of tumour tissue had to be available for biomarker analysis.
At baseline, median patient age was 63 years, 76 % of patients were male, 92 % of patients were current or former smokers, 19 and 80 % of patients had stage IIIB and IV disease, 24 and 76 % of patients had an ECOG performance status of 0 or 1, and 6 % of patients had brain metastases [25].
Patients were randomized to receive intravenous nivolumab 3 mg/kg every 2 weeks or intravenous docetaxel 75 mg/m2 every 3 weeks [25]. Treatment continued until disease progression or discontinuation because of toxicity or other reasons. The primary endpoint was OS; the trial was terminated early on the recommendation of an independent data and safety monitoring committee as the prespecified interim analysis showed that OS significantly favoured nivolumab versus docetaxel. The minimum duration of follow-up was ≈11 months.
Nivolumab was more effective than docetaxel in the second-line treatment of advanced, squamous NSCLC [25]. At the time of the interim analysis, the risk of death was significantly reduced by 41 % with nivolumab versus docetaxel (Table 2) [25]. The median OS was 9.2 months with nivolumab and 6.0 months with docetaxel, with 1-year OS rates of 42 and 24 %, respectively, and 18-month OS rates of 28 and 13 % respectively (Table 2) [25, 27]. Hazard ratios for OS favoured nivolumab versus docetaxel across various prespecified subgroups, apart from the ‘rest-of-world’ geographic region (i.e. Argentina, Australia, Chile, Mexico and Peru) and patients aged ≥75 years.
The confirmed ORR was significantly higher in nivolumab than in docetaxel recipients (Table 2) [25]. A complete response was seen in 1 % of nivolumab recipients and 0 % of docetaxel recipients, and a partial response occurred in 19 and 9 % of patients in the corresponding treatment groups. At the time of analysis, the median duration of response had not yet been reached in nivolumab recipients and was 8.4 months in docetaxel recipients.
The risk of death or disease progression was significantly reduced by 38 % with nivolumab versus docetaxel (Table 2) [25]. The median PFS was 3.5 months with nivolumab and 2.8 months with docetaxel. One-year [25] and 18-month [27] PFS rates are shown in Table 2.
PD-L1 expression was neither prognostic nor predictive of OS, PFS or ORR [25, 27].
During treatment, health status and lung cancer symptoms tended to improve with nivolumab and remain stable with docetaxel (analyses available as abstracts [28, 29] and a slide presentation [29]). Health status [28] and lung cancer symptoms [29] were assessed for up to 54 weeks with nivolumab and 18 weeks with docetaxel, as there were fewer than ten on-treatment patients in each group after these time points.
In terms of health status, nivolumab recipients had significant (p ≤ 0.05) improvements from baseline in the EuroQOL EQ-5D index score at weeks 16–30 and 42–54 (with changes at weeks 42–54 exceeding the minimally important difference) and in the EQ visual analogue scale (EQ-VAS) score at weeks 12, 20–36 and 48 (with changes at weeks 24–36 and 48 exceeding the minimally important difference). With docetaxel, there were no significant changes from baseline to week 18 in the EQ-5D index or EQ-VAS scores [28].
When assessed using the Lung Cancer Symptom Scale (LCSS), 20.0 % of nivolumab recipients and 21.9 % of docetaxel recipients had clinically meaningful symptom improvements by week 12 of treatment [29]. In terms of the LCSS average symptom burden index (assessing anorexia, fatigue, dyspnoea, pain, haemoptysis and cough), nivolumab recipients had significant (p < 0.05) improvements from baseline at weeks 24–54, whereas no significant change from baseline was seen in docetaxel recipients through week 18. A significant (p < 0.05) difference favouring nivolumab versus docetaxel was seen for the change in the LCSS average symptom burden index at weeks 30, 36 and 42. In addition, the time to deterioration in anorexia significantly (p = 0.009) favoured nivolumab versus docetaxel recipients. Based on the LCSS 3-item index (assessing symptom distress, interference with activity level and health-related quality of life), symptom burden significantly (p < 0.05) improved from baseline in nivolumab recipients at weeks 24, 42, 48 and 54, whereas no significant change from baseline was seen in docetaxel recipients through week 18. The time to deterioration in the LCSS 3-item index and in each of the three items significantly (p < 0.05) favoured nivolumab versus docetaxel recipients [29].
