Abstract
Introduction
The inclusion of herbal medicinal products and herbal supplements in pharmacovigilance systems is important because a systematic approach of collecting and analyzing adverse drug reactions related to these products will help practitioners, patients, and regulators to gain more knowledge and prevent harm.
Objective
We aimed to categorize the adverse drug reaction reports on herbal medicinal products and herbal supplements submitted to the Pharmacovigilance Centre Lareb between 1991 and February 2021 on the basis of their regulatory status, herbs included, and adverse drug reactions involved.
Methods
We categorized products on the basis of their registration status and herbal ingredients. The products were then categorized according to the Herbal Anatomical Therapeutic Chemical Classification System. We used descriptive statistics in Microsoft Excel 2019. Pivot tables were used for the analysis and presentation of the data.
Results
Until February 2021, a total of 789 reports of herbal medicinal products and herbal supplements were received by Lareb. In these reports, a total of 823 herbal products were labeled as suspect. These products caused a total of 1727 adverse drug reactions. Of the 823 products, 229 were registered as a medicine, and 594 were on the market as a herbal supplement. Of the 823 herbal products, 522 reports concerned single-herb products, 256 reports concerned combination products, 27 reports concerned vitamin products containing herbal ingredients, and 18 reports concerned product issues. Approximately 15% of reports concerned serious adverse drug reactions, and adulterated products harbored a high risk of causing serious adverse drug reactions.
Conclusions
Analysis of the herbal medicinal products and herbal supplements in the Dutch pharmacovigilance database revealed a variety of suspected herbal ingredients. The reports provide insight into the variety of herbal products used in the Netherlands and the adverse reactions associated with their use. Pharmacovigilance of herbal products is essential to ensure their safe use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A systematic approach of collecting and analyzing adverse drug reactions related to herbal products will help practitioners, patients, and regulators to gain more knowledge and prevent harm. |
Analysis of the herbal products in the Dutch pharmacovigilance provided insight into the variety of herbal products used in the Netherlands and the adverse reactions associated with their use. |
Pharmacovigilance of herbal products, including herbal supplements, is essential to ensure their safe use. |
1 Introduction
The use of herbal products is widespread, with large groups of people worldwide relying on them for at least parts of their primary healthcare, including self-care [1]. Herbal products are marketed as herbal medicinal products (HMPs), foods, or as (food) supplements.
Herbal medicinal products exclusively contain one or more herbal substances or one or more herbal preparations or one or more such herbal substances in combination with one or more such herbal preparations as active ingredients. A herbal substance is raw plant material, and a herbal preparation is a processed herbal substance (e.g., extract) [2].
In the European Union (EU), there are several options for marketing a herbal product as a medicinal product. If there are adequate clinical data to substantiate the efficacy of the herbal product, an application can be submitted for marketing authorization. There are two types of marketing authorizations: a full authorization, which is based on a marketing authorization holder’s own clinical data, or a well-established use authorization, which is based on published clinical data [3].
Since 2004, there is a third option in the EU for herbal products to gain market access as a medicinal product. The procedure, which is laid down in EU Directive 2004/24/EC, allows the approval of herbal medicinal products on the basis of long-standing medicinal use. Demonstration of clinical efficacy is not required. It involves the assessment of mostly bibliographic safety and efficacy data. [4, 5]. It is a simplified registration procedure that is restricted to herbal products that do not require medicinal supervision and that have been in medicinal use for at least 30 years, including 15 years in the EU. These traditional herbal medicinal products have to fulfill all requirements for quality and safety. The EU Directive 2004/24/EC on traditional herbal medicinal products was implemented in the Netherlands in 2005.
In addition to HMPs, there are also numerous herbal products on the market that fall within the gray area of nonregistered health-enhancing products, including (food) supplements and herbal health products. These products are further designated ‘herbal supplements’ in this paper.
In contrast to herbal medicinal products, herbal supplements do not require pre-market approval. Consequently, their quality and safety are less guaranteed [6, 7]. They are marketed in the same pharmaceutical forms (such as tablets, tablets, capsules of liquid) as medicinal products. Because of their appearance and health-related claims, consumers often wrongfully consider them as medicines.
