Abstract
Introduction
Surveillance of drug safety during pregnancy is a special interest of pharmacovigilance (PV). The role that national PV centres take in this field is, however, unclear.
Aim
The aim of this study was to provide insight into current activities, future intentions and need for support of national PV centres in the field of drug safety during pregnancy.
Method
A web-based questionnaire was used to ask PV centres about their current activities concerning the surveillance of drug safety during pregnancy, their intentions to implement or improve activities and need for support. For these three main topics, questions were posed about spontaneous adverse drug reaction (ADR) reporting, additional activities to obtain information, signal detection and informing healthcare professionals and the public.
Results
The questionnaire was sent to PV centres of 172 countries. Response was 40%. In general, the PV centres received limited numbers of reports of ADRs in the (unborn) child, related to drug exposure during pregnancy. Signal detection in pregnancy cases is carried out by 8 out of 58 PV centres (13.5%). Most PV centres mention they have intentions to implement or improve activities, mainly for spontaneous reporting (69.4%) and methods for signal detection (67.2%). Support was needed for all topics of the questionnaire.
Conclusion
Current activities of national PV centres concerning drug safety during pregnancy are limited. The majority of PV centres are, however, willing to improve or implement activities. Programmes should be set up in order to support and stimulate PV centres with these activities. The aim of all these activities is to increase knowledge about the safety of drugs during pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is a large difference in current activities on surveillance of drug safety during pregnancy between national pharmacovigilance (PV) centres. |
About 70% of PV centres of low/middle-income countries and 60% of upper-middle/high-income countries do not have specific pregnancy-related questions on their ADR reporting form. |
The majority of PV centres are willing to improve activities concerning the surveillance of drug safety during pregnancy. |
Support is needed for the entire process of the surveillance of drug safety during pregnancy; collecting the right information, performing signal detection and informing healthcare professionals and the public about knowledge of drug safety during pregnancy. |
Support could result in collecting more specific pregnancy data, like data on time of the exposure in relation to the outcome. This would result in high-quality analysis. |
1 Introduction
1.1 Drug-Induced Birth Defects
Surveillance of drug safety during pregnancy is a special interest of pharmacovigilance (PV). When a drug is used during pregnancy, not only the mother, but also embryo or the foetus may be exposed to the drug. This can result in adverse effects in the (unborn) child (Box 1 in “Appendix”). Although the safety of a drug is tested before it enters the market, due to ethics, pregnant women are almost never included in those clinical trials. The unfortunate reality is, therefore, that we learn about most teratogenic effects only after a drug has been marketed, and after it has been used by pregnant women [1].
The well-known historical example is that of thalidomide in the early 1960s. Thousands of congenitally malformed infants were born as a result of exposure in utero to an unsafe drug promoted for use in pregnant women [2]. Besides malformations, like phocomelia in thalidomide-exposed children, teratogens can also increase the incidence of miscarriage. An example is the case of mycophenolate mofetil [3]. There are also examples in which the symptoms are noticed later on in the child’s life, which is the case of diethylstilbestrol (DES), used to prevent miscarriages. At least 25% of daughters, whose mothers had been using DES during pregnancy, had genital tract anomalies including vaginal adenosis and they had an increased chance for developing clear cell adenocarcinoma of the vagina and cervix [4]. Recently, a potential safety issue concerning dolutegravir and neural tube defects was disseminated. The issue was identified from a preliminary unscheduled analysis of an ongoing observational study in Botswana, which found four cases of neural tube defects out of 426 women who became pregnant while taking the HIV antiretroviral medicine dolutegravir. This gave a risk of approximately 0.9% for developing neural tube defects, compared with a 0.1% risk in infants born to women taking other antiretroviral drugs at the time of conception [5].
These cases highlight the importance of systematic surveillance of drug safety during pregnancy. Although for some drugs there is knowledge about the safety of use during pregnancy, for most drugs this information is unknown. Yet, for patients and physicians, knowledge on drug safety during pregnancy is needed in order to make prescribing decisions concerning the safety of both the woman and the unborn child.