5 Tolerability of Nivolumab
Intravenous nivolumab had a manageable adverse event profile in patients with advanced, squamous NSCLC [23–25], and was better tolerated than intravenous docetaxel in the CheckMate 017 trial [25]. In CheckMate 017, treatment-related adverse events (any grade) were reported in 58 % of nivolumab recipients and 86 % of docetaxel recipients and treatment-related grade 3 or 4 adverse events were reported in 7 and 55 % of patients in the corresponding treatment groups [25].
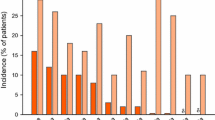
The treatment-related adverse events (any grade) reported most frequently in nivolumab recipients (e.g. fatigue, decreased appetite, asthenia, nausea and diarrhoea) all occurred with a numerically lower incidence than in docetaxel recipients (Fig. 1) [25]. The most commonly reported adverse events (any grade) in patients receiving docetaxel included fatigue, neutropenia, nausea, anaemia and alopecia (Fig. 1). With nivolumab, treatment-related grade 3 or 4 fatigue, decreased appetite and leukopenia each occurred in 1 % of patients. The most commonly reported treatment-related grade 3 or 4 adverse events with docetaxel were neutropenia (30 % of patients), febrile neutropenia (10 %), fatigue (8 %), asthenia (4 %) and leukopenia (4 %).
Tolerability of intravenous nivolumab versus intravenous docetaxel in the second-line treatment of advanced, squamous non-small cell lung cancer. Incidence of treatment-related adverse events (any grade) occurring in ≥10 % of patients in either treatment arm in the CheckMate 017 trial [25]. The safety analysis included patients who had received at least one dose of study drug. δ = an incidence of 0 %
Treatment-related serious adverse events of any grade were reported in 7 % of nivolumab recipients and 24 % of docetaxel recipients, with treatment-related serious grade 3 or 4 adverse events reported in 2 and 19 % of patients in the corresponding treatment groups [25]. No treatment-related deaths occurred in patients receiving nivolumab, whereas three deaths (2 % of patients) were attributed to docetaxel.
Discontinuation because of treatment-related adverse events occurred in 3 % of nivolumab recipients (most commonly because of pneumonitis) and in 10 % of docetaxel recipients (most commonly because of peripheral neuropathy and fatigue) [25].
Nivolumab has been associated with immune-mediated adverse reactions, including immune-mediated pneumonitis, colitis, hepatitis, nephritis and renal dysfunction, and hypothyroidism and hyperthyroidism [9]. It should be noted that patients with autoimmune disease were excluded from CheckMate 063 [23] and 017 [25]. Patients were monitored for immune-mediated adverse reactions during the trials, and protocols were in place to manage any immune-mediated adverse reactions that occurred [25] (see also Sect. 7).
In CheckMate 017, treatment-related select adverse events (any grade) included pneumonitis (5 % of nivolumab recipients vs. 0 % of docetaxel recipients), hypothyroidism (4 vs. 0 %), diarrhoea (8 vs. 20 %), colitis (1 vs. 0 %), increased alanine aminotransferase (ALT) levels (2 vs. 1 %), increased aspartate aminotransferase (AST) levels (2 vs. 1 %), tubulointerstitial nephritis (1 vs. 0 %), increased blood creatinine levels (3 vs. 2 %), acute renal failure (0 vs. 1 %), rash (4 vs. 6 %), pruritus (2 vs. 0 %), infusion-related reactions (1 vs. 1 %) and hypersensitivity (0 vs. 2 %) [25]. Treatment-related pneumonitis, colitis and tubulointerstitial nephritis of grade 3 severity each occurred in 1 % of nivolumab recipients, whereas skin-related adverse events and infusion-related reactions were all of grade 1 or 2 severity. Immune-modulating medication was administered to 18–83 % of patients experiencing treatment-related select endocrine, gastrointestinal, pulmonary, renal or skin adverse events [25].