In the Netherlands, herbal supplements fall under the Commodities Act [8]. The Commodities Act applies to all products used by consumers, both food and non-food. It is linked to a large number of separate regulations and decrees that contain rules for specific foods and consumer products. The Act is a framework act that provides general rules on public health, product safety, fair trading, and adequate information [9]. The health claims on these products are assessed by the European Food Safety Authority.
Herbal medicinal products and herbal supplements are often perceived by the lay public as less toxic than conventional medicines [10, 11]. However, both contain pharmacologically active ingredients and therefore can give rise to adverse drug reactions (ADRs). The surveillance of ADRs related to nonregistered herbal supplements is more complex than that related to registered medicinal products [12]. Challenges include the regulatory status of herb-containing products, the limited knowledge of such products among healthcare workers (pharmacists, physicians), the safety assessment, and inadequate quality control [1]. In addition, information provided by the manufacturers of herbal supplements about the exact composition of the product is sometimes deficient or incomplete.
The inclusion of HMPs and herbal supplements in pharmacovigilance systems is important because a systematic approach of collecting and analyzing ADRs related to these products will help practitioners, patients, and regulators to gain more knowledge and prevent harm [13]. In the Netherlands, the Pharmacovigilance Centre Lareb maintains the spontaneous reporting system (SRS) for ADRs. In this SRS, reports on authorized/registered medicines and vaccines are collected and analyzed with the goal of finding safety signals and providing information on ADRs to healthcare professionals and the public. This includes pharmacovigilance for HMPs. In addition, reports on other categories of products, such as pharmacy-compounded products, vitamins, and herbal supplements, are received and handled. The aim of this study was to categorize the suspected ADR reports on HMPs and herbal supplements submitted to Lareb on the basis of their regulatory status, herbs included, and ADRs involved.
2 Methods
2.1 Database and Dataset
The dataset for this study contained HMP and herbal supplement reports on ADRs from the SRS in the Netherlands received since the establishment of Lareb in 1991 until February 2021. Herbal medicinal product reports can include products that are licensed as a medicine, for example, a Hypericum perforatum product [14]. These reports have been received directly from healthcare professionals and consumers or indirectly via Marketing Authorization Holders. The database itself adheres to E2B (R3) Electronic Transmission of Individual Case Safety Reports guidelines [15], and reports of registered drugs and nonregistered products can be stored, coded, assessed, and shared with other international databases.
The legal status of the herbal products, registered as a medicinal product or as a herbal supplement, determines how they are stored in the database. Medicinal products are authorized/registered through the Dutch Medicines Evaluation Board (MEB). These products are coded according to the Dutch Drug Database ‘G-Standard,’ which is used by all parties in the healthcare field in the Netherlands and contains all the products that are dispensed by or used in the pharmacy [16]. For herbal supplements (covered by the Commodities Act), a separate ‘product module’ of the database has been developed by Lareb in which the ingredients, product group, and brand name can be coded [17].
Adverse drug reactions were coded with the Medical Dictionary for Regulatory Activities (MedDRA®), a standardized and hierarchical medical terminology. The reported ADRs were grouped on System Organ Classes (SOCs) and Preferred Terms (PTs) [18].
We included all herbal products in the database regardless of legal status, except for homeopathic products (which are often based on herbal substances). Some herbal products for which reports were received contained additional ingredients, such as vitamins and minerals. These substances were included in the assessment of the product.
2.2 Data Extraction
A list of HMPs was received from the Dutch MEB on 21 February, 2021, and through an SQL query, the set of registered herbal products in the Lareb database was extracted. This was combined with a search in the product module for herbal supplements. The products in this latter category were checked manually for the completeness of herbal ingredients, using official sites or patient leaflets where possible. For each report, we extracted the Database ID number, the qualification of the reporter, the year of reporting, the proprietary name , the reported use or indication, the concomitant medication, the age and sex of the patient (user), the ADR description of the reporter, the MedDRA® PT and SOC, the seriousness of the reaction according to Council for International Organizations of Medical Sciences criteria [19], the action that was taken with the product, the outcome of the event, the ingredients of the product (excluding excipients), and a summary and narrative of the report.