1.2 Important Role of National Pharmacovigilance (PV) Centres
The thalidomide disaster triggered the formation of a resolution at the 16th World Health Assembly which called for “a systematic collection of information on serious adverse drug reactions during the development and particularly after medicines have been made available for public use”. This led to a global initiative and the formation of the WHO Programme for International Drug Monitoring (PIDM). On a national level, countries appointed PV focal points, which in most countries have led to development of PV centres. As of January 2016, 123 countries have joined the WHO PIDM, and in addition, 28 associate members are awaiting full membership [6].
PV centres maintain the national spontaneous reporting system to which possible adverse drug reactions (ADRs), observed in daily practice, can be reported. Using this system, they also monitor the safety of drugs during pregnancy. Experience has taught us that these systems have only detected a limited number of teratogenic effects, among others, because of low reporting rates.
Given the importance of the safety of drugs used during pregnancy, we believe that it is important that spontaneous reporting concerning drug use during pregnancy is encouraged. A spontaneous reporting system is a relatively inexpensive method that can be used during the entire lifecycle of a drug, in the entire population. PV could therefore play a role in finding signals of risks of drug safety during pregnancy [7,8,9]. Although this system also has shortcomings, like missing data, lack of control groups and selection bias, studies in Europe and the US have shown that the majority of new drug safety signals were triggered by spontaneous reports [9, 10]. The aim of this study is to provide insight into current activities, future intentions and need for support of national PV centres in the field of drug safety during pregnancy. In addition, for these topics, differences between low/low-middle income (LMI) countries and upper-middle/high income (UMHI) countries were explored.
2 Method
2.1 Study Design
For this study, a web-based questionnaire was used to ask national PV centres about their current activities concerning the surveillance of drug safety during pregnancy, their intentions to implement or improve activities and need for support. In addition, a retrospective observational analysis of data from the WHO global database of Individual Case Safety Reports (VigiBase) [11] was performed to get an impression of the number of ADR reports in the (unborn) child related to a drug used by the mother during pregnancy.
2.2 Analysis WHO Global Database
All reports of 2015 with MedDRA® terms [12] belonging to the system organ class (SOC) Congenital, familial and genetic disorders were selected from VigiBase [11]. The number of reports per country was analysed. In addition, this analysis was performed specifically for countries for which the PV centre responded to the web-based questionnaire.
2.3 Web-Based Questionnaire
The web-based questionnaire contained three topics: (1) the national PV centre’s current activities concerning the surveillance of drug safety during pregnancy, (2) their intentions to implement or improve activities and (3) need for support. For these three topics, questions were posed about spontaneous ADR reporting and additional activities to obtain information, signal detection and dissemination of information. The questionnaire can be found in Electronic Supplementary Material 1.
The questionnaire was designed by a group of experts from The Netherlands Pharmacovigilance Centre Lareb; both PV experts and experts specifically in the field of teratology. After development, the questionnaire was tested by two colleagues with different degrees of experience in PV.
The PV centres of 172 countries were approached to complete the questionnaire. These were PV centres of countries that are full or associate members of the WHO PIDM and other contact persons from PV centres. In the period from September 2016 until July 2017, the questionnaire was sent using a web-based SurveyMonkey® package (SurveyMonkey, Palo Alto, CA, USA) [13]. A reminder was sent to non-responders after 3 weeks. Data collection closed September 2017.
Data from all PV centres that (partly) responded to the questionnaire were included for analysis. Each PV centre’s country of origin was categorized into LMI or UMHI country, using the World Bank list of economies (January 2015) [14]. Data were analysed by descriptive statistics, using Microsoft® Excel® 2013.
3 Results
3.1 Respondents
The questionnaire was sent to the national PV centres of 172 countries. Countries were from the following regions: 45 (25%) Africa, 46 (27%) Asia, 43 (25%) Europe, 20 (12%) North America, 13 (8%) South America and 5 (3%) Oceania.