Longer-term (18 months) follow-up in CheckMate 063 [26] and 017 [27] did not reveal any unexpected safety signals in nivolumab recipients, with nivolumab maintaining a favourable adverse event profile versus docetaxel in CheckMate 017 [27]. The majority of nivolumab recipients who developed treatment-related select adverse events experienced them within the first 3–6 months of treatment [26, 27].
Nivolumab has low immunogenic potential. In CheckMate 063, 11 of 101 (10.9 %) evaluable patients were positive for anti-drug antibodies at baseline (analysis available as a poster) [21]. Post-baseline, 12 (11.9 %) patients were anti-drug antibody positive, although none of the patients were persistent positive. None of the patients were positive for neutralizing antibodies. Of the four evaluable patients in this trial who experienced hypersensitivity or infusion reactions, one was positive for anti-drug antibodies at baseline [21].
6 Dosage and Administration of Nivolumab
Nivolumab is approved in the USA for the treatment of patients with metastatic squamous NSCLC with progression on or after platinum-based chemotherapy [8] and in the EU for the treatment of adults with locally advanced or metastatic squamous NSCLC after prior chemotherapy [9]. The recommended dosage of nivolumab is 3 mg/kg administered by intravenous infusion over 60 min every 2 weeks [8, 9]. Treatment should continue until disease progression or unacceptable toxicity [8, 9]. Local prescribing information should be consulted for more information concerning warnings, precautions and management of adverse reactions related to nivolumab.
7 Place of Nivolumab in the Management of Advanced Squamous NSCLC
Second-line treatment remains substantially underutilized in patients with metastatic, squamous NSCLC [30]. Indeed, until recently, little progress had been made in the management of previously-treated, advanced, squamous NSCLC, with docetaxel remaining the standard second-line treatment option for over a decade, despite being generally associated with only modest improvements in OS [25, 31]. Historically, NSCLC had not been considered an immune responsive condition [6, 32]. However, improved understanding of the interaction between the immune system and tumours led to the development of immune checkpoint inhibitors such as nivolumab and pembrolizumab [32]. Nivolumab was the first PD-1 immune checkpoint inhibitor approved for use in NSCLC. Pembrolizumab is a humanized IgG4 monoclonal antibody that also targets the PD-1 receptor [33], and was recently approved in the USA for use in previously-treated patients with metastatic squamous or nonsquamous NSCLC whose tumours express PD-L1 [34] (PD-L1 positivity is defined as a tumour proportion score of ≥50 % using the FDA-approved companion diagnostic test [35]).
Intravenous nivolumab was more effective than intravenous docetaxel in the second-line treatment of advanced, squamous NSCLC, according to the results of the CheckMate 017 trial (Sect. 4.2). The risk of death was significantly lower with nivolumab than with docetaxel and the ORR was significantly higher. Moreover, the responses seen with nivolumab were durable, which translated to a significant PFS benefit [25]. Although comparisons across trials should be made with caution, the 1-year OS rate of 42 % achieved with nivolumab in CheckMate 017 is higher than would historically be expected in patients with advanced NSCLC receiving subsequent therapy [36–38]. A limitation of CheckMate 017 is that it only enrolled patients with an ECOG performance status of 0 or 1 [39]. Data on the use of nivolumab in patients with an ECOG performance status of 2 or 3 would be of interest. Eight percent of patients with previously-treated advanced squamous or nonsqumaous NSCLC in the ongoing CheckMate 153 trial had an ECOG performance status of 2 [40]. Early results indicate that nivolumab recipients with an ECOG performance status of 2 had a similar treatment response and adverse event profile to nivolumab recipients with an ECOG performance status of 0–1 [40].
The OS benefit seen with nivolumab versus docetaxel was maintained across various prespecified subgroups in CheckMate 017 (Sect. 4.2). Interestingly, a retrospective exploratory analysis of a phase I study in patients with advanced NSCLC found an ORR of 30 % in nivolumab recipients with a smoking history of >5 pack-years versus 0 % in those with a smoking history of ≤5 pack-years [22]. More information is needed to explain this finding, although a possible reason is that smoking-associated lung cancer has a higher mutational load, resulting in increased immunogenicity [22].