2.3 Categorization Herbals
In addition to the designation of their regulatory status (registration through the MEB or on the market as herbal supplements), the products were divided into four categories:
-
(I)
Single-herb products: products containing one herbal ingredient;
-
(II)
Combination products: products containing multiple herbal ingredients;
-
(III)
Vitamin products: products containing vitamins and/or minerals in addition to one or more herbal ingredients;
-
(IV)
Product issue: (illegal) products that are contaminated, adulterated, or considered as counterfeit herbal products.
For products in category IV, the suspected contamination or adulteration can be further analyzed by the National Institute for Public Health and the Environment (RIVM) if a sample of the product is available.
The products were then categorized according to the Herbal Anatomical Therapeutic Chemical Classification (HATC) system. The HATC system divides herbal products into groups based on their therapeutic use. The main anatomical groups are the same as those in the Anatomical Therapeutic Chemical system applied to conventional medicines [20, 21]. As not all products could be placed in this categorical system, the categories Prophylaxis and Product issues were added. The Prophylaxis category concerns products that are used for general health benefits, not for the treatment of a disease. The Product issues category is reserved for contaminated, adulterated, and counterfeit herbal products. This category is considered distinct from the other categories, as the ADRs are caused by the product issue instead of the herbal ingredient(s).
Some herbal ingredients, such as Ginkgo biloba leaves and leaf extracts, have multiple indications. In these cases, the most commonly reported indication was used. Additionally, many products contain multiple herbs, which makes categorization into a single category more difficult and complicated. In the case where an EU herbal monograph of the herb of herbal preparation was available (via emea.europa.eu), the principle indication mentioned in the monograph was used for categorization. A herbal monograph contains the HMPC’s scientific opinion on safety and efficacy data about a herbal substance and its preparations intended for medicinal use [22]. In the absence of an EU monograph, the indication mentioned in the report or a main indication mentioned in the literature was used. Finally, we specified whether the product was related to Ayurvedic medicine or traditional Chinese medicine.
2.4 Statistical Analysis
We used descriptive statistics in Microsoft Excel 2019. Pivot tables were used for the analysis and presentation of the data.
3 Results
3.1 Characteristics of the Reports
From 1991 to February 2021, a total of 789 reports of HMPs and herbal supplements were received by Lareb (Table 1). The number of reports received per year is shown in Fig. 1.
In these reports, a total of 823 herbal products were labeled as suspected. The products caused a total of 1727 ADRs. Of the 823 products, 229 were registered as a medicine, and 594 were on the market as a herbal supplement.
Of the 823 herbal products, 522 reports concerned single-herb products, 256 reports concerned combination products, 27 reports concerned vitamin products containing herbal ingredients, and 18 reports concerned product issues.
The 522 single-herb products contained a total of 86 different herbal ingredients. Of these products, Monascus purpureus, or red yeast rice (RYR), was the most common (n = 119), followed by Mentha piperita (n = 53), H. perforatum (n = 34), Valeriana officinalis (n = 32), and Plantago ovata (n = 29) (Fig. 2).
The 256 combination products contained a total of 261 different herbal ingredients. Of these ingredients, Melissa officinalis was the most commonly reported (n = 44), followed by Matricaria chamomilla (n = 42), M. piperita (n = 42), Angelica sinensis (n = 41), Silybum marianum (n = 40), and Glycyrrhiza glabra (n = 39) (Fig. 3).
The 27 vitamin products contained a total of 47 different herbal ingredients. Of these ingredients, Panax ginseng was the most commonly reported (n = 8), followed by various algae (n = 5), Camellia sinensis (n = 5), Vitis vinifera (n = 5), and G. biloba (n = 4). The remaining 42 herbal ingredients were reported only once or twice.
Product issues included contaminated, adulterated, or counterfeit herbal products. Over the years, a total of 18 cases of product issues or suspected product issues were reported (Table 2). Twelve products had product issues (e.g., adulteration and contamination, involving a single substance), and six had product issues involving multiple substances. The most common product issue was adulteration with sibutramine (n = 8). Other adulterants and contaminants found were meloxicam, paracetamol, dexamethasone, chlorphenamine, phenylbutazone, diclofenac, furosemide, an unknown pesticide, and lead. In ten cases, contamination or adulteration was confirmed with laboratory results from the RIVM; in six cases, there was an already known association between the product and the product issue, and in the remaining two cases, the product issue was suspected.