69 PV centres (40%) filled out the questionnaire. This concerned 26 LMI and 43 UMHI countries. LMI countries were Armenia, Burkina Faso, Burundi, Cabo Verde, Cambodia, Ethiopia, Guatemala, Guinee Bissau, Kyrgyzstan, Lao People’s Democratic Republic, Liberia, Morocco, Myanmar, Nigeria, Paraguay, Philippines, Republic of Moldova, Republic of Benin, Sierra Leone, South Sudan, Sudan, Tanzania, Uganda, Union of the Comoros, Vietnam and Zambia. UMHI countries were: Angola, Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, China, Croatia, Cuba, Cyprus, Denmark, Estonia, Finland, France, Germany, Ireland, Italy, Jordan, Kazakhstan, Latvia, Libya, Macedonia, Montenegro, The Netherlands, New Zealand, Oman, Portugal, Republic of Belarus, Saudi Arabia, Serbia, Singapore, Slovenia, South Africa, St. Lucia, St. Vincent and the Grenadines, Sweden, Switzerland, Thailand, Tunisia, United Kingdom and Uruguay.
3.2 Analysis WHO Global Database
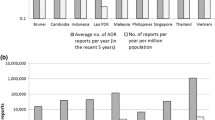
In 2015, 106 countries had sent a total of 2,045,857 reports to VigiBase. On average, 0.3% of these reports (6784/2045857) concerned an ADR coded as Congenital, familial and genetic disorders. These reports came from 61 countries (see Electronic Supplementary Material 2). The rate of spontaneous reports belonging to the SOC Congenital, familial and genetic disorders per total number of reports was the highest for Cyprus (2/4) and Malta (4/102). The US has the highest number of reports in total (1,160,688) as well as specifically for the SOC Congenital, familial and genetic disorders (4994).
For the PV centres participating in the questionnaire study, the absolute number of ADRs belonging to the SOC Congenital, familial and genetic disorders is demonstrated in categories in Fig. 1. Some PV centres received a high number of reports. These were both LMI and UMHI countries. Generally, there was not much difference between LMI and UMHI countries.
3.3 Web-Based Questionnaire
3.3.1 Current Activities
Collecting and assessing information about teratogenic effects of drugs is in need of a specific approach. A specific set of information is crucial to make a proper analysis; for instance, information about disease of the mother, accurate period of drug use and precise description of the birth defect. Therefore, specific questions about pregnancy in case of an ADR in the (unborn) child due to drug use during pregnancy are preferable. Results show 43.9% (25/57) of the PV centres mentioned that they have such additional questions on their reporting forms. This concerns seven PV centres of LMI countries (26.9% of total) and 18 of UMHI countries (41.9% of total).
Ten PV centres mentioned engaging in additional activities to obtain information about pregnancy-related ADRs. This ranges from active follow-up on missing information in ADR reports to collaboration with (national) pregnancy registries. Furthermore, 15 PV centres mentioned that there is another organisation in their country that performs specific activities regarding surveillance of drug exposure during pregnancy; for example, by using a pregnancy register, congenital malformation register, or healthcare register. The Netherlands Pharmacovigilance Centre Lareb seemed to be the only responding PV centre that has a pregnancy registry, called ‘pREGnant’. This registry aims to include and monitor pregnant women independently on drug use, and specifically focuses on drug-related pregnancy outcomes. Data are obtained using six online questionnaires, filled in by the woman before 17 weeks of pregnancy, at 17 and 34 week of pregnancy and 2, 6 and 12 months after childbirth. Starting from January 2014, pREGnant currently has included over 3000 women.
Signal detection in pregnancy cases is currently carried out by 8 out of 58 PV centres (13.5%). These are all UMHI countries. All eight PV centres use the case-by-case methods for signal detection, meaning that each incoming report is assessed individually by a PV assessor and further analysed as needed. In addition, three PV centres carry out a statistical disproportionality analysis.