Based on the results of CheckMate 017, US National Comprehensive Cancer Network (NCCN) guidelines include nivolumab as a category 1 option for the treatment of patients with advanced, squamous NSCLC and an ECOG performance status of 0–1 who have progressed following platinum-based chemotherapy [2]. Guidelines from the European Society of Medical Oncology predate the approval of nivolumab [1]. Erlotinib is also included in NCCN guidelines as an option in this treatment setting, with category 2b options including docetaxel, gemcitabine, and ramucirumab plus docetaxel [2].
Intravenous nivolumab was better tolerated than intravenous docetaxel in the CheckMate 017 trial (Sect. 5). In terms of treatment-related select adverse events, immune-mediated pneumonitis is of particular concern in patients with lung cancer, given that they may already have poor lung reserve because of smoking or metastatic disease [32]. Across clinical trials, fatal pneumonitis was reported in 5 of 691 (0.7 %) patients with solid tumours who received nivolumab [22]. Fatal pneumonitis was reported early in the nivolumab programme before management guidelines were implemented (e.g. two cases of fatal pneumonitis occurred in patients with NSCLC before pneumonitis was recognized as a nivolumab-associated toxicity [22]). No cases of fatal pneumonitis were reported among nivolumab recipients in phase III trials in patients with previously-treated, advanced NSCLC [25, 41]. Early recognition and treatment of pneumonitis is critical, given that early-grade pneumonitis is typically reversible with dose delay or treatment discontinuation and/or administration of corticosteroids [7]. The US prescribing information [8] and the EU SmPC [9] recommend that nivolumab be withheld in patients with grade 2 pneumonitis; it can be resumed when pneumonitis recovers to grade 0 or 1 [8] or when symptoms resolve, radiographic abnormalities improve and management with corticosteroids is complete [9]. Nivolumab should be permanently discontinued in patients with grade 3 or 4 pneumonitis [8, 9].
Immune-mediated colitis, hepatitis, nephritis and renal dysfunction, and hypothyroidism and hyperthyroidism have also been reported in nivolumab recipients (Sect. 5). Management guidelines for immune-mediated adverse events, including recommendations for the use of corticosteroids, were reported in association with a phase III trial comparing the efficacy of nivolumab with that of dacarbazine in patients with metastatic melanoma (CheckMate 066) [42]. US prescribing information [8] and the EU SmPC [9] recommend that nivolumab be withheld in patients with grade 2 or 3 colitis and permanently discontinued in patients with grade 4 colitis. Nivolumab may also need to be withheld or discontinued in patients with immune-related hepatitis (depending on the degree of elevation in ALT, AST and bilirubin levels) or with immune-mediated nephritis and renal dysfunction (depending on the degree of elevation in creatinine levels) [8, 9].
Longer-term tolerability data from CheckMate 063 [26] and 017 [27] did not reveal any unexpected safety signals in nivolumab recipients, with most treatment-related select adverse events occurring within the first 3–6 months of treatment.
None of the nivolumab recipients in CheckMate 063 developed neutralizing antibodies (Sect. 5), although neutralizing antibodies have been reported infrequently in other studies [21]. The development of neutralizing antibodies did not appear to be associated with a loss of efficacy or an increased risk of acute infusion reactions or hypersensitivity events [21]. In addition, the development of anti-drug antibodies did not appear to alter the safety profile or clearance of nivolumab [9].
In terms of ongoing trials, early results from CheckMate 153 (which is primarily examining the safety of nivolumab in patients with previously-treated, advanced squamous or nonsquamous NSCLC) are consistent with the findings of other trials, with no new safety signals detected [40]. Another trial, CheckMate 171, is examining the incidence of high-grade treatment-related select adverse events in patients with previously-treated, advanced squamous NSCLC who are receiving nivolumab (NCT02409368).