Consumers reported the most on herbal products (n = 534), followed by physicians (n = 167), pharmacists (n = 100), and other health professionals (n = 19). The qualification of the reporter was unknown in three case. Herbal practitioners are also able and invited to report, but this group is hardly represented among reporters.
In 540 of the 823 cases, product use was discontinued by the user. The dose and/or dose frequency was reduced in 19 of the cases. In the remaining 264 cases, the dose remained unchanged, the subsequent action was not reported or not applicable, or the subsequent action taken was unknown.
Of the 1727 reported ADRs, 57% (989) were resolved, were recovered from, or were recovered from with sequelae remaining at the time of reporting. Of the remaining ADRs, 21% (369) were not recovered from. The outcome of 20% (345 ADRs) was unknown, and the outcome of 1% (16 ADRs) was not reported on the reporting form. Eight outcomes were fatal. These fatal outcomes concerned six individual cases.
3.2 Adverse Drug Reactions
The most frequently reported ADRs were SOC gastrointestinal disorders (n = 354), followed by nervous system disorders (n = 226), skin and subcutaneous disorders (n = 185), general disorders and administration-site conditions (n = 188), and psychiatric disorders (n = 137). Table 3 depicts the frequency for each SOC, along with the three most commonly reported ADRs at the MedDRA® PT level.
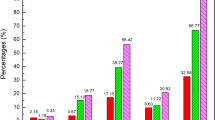
In the 789 individual reports, a total of 115 serious cases were reported. The product categories with the most serious cases were the categories Vitamin products and Product issue with 27% and 28%, respectively. The percentage of serious cases reported with Single-herb products and Combination products was comparable, with 14% for both categories. The Electronic Supplementary Material shows an overview with all ADRs on MedDRA® PT level for the separate reports.
3.3 Pharmacotherapeutic Categorization
The HMPs were categorized according to the HATC index. The most commonly reported HATC categories were Alimentary tract and metabolism (n = 183), Nervous system (n = 173), Cardiovascular system (n = 140), Genitourinary system and sex hormones (n = 107), and Respiratory system (n = 76), see Table 4. Eighteen Ayurvedic products and four traditional Chinese medicine products were reported.
4 Discussion
Analysis of the HMPs and herbal supplements in the Dutch pharmacovigilance database revealed a variety of suspected herbal ingredients, and these reports provide insight into the products used and herb-related adverse reactions in the Netherlands. Reports concerned two times more herbal supplements than (registered/authorized) HMPs. Compared with HMPs, herbal supplements have a substantially larger market share. This could explain the number of reports on herbal supplements. Unfortunately, no clear figures on the usage of herbal products specifically for the Netherlands are available. Data from the Dutch National Food Consumption Surveys were used to obtain an impression on the consumption of food supplements containing herbs or other botanical ingredients in the Netherlands. The prevalence of users of the above-mentioned products in the Dutch population was approximately 10% for men, 17% for women, and 13% for children. The most frequently used botanicals were Echinacea purpurea, G. biloba, cranberry (Vaccinium macrocarpon), Panax ginseng, and algae [23]. Additionally, it should be noted that registration as a traditional herbal medicinal product in the Netherlands has only been possible since 2005 and that Lareb started in 1991 to collect ADRs of herbal products [4].
Single-herb ingredient products have been reported twice as much as combination products. This is partially attributed to the RYR and M. piperita products, commonly used in the Netherlands, accounting for approximately one-third of the total number of reports.
A total of 18 reports about product issues, including contaminated and adulterated products, were found to be related to supplements used for slimming and to imported products. These reports demonstrate the need for rigorous monitoring of the quality of these products. The identification of such products has led to multiple regulatory actions in the past [24, 25].
A total of 1727 ADRs have been reported. The most common SOC was gastrointestinal disorders. A total of 115 cases with serious ADRs were reported, with 15% of all cases concerning herbal products. This is considerably lower than the percentage of serious reports found for conventional medicines (approximately 30% serious reports in total). The relatively high rate of serious ADRs within the product issue category is more or less expected because herbal supplements may contain undisclosed illegally added compounds leading to unexpected ADRs.