PV centres can inform healthcare professionals and the public about the safety of drugs during pregnancy. Thirty-eight PV centres mentioned that information about the safety of drugs during pregnancy is disseminated to healthcare professionals and 28 PV centres disseminate information (also) to the general public (67.9% and 50.0%, respectively, total response 56 countries). Of these, about 35% were from LMI countries and 65% from UMHI countries. Methods used for dissemination of information are demonstrated in Figs. 2 and 3. When methods for dissemination of information are compared between LMI and UMHI countries, some methods stand out. A website is more often used by UMIC countries than LMI countries: for healthcare professionals, seven PV centres versus three and for patients, eleven versus five. Lectures for healthcare professionals are used by nine PV centres of UMHI countries and for one from an LMI country. Lectures are not used for patients. Newsletters for the public are more often used by UMHI countries (5 vs 3 in LMI countries). For healthcare professionals, nine and eight PV centres of UMHI and LMI countries, respectively, use newsletters. The drug information centre and teratology information centres are more often used to disseminate information to the public in LMI countries (6 vs 4 PV centres in UMHI countries), while in UMHI countries, these systems are more often used for healthcare professionals (11 vs 7 for PV centres of LMI countries.
The PV centres that disseminate information include five centres that also perform signal detection. For this reason, in general, the information PV centres disseminate about the safety of drug use during pregnancy will mostly be information found in literature or received by other organisations, which is also very important.
3.3.2 Intentions for Improvement or Implementation of Activities
Most PV centres have intentions to improve or implement activities concerning surveillance of drug use during pregnancy. Forty-three out of 62 (69.4%) PV centres mentioned that they are considering implementing or improving activities concerning spontaneous reporting about drug exposure during pregnancy. Thirty-three out of 58 PV centres (56.9%) mentioned that they are considering implementing or improving a programme to collect pregnancy outcome after exposure to drugs during pregnancy. Methods for signal detection in pregnancy cases was mentioned by 39 out of 58 PV centres (67.2%). Thirty-two out of 52 PV centres (61.5%) are considering improving or implementing activities on information dissemination on drug safety during pregnancy.
PV centres of LMI countries have more intentions to improve or implement activities. For example, 84.0% (21/25) of PV centres from LMI countries are considering implementing or improving activities concerning spontaneous reporting about drug exposure during pregnancy, compared with 59.5% (22/37) of UMHI countries. Also 82.6% (19/23) of PV centres from LMI countries are considering implementing or improving a programme to collect pregnancy outcome after drugs exposure during pregnancy, compared with 40.0% (14/35) of UMHI countries.
3.3.3 Need for Support
A total of 75.8% (47/62) of PV centres mentioned that they would need support for activities relating to spontaneous reporting about drug exposure during pregnancy and 77.4% (48/62) with setting up a programme to collect pregnancy outcome after exposure to drugs during pregnancy. Also for signal detection and dissemination of knowledge, support was needed by 83.5% (45/54) and 79.2% (42/53) of the PV centres, respectively. Specific topics of needed support are described in Table 1. The level of interest for several forms of support is presented in Fig. 4. All proposed forms of support were found to be important/very important by the majority of PV centres.
Comparing the percentage of needed support between LMI and UMHI countries, the percentage for LMI countries is slightly higher. For example, support for the case-by-case method was 62% (16) of PV centres from LMI countries versus 47% (20) of PV centres from UMHI countries. The percentage of needed support was comparable between PV centres from LMI and UMHI countries, for example for setting up a Drug Information Centre or Teratology Information Service, newsletter design and disproportionality analysis.
4 Discussion
This study indicates that current activities of national PV centres in the field of drug safety during pregnancy are limited. PV centres receive a limited number of spontaneous reports of ADRs in the (unborn) child related to drug exposure by the mother during pregnancy. Also, the majority of PV centres do not have additional questions specifically about the pregnancy present on their ADR reporting form. Only eight PV centres perform signal detection on their pregnancy data. Nevertheless, the majority of PV centres are willing to improve or implement activities concerning the surveillance of drug safety during pregnancy.