There is a need to establish predictive biomarkers in order to identify which patients are most likely to respond to nivolumab [43]. Results have been mixed regarding the potential of PD-L1 as a predictive biomarker. Some studies indicated that PD-L1 expression may be predictive of response to nivolumab [5, 13, 14]. For example, in patients with advanced solid tumours, an objective response was seen in 9 of 25 (36 %) nivolumab recipients whose tumours expressed PD-L1 and in none of the 17 nivolumab recipients whose tumours did not express PD-L1 [14]. However, subgroup analysis in nivolumab recipients with heavily-treated, advanced NSCLC revealed no clear association between PD-L1 expression and response or survival [22]. Moreover, PD-L1 expression was neither prognostic nor predictive of efficacy in the CheckMate 017 trial (Sect. 4.2). Factors that may affect the sensitivity and specificity of PD-L1 detection include the type of assay used and the quality of the tissue sample [7, 44]. For example, archival or recent biopsy samples could be submitted for PD-L1 biomarker analysis in CheckMate 017 [25]. Given that a proportion of patients with PD-L1-negative tumours also derive benefit from nivolumab [43], NCCN guidelines do not currently recommend PD-L1 testing in patients with metastatic NSCLC prior to starting nivolumab therapy [2].
First-line monotherapy with nivolumab also showed therapeutic potential in advanced NSCLC in a phase I trial [45]. CheckMate 026 is a phase III trial examining the efficacy of first-line therapy with nivolumab versus platinum-based doublet chemotherapy in patients with stage IV or recurrent PD-L1-positive NSCLC (NCT02041533).
In terms of other trials, the phase I CheckMate 012 trial is examining nivolumab administered alone or in combination with various other agents in patients with advanced NSCLC (NCT01454102) [46–49]. Combination therapy regimens showing therapeutic potential in patients with chemotherapy-naïve, advanced NSCLC include nivolumab plus platinum-based doublet chemotherapy [47], nivolumab plus ipilimumab [48], and nivolumab plus erlotinib in epidermal growth factor receptor mutation-positive nonsquamous NSCLC [46, 47]. CheckMate 012 also indicates that maintenance therapy with nivolumab with or without bevacizumab may be beneficial in patients who did not progress following first-line platinum-based chemotherapy [49]. Whilst combining nivolumab with other agents has the potential to improve antitumour activity, the incidence of adverse events (including pneumonitis) may also be increased [7].
CheckMate 227 is an ongoing phase III trial comparing the efficacy and safety of nivolumab alone, nivolumab plus ipilimumab, and platinum-based doublet chemotherapy in patients with stage IV or recurrent NSCLC who have not received prior systemic antineoplastic therapy (NCT02477826).
The phase III CheckMate 057 trial compared the efficacy and safety of nivolumab (n = 292) with that of docetaxel (n = 290) in previously-treated patients with advanced, nonsquamous NSCLC [41]. At the interim analysis, median OS (primary endpoint) was significantly longer with nivolumab than with docetaxel (12.2 vs. 9.4 months) (HR 0.73; 96 % CI 0.59–0.89; p = 0.002), with a 1-year OS rate of 51 and 39 %, respectively, and an 18-month OS rate of 39 and 23 %, respectively. ORR was significantly higher with nivolumab than with docetaxel (19 vs. 12 %; p = 0.02), with no significant between-group difference in PFS [41]. NCCN guidelines include nivolumab as an option for subsequent therapy in patients with metastatic nonsquamous NSCLC who have progressed following platinum-based therapy [2]. Nivolumab was recently approved in the USA for use in previously-treated patients with advanced, nonsquamous NSCLC [11], and has been submitted for approval in this indication in the EU.
In conclusion, nivolumab represents an important advance in previously-treated, advanced, squamous NSCLC. It is more effective and better tolerated than docetaxel, and has a manageable adverse event profile. Thus, nivolumab will most likely become the new standard of care for the second-line treatment of advanced, squamous NSCLC.
Data selection sources:
Relevant medical literature (including published and unpublished data) on nivolumab was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 5 October 2015], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Nivolumab, Opdivo, BMS-936558, ONO-4538, non-small cell, lung, cancer, carcinoma.
Study selection: Studies in patients with non-small cell lung cancer who received nivolumab. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii27–39.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer (version 7.2015). 2015. http://www.nccn.org/. Accessed 12 Oct 2015.