The classification of HMPs into different pharmacotherapeutic categories according to the HATC system presented us with a number of difficulties [17]. First, some products contained many different herbs with different indications, making division into a single area difficult. For instance, traditional Chinese medicines cannot be classified with the HATC system. Second, not all herbal products could be divided into the HATC coding system. Third, the undeclared ingredients that may occur in herbal supplements were difficult to take into account [26]. With the addition of the categories ‘Prophylaxis’ and ‘Product Issue,’ it became possible to divide all products into an appropriate category. However, multiple categories could still have applied to a single product. Women reported ADRs related to HMPs and herbal supplements twice as much as men because women use more herbal products and/or because they are more likely to experience and/or identify and report ADRs than men [27,28,29,30].
Lareb has been receiving reports on herbal products since 1996. The number of reports of herbal products per year steadily rose until 2018, after which the number roughly remained at the same level between 2019 and 2020. Signals on these reports are shared with other organizations in the Netherlands, such as the Netherlands Food and Consumers Products Safety Authority (NVWA), the Healthcare Inspectorate, the RIVM, and the MEB, depending on the regulatory status of the product [6]. Consumers were the most common reporters (66% of reports) with physicians and pharmacists at distant second and third positions at 19% and 13%, respectively. This is encouraging because consumers are able to report on ‘blind spots’ of the pharmacovigilance system, such as herbal products [31]. Previous qualitative research from Canada found that consumers generally do not feel comfortable reporting their suspected ADRs related to herbal products to healthcare professionals or to regulatory authorities [32]. It is yet unclear if this is also the case in the Netherlands.
Reporting by healthcare professionals could be increased by making physicians and pharmacists more aware of herbal products as a potential cause for ADRs. In general, knowledge about herbal products is limited among healthcare workers in the Netherlands, and there is skepticism about the appropriateness of their use. Furthermore, in many cases, a physician or a pharmacist will not be aware of a patient’s use of herbal products, as these are usually over-the-counter products obtained beyond the drug monitoring systems. Finally, it is important that a solid pharmacovigilance system be in place for these products and that regulatory authorities can take appropriate measures to protect public health if safety signals are found.
4.1 Strengths and Limitations
The strength of this study is the systematic approach in identifying and analyzing reports of herbal products in the Dutch pharmacovigilance database. An SRS is inherently related to some limitations, such as under-reporting and selective reporting for certain products or issues. Under-reporting of HMPs and herbal supplements sold over the counter is a major issue, as the use of herbals may not be known to the physician or pharmacist [32, 33]. In addition, the occurrence of ADRs may be attributed less obviously to herbal products than to conventional medicines. We expect that the reports on herbal products are a reflection of the ADRs that occur in daily practice; however, the complete range of herbal products used could be substantially different because of under-reporting. Many consumers and healthcare providers are probably also unaware that Lareb also collects and analyzes reports about herbal products. More publicity for this task can hopefully change this in the future.
For the identification of the product used and the plant materials involved, Lareb relies on information provided by the patient or the healthcare professional. On the reporting form used, consumers can add a link to the website where a product was bought, and they can upload photos of the product and the product leaflet if available. If Lareb receives a case on an unknown product, it is possible to ask follow-up questions to the reporter. However, we rely on information available about the product online knowing that this may be incomplete or incorrect. In addition, reporters can sometimes mention ambiguous and alternative plant names [34]. At present, we do not use taxonomic and pharmacognostic expertise for the identification of plants [35]. Botanical identification can only be applied if a herbal medicinal product or herbal supplement contains (powdered) plant material (herbal substance). In the case of a herbal preparation (e.g., an extract), chromatographic analysis should be used. When a product is suspected of containing illegal or otherwise harmful ingredients, an analysis can be performed by the RIVM. For instance, the content of monacolin K (= lovastatin) and other monacolins in RYR products has been investigated [36].
All reports on HMPs and herbal supplements in the Lareb database were individually assessed for causality. Causality assessment regarding herbal products is more difficult than with other medicines [12]. It is beyond the scope of this study to describe the causality assessment of individual reports. However, in previous publications, Lareb highlighted the relationship between many groups of herbal products and their ADRs or interactions [37,38,39,40,41], for instance, for ADRs related to RYR [38, 41], hop-containing and soy-containing products and postmenopausal bleeding [39, 40], Actaea racemosa (black cohosh) and hepatitis [42], drug–herb interactions with H. perforatum (St John’s wort), and other drug–herb interactions [41]. The interactions reported for St. John’s wort included five reports concerning interactive effects with oral contraceptives. Liver enzyme-inducing substances contained in St. John’s wort (inducing cytochrome P450 3A4 isoenzymes and a P-glycoprotein pump) are seen to lower estrogen and progestogen levels, making the contraceptive less reliable. Other reports concerned interactions with antidepressants, which are also metabolized (at least partially) via cytochrome P450 3A4. For G. biloba, four interactions with vitamin K antagonists, one with an antiviral medicine, and one interaction with anti-epileptic drugs have been reported [41].
5 Conclusions
Analysis of the HMPs and herbal supplements in the Dutch pharmacovigilance database revealed a variety of suspected herbal ingredients. The reports provide insight into the variety of herbal products used in the Netherlands and the adverse reactions associated with their use. A trend is visible toward more frequent reporting for herbal products over the years. Approximately 15% of reports concerned serious ADRs, and adulterated products harbored a high risk of causing serious ADRs. It is important that consumers and healthcare workers are informed about possible risks related to the use of herbal products. In addition, physicians and pharmacists should be alert regarding the use of herbal products and should always inquire whether a patient is using any. This action is also relevant to prevent possible drug–herb interactions. The reporting of ADRs caused by all herbal products contributes to the knowledge about their safety, makes it possible to generate safety signals, and supports the (early) discovery of possibly illegal ingredients present in herbal supplements. Pharmacovigilance of herbal products, including herbal supplements, is essential to ensure their safe use.
References
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. https://doi.org/10.3389/fphar.2013.00177.
European Medicines Agency (EMA). Herbal medicinal products; 2020. https://www.ema.europa.eu/en/human-regulatory/herbal-medicinal-products. Accessed 11 Nov 2021.
Borg JJ, Laslop A, Pani L, Maciulaitis R, Melchiorri D. Reflections on decisions made on the well-established use of medicinal products by EU regulators and the ECJ. Sci Pharm. 2014;82(3):541–54. https://doi.org/10.3797/scipharm.1402-14.
van Galen E. Traditional herbal medicines worldwide, from reappraisal to assessment in Europe. J Ethnopharmacol. 2014;158 Pt B:498–502. https://doi.org/10.1016/j.jep.2014.07.013.
Kroes BH. The legal framework governing the quality of (traditional) herbal medicinal products in the European Union. J Ethnopharmacol. 2014;158 Pt B:449–53. https://doi.org/10.1016/j.jep.2014.07.044.
de Boer A, Geboers L, van de Koppel S, van Hunsel F. Nutrivigilance: reporting adverse events of non-registered products in the Netherlands. Curr Dev Nutr. 2021;5(Suppl 2):1265. https://doi.org/10.1093/cdn/nzab056_003.
Calapai G. European legislation on herbal medicines: a look into the future. Drug Saf. 2008;31(5):428–31. https://doi.org/10.2165/00002018-200831050-00009.
Rijksoverheid. Warenwetbesluit algemene productveiligheid; 1993. https://wetten.overheid.nl/BWBR0006158/2020-06-09. Accessed 11 May 2022.
Netherlands Enterprise Agency. Commodities Act; 2022. Accessed 25 Mar 2022.
Snyder FJ, Dundas ML, Kirkpatrick C, Neill KS. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81–95. https://doi.org/10.1080/01639360802634043.
Tengku Mohamad TAS, Islahudin F, Jasamai M, Jamal JA. Preference, perception and predictors of herbal medicine use among Malay women in Malaysia. Patient Prefer Adherence. 2019;13:1829–37. https://doi.org/10.2147/ppa.S227780.
Barnes J. Pharmacovigilance of herbal medicines: a UK perspective. Drug Saf. 2003;26(12):829–51.