Zielinski C, Knapp S, Mascaux C, et al. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol. 2013;24(5):1170–9.
Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2(9):846–56.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74.
Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112(9):1421–7.
Brahmer JR, Hammers H, Lipson EJ. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future Oncol. 2015;11(9):1307–26.
Bristol-Myers Squibb Company. Opdivo (nivolumab) injection, for intravenous use: US prescribing information. 2015. http://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed 12 Oct 2015.
European Medicines Agency. Nivolumab BMS: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 12 Oct 2015.
European Medicines Agency. Opdivo (nivolumab): EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 12 Oct 2015.
Food and Drug Administration. FDA expands approved use of Opdivo in advanced lung cancer. 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm466413.htm. Accessed 12 Oct 2015.
Berman D, Korman A, Peck R, et al. The development of immunomodulatory monoclonal antibodies as a new therapeutic modality for cancer: the Bristol-Myers Squibb experience. Pharmacol Ther. 2015;148:132–53.
Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Brahmer J. Treatment targeting PD1/PDL1 and toxicity [abstract no. MS09.2]. J Thorac Oncol. 2013;8(Suppl 2):S53–4.
Passey C, Simon J, Roy A, et al. Assessment of drug interaction potential by nivolumab using cytokine modulation data [abstract no. 118 plus poster]. In: American Society for Clinical Pharmacology and Therapeutics Annual Meeting. 2015.
Agrawal S, Williams D, Waxman I, et al. Absence of QT prolongation effect by nivolumab or ipilimumab in patients with solid tumors [abstract no. 117 plus poster]. In: American Society for Clinical Pharmacology and Therapeutics Annual Meeting. 2015.
Feng Y, Bajaj G, Wang X, et al. Model-based analysis of nivolumab to support clinical pharmacology profiling in subjects with solid tumors [abstract]. Clin Pharmacol Ther. 2015;97(Suppl 1):S41–2.
Agrawal S, Feng Y, Kollia G, et al. Clinical pharmacokinetics (PK) of BMS-936558, a fully human anti-PD-1 monoclonal antibody [abstract no. TPS2622]. J Clin Oncol. 2012;30(15 Suppl).
Feng Y, Wang X, Agrawal S, et al. Nivolumab exposure-response (E-R) analysis for clinical development of nivolumab in advanced refractory squamous non-small cell lung cancer [abstract no. QP-19]. Clin Pharmacol Ther. 2015;97(Suppl 1):S7.
Agrawal S, Roy A, Feng Y, et al. Immunogenicity of nivolumab and its impact on pharmacokinetics and safety in subjects with metastatic solid tumors [abstract no. 115 plus poster]. In: American Society for Clinical Pharmacology and Therapeutics Annual Meeting. 2015.
Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–12.
Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–65.
Sakai H, Nishio M, Hida T, et al. Phase II studies of nivolumab in patients with advanced squamous (SQ) or non-squamous (NSQ) non-small cell lung cancer (NSCLC) [abstract no. 521 plus poster]. Eur J Cancer. 2015;51(Suppl 3):S110.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Horn L, Rizvi N, Mazières J, et al. Longer-term follow-up of a phase 2 study (CheckMate 063) of nivolumab in patients with advanced refractory squamous (SQ) non-small cell lung cancer (NSCLC) [abstract plus slide presentation]. In: 16th World Conference on Lung Cancer. 2015.
Reckamp K, Spigel DR, Rizvi N, et al. Phase 3, global, randomized trial (CheckMate 017) of nivolumab vs docetaxel in advanced squamous (SQ) cel non-small cell lung cancer (NSCLC) [abstract plus slide presentation]. In: 16th World Conference on Lung Cancer. 2015.
Reck M, Coon C, Taylor F, et al. Evaluation of overall health status in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 017 [abstract no. 3011]. Eur J Cancer. 2015;51(Suppl 3):S599.
Gralla RJ, Coon C, Taylor F, et al. Evaluation of disease-related symptoms in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel [abstract plus slide presentation]. In: 16th World Conference on Lung Cancer. 2015.
Sacher AG, Le LW, Lau A, et al. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: are patients undertreated? Cancer. 2015;121(15):2562–9.