Menniti-Ippolito F, Mazzanti G, Santuccio C, Moro PA, Calapai G, Firenzuoli F, et al. Surveillance of suspected adverse reactions to natural health products in Italy. Pharmacoepidemiol Drug Saf. 2008;17(6):626–35. https://doi.org/10.1002/pds.1566.
Dutch Medicines Evaluation Board. SmPC Hypericum perforatum Laif®; 2022. https://www.geneesmiddeleninformatiebank.nl/smpc/h103963_smpc.pdf. Accessed 25 Mar 2022.
ICH guideline E2B (R3) on electronic transmission of individual case safety reports (ICSRs): data elements and message specification: implementation guide European Medicines Agency; 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-4.pdf. Accessed 11 May 2022.
Z Index. About Z-index; 2018. https://www.z-indexnl/english. Accessed 11 May 2022.
Hunsel F, Skalli S. Chapter 9. Coding reports involving herbal medicines in a pharmacovigilance database. In: Jo Barnes, editor. Pharmacovigilance of herbal medicines. Springer; 2022 (in press).
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Welcome to MedDRA; 2021. https://www.meddra.org/. Accessed 11 May 2022.
CIOMS Working Group VIII. Practical aspects of signal detection in pharmacovigilance: report of CIOMS Working Group VIII. Report No.: 9290360828, Geneva; 2010.
Farah MH, Edwards R, Lindquist M, Leon C, Shaw D. International monitoring of adverse health effects associated with herbal medicines. Pharmacoepidemiol Drug Saf. 2000;9(2):105–12. https://doi.org/10.1002/(SICI)1099-1557(200003/04)9:2%3c105::AID-PDS486%3e3.0.CO;2-2.
The Herbal Anatomical Therapeutic Chemical Classification System. Uppsala Monitoring Centre. https://www.who-umc.org/whodrug/whodrug-portfolio/whodrug-global/herbal-atc/. Accessed 1 May 2021.
European Medicines Agency (EMA). European Union monographs and list entries; 2022. https://www.ema.europa.eu/en/human-regulatory/herbal-products/european-union-monographs-list-entries#:~:text=A%20European%20Union%20(EU)%20herbal,preparations%20intended%20for%20medicinal%20use. Accessed 25 Mar 2022.
Jeurissen SMF, Buurma-Rethans EJM, Beukers MH, Jansen-van der Vliet M, van Rossum CTM, Sprong RC. Consumption of plant food supplements in the Netherlands. Food Funct. 2018;9(1):179–90. https://doi.org/10.1039/c6fo01174h.
van Hunsel F, Venhuis BJ, Keizers PH, Kant A. A “natural” weight loss product containing sibutramine. Drug Test Anal. 2016;8(3–4):311–4. https://doi.org/10.1002/dta.1925.
van Hunsel F, van Grootheest K. Adverse drug reactions of a slimming product contaminated with sibutramine. Ned Tijdschr Geneeskd. 2011;155(42):A3695.
Kim M, Woo Y, Han C-H. Current status of the spontaneous reporting and classification/coding system for herbal and traditional medicine in pharmacovigilance. Integr Med Res. 2021;10(1): 100467. https://doi.org/10.1016/j.imr.2020.100467.
de Vries ST, Denig P, Ekhart C, Burgers JS, Kleefstra N, Mol PGM, et al. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol. 2019;85(7):1507–15. https://doi.org/10.1111/bcp.13923.
Zhang Y, Leach MJ, Hall H, Sundberg T, Ward L, Sibbritt D, et al. Differences between male and female consumers of complementary and alternative medicine in a national US population: a secondary analysis of 2012 NIHS data. Evid Based Complement Alternat Med. 2015;2015: 413173. https://doi.org/10.1155/2015/413173.
Rhee TG, Harris IM. Gender differences in the use of complementary and alternative medicine and their association with moderate mental distress in U.S. adults with migraines/severe headaches. Headache. 2017;57(1):97–108. https://doi.org/10.1111/head.12986.
Stjernberg L, Berglund J, Halling A. Age and gender effect on the use of herbal medicine products and food supplements among the elderly. Scand J Prim Health Care. 2006;24(1):50–5. https://doi.org/10.1080/02813130500475522.
van Hunsel F, Harmark L, Rolfes L. Fifteen years of patient reporting: what have we learned and where are we heading to? Expert Opin Drug Saf. 2019;18(6):477–84. https://doi.org/10.1080/14740338.2019.1613373.
Walji R, Boon H, Barnes J, Austin Z, Welsh S, Baker GR. Consumers of natural health products: natural-born pharmacovigilantes? BMC Complement Altern Med. 2010;10:8. https://doi.org/10.1186/1472-6882-10-8.
Vlieger AM, van de Putte EM, Hoeksma H. The use of complementary and alternative medicine in children at a general paediatric clinic and parental reasons for use. Ned Tijdschr Geneeskd. 2006;150(11):625–30.
Dauncey EA, Irving JTW, Allkin R. A review of issues of nomenclature and taxonomy of Hypericum perforatum L. and Kew’s medicinal plant names services. J Pharm Pharmacol. 2019;71(1):4–14. https://doi.org/10.1111/jphp.12831.
Chen G, Sun W. The role of botanical gardens in scientific research, conservation, and citizen science. Plant Divers. 2018;40(4):181–8. https://doi.org/10.1016/j.pld.2018.07.006.
Venhuis BJ, van Hunsel F, van de Koppel S, Keizers PH, Jeurissen SM, De Kaste D. Pharmacologically effective red yeast rice preparations marketed as dietary supplements illustrated by a case report. Drug Test Anal. 2016;8(3–4):315–8. https://doi.org/10.1002/dta.1929.
van Hunsel FP, van Grootheest AC. Adverse reactions to herbal remedies: analysis of reported adverse reactions in the Netherlands. Ned Tijdschr Geneeskd. 2013;157(47):A6615.
Vrolijk MF, van de Koppel S, van Hunsel F. Red yeast rice (Monascus purpureus) supplements: case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br J Clin Pharmacol. 2021;87(4):2146–51. https://doi.org/10.1111/bcp.14599.
van Hunsel F, van de Koppel S, van Puijenbroek E. Post-menopausal vaginal hemorrhage related to the use of a hop-containing phytotherapeutic product. Drug Saf Case Rep. 2015;2(1):14. https://doi.org/10.1007/s40800-015-0016-2.
van Hunsel FP, Kampschöer P. Postmenopausal bleeding and dietary supplements: a possible causal relationship with hop- and soy-containing preparations. Ned Tijdschr Geneeskd. 2012;156(41):A5095.
de Boer A, van Hunsel F, Bast A. Adverse food-drug interactions. Regul Toxicol Pharmacol. 2015;73(3):859–65. https://doi.org/10.1016/j.yrtph.2015.10.009.
van de Meerendonk HW, van Hunsel FP, van der Wiel HE. Autoimmune hepatitis induced by Actaea racemosa: side affects of an herb extract. NedTijdschr Geneeskd. 2009;153(6):246–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this study.
Conflict of interest
The authors declare no conflicts of interest that are directly relevant to the content of this study. Florence P.A.M. van Hunsel is a member of the ISoP Special Interest Group on Herbal and Traditional Medicines. This study was presented as a poster at the Annual ISoP Meeting 2021.
Ethics approval
Ethics approval was not needed for this study.
Consent to participate
No approval or consent was needed for this study.
Consent for publication
No approval or consent was needed for this study.
Availability of data and material
The datasets for this manuscript are not publicly available because of the Lareb data-protection policy. Requests to access the datasets should be directed to the first author and will be granted on reasonable request.
Code availability
The SQL statements for the data used in this article are not publicly available because of the Lareb data-protection policy. Requests to access the datasets should be directed to the first author and will be granted on reasonable request.
Author contributions
The original study protocol was designed by all authors. The dataset was established by DK with the help of SK. Data analysis was performed by DK with input from the other authors. The design of the manuscript was determined by all authors. All authors contributed to the final data analysis and to manuscript drafting and revision. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Hunsel, F.P.A.M., van der Kooi, D., van de Koppel, S. et al. Analysis of Reports on Adverse Drug Reactions Related to Herbal Medicinal Products and Herbal Supplements in the Netherlands Received by the National Pharmacovigilance Centre Lareb. Drug Saf 45, 651–661 (2022). https://doi.org/10.1007/s40264-022-01180-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01180-5