Al-Farsi A, Ellis PM. Treatment paradigms for patients with metastatic non-small cell lung cancer, squamous lung cancer: first, second, and third-line. Front Oncol. 2014;4:157.
Sundar R, Cho B-C, Brahmer JR, et al. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7(2):85–96.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Merck & Co Inc. Keytruada (pembrolizumab): US prescribing information. 2015. http://www.keytruda.com. Accessed 12 Oct 2015.
Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). 2015. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm. Accessed 12 Oct 2015.
Penrod JR, Korytowsky B, Petrilla A, et al. Survival of U.S. Medicare patients with advanced non-small cell lung cancer by line of therapy [abstract no. 6582]. J Clin Oncol. 2014;32(15 Suppl).
Massarelli E, Andre F, Liu DD, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. 2003;39(1):55–61.
Scartozzi M, Mazzanti P, Giampieri R, et al. Clinical predictive factors for advanced non-small cell lung cancer (NSCLC) patients receiving third-line therapy: selecting the unselectable? Lung Cancer. 2010;68(3):433–7.
European Medicines Agency. Nivolumab BMS: assessment report. 2015. http://www.ema.europa.eu. Accessed 12 Oct 2015.
Hussein M, McCleod M, Chandler J, et al. Safety and efficacy of nivolumab in an ongoing trial of a PD-L1+/− patient population with metastatic non-small cell lung cancer [abstract plus slide presentation] In: 16th World Conference on Lung Cancer. 2015.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med. 2015. doi:10.1056/NEJMoa1507643.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30.
Rajan A, Gulley JL. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2014;3(6):403–5.
Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology (Williston Park, NY). 2014;28(Suppl 3):39–48.
Gettinger SN, Hellmann MD, Shepherd FA, et al. First-line monotherapy with nivolumab (NIVO) in advanced non-small cell lung cancer (NSCLC): safety, efficacy, and biomarker analysis [abstract no. 3096]. Eur J Cancer. 2015;51(Suppl 3):S632.
Gettinger S, Chow LQ, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (PTS) with epidermal growth factor receptor mutant (EGFR MT) advanced non-small cell lung cancer (NSCLC) [abstract no. 171]. Int J Radiat Oncol Biol Phys. 2014;90(5 Suppl):S34–5.
Gettinger S, Rizvi N, Chow LQ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) or erlotinib (ERL) in advanced non-small cell lung cancer (NSCLC) [abstract no. 1054PD]. Ann Oncol. 2014;25(Suppl 4).
Hellmann MD, Rizvi N, Gettinger SN, et al. Safety and efficacy of first-line nivolumab (NIVO) and ipilimumab (IPI) in non-small cell lung cancer (NSCLC) [abstract no. 3097]. Eur J Cancer. 2015;51(Suppl 3):S632.
Rizvi NA, Antonia SJ, Shepherd FA, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) maintenance as monotherapy or in combination with bevacizumab (BEV) for non-small cell lung cancer (NSCLC) previously treated with chemotherapy [abstract]. Int J Radiat Oncol Biol Phys. 2014;90(5 Suppl):S32.
Acknowledgments
During the peer review process, the manufacturer of nivolumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Gillian Keating is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: J. G. Aerts, Department of Pulmonary Medicine, Erasmus MC Cancer Institute, Rotterdam, The Netherlands; C. Chouaïd, Service de Pneumologie, GRC Onco Paris Est, UPEC, CHI Créteil, Créteil, France; L. Crinò, Division of Medical Oncology, Santa Maria della Misericordia Hospital, Azienda Ospedaliera di Perugia, Perugia, Italy; J. Mazières, Thoracic Oncology Department, Larrey Hospital, University Hospital of Toulouse, University of Toulouse III (Paul Sabatier), Toulouse, France; A. Pluzanski, Lung Cancer Department, Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology, Warsaw, Poland.
Rights and permissions
About this article
Cite this article
Keating, G.M. Nivolumab: A Review in Advanced Squamous Non-Small Cell Lung Cancer. Drugs 75, 1925–1934 (2015). https://doi.org/10.1007/s40265-015-0492-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0492-9