There may be several reasons to explain the low number of spontaneous reports related to pregnancy received by a PV centre. The reporting form may not always be suitable to report adverse outcomes in the (unborn) child related to drug use during pregnancy by the mother. But also, the lack of awareness and other organisations that follow pregnancy outcomes may influence the reporting rate. As post-marketing surveillance is the main data source for safety of drugs in pregnancy, a strong post-market surveillance system is needed to be able to monitor the safety of drugs used during pregnancy. Although for some drugs it is known whether or not it is safe to use during pregnancy, for many drugs this information is still missing. Lack of information may lead to pregnant women and physicians having unrealistically high perceptions of teratogenic drug effects [15]. In addition, it is difficult for people to make complex prescribing decisions, particularly when the risks are uncertain [16]. It is therefore important to gain more knowledge about the safety of drugs during pregnancy and on how to make benefit–harm decisions.
4.1 Collecting Data
National spontaneous reporting systems should be optimized in such a way that they stimulate the reporter to provide proper information about the pregnancy in case of pregnancy-related ADRs, for example by using structured question fields on the reporting form. In addition to the spontaneous reporting system, PV centres could consider the use of cohort event monitoring (CEM) such as pregnancy registries. CEM and case–control surveillance are the two main approaches used for the purpose of identifying teratogens in the post-marketing setting [1]. CEM can be used alongside clinical practice and combines the strengths of the pharmacoepidemiological as well as the clinical PV approach of drug safety surveillance [17,18,19]. In CEM, a group of patients is monitored for ADRs while treated with a specific drug, or group of drugs. CEM can be used for several purposes, such as to characterise known ADRs, identify risk factors and interactions and for signal detection [20]. Most importantly, in the context of drug safety during pregnancy, one is able to follow the mother and child during and after pregnancy. This provides the high-quality information that is needed to make a proper analysis. PV centres can take the initiative to start a CEM themselves, but they can also work together with other organisations in order to share knowledge, for example existing pregnancy registries [21, 22].
4.2 Signal Detection
This study showed that only a limited number of PV centres actually perform signal detection on their data. These were all PV centres in UMHI countries. This indicates the need for training in signal detection. Besides signal detection on a national basis, it is also advisable to explore the possibility of performing signal detection on an international basis, for example by using the WHO global database of Individual Case Safety Reports, VigiBase, which is maintained by the WHO collaborating centre for International Drug Monitoring, the Uppsala Monitoring Centre. It is the largest database of its kind in the world, with over 16 million reports of suspected ADRs (January 2018). A study by Montastruc et al. already demonstrated that possible new signals of congenital malformations following exposure to antipsychotic drugs during pregnancy could be detected using this database [23].
4.3 Support
It is important that PV centres are supported in order to actually accomplish intended improvements and implementation of activities. This study demonstrates that support is needed for the entire process; collecting the right information, performing signal detection and informing healthcare professionals and the public about drug safety during pregnancy. The need for support was slightly higher for PV centres from LMI countries compared with UMHI countries.
In order to support PV centres, The Netherlands Pharmacovigilance Centre Lareb, under the guidance of WHO, is developing a Pregnancy PV Toolkit. This toolkit will provide a collection of resources and information needed for the practice of drug safety surveillance during pregnancy.
4.4 Strengths and Limitations of this Study
Due to the high number of countries that participated in this questionnaire, this overview provided insight into the current activities around drug safety surveillance during pregnancy by national PV centres from LMI and UMHI countries. Selection bias should be kept in mind; colleagues of the national PV centres that contributed to the questionnaire might be more interested in the field of pregnancy or need more support compared with the non-responding PV centres, resulting in positive thoughts about intentions for improvement and appreciated support. In addition, the questionnaire was only available in English. This may have prevented some PV centres from participating. Because of the mostly categorical questions used in the questionnaire, more research is necessary in order to get an in-depth view of activities of the PV centres and specific support they would need to improve or implement activities around the safety of drugs during pregnancy in their specific settings.
4.5 Future Studies
There are many initiatives around the world to monitor the safety of drugs during pregnancy, such as pregnancy registries [21, 22]. Future studies should look into how information about safety during pregnancy can best be documented, how data from different sources can be brought together, and how new knowledge should be communicated with patients and healthcare professionals. Future research could also include male exposures resulting in adverse outcomes to the child via sperm, a field even less explored.
Concerning support, more in-depth studies are needed to explore what methods are most effective for all specific domains for which support is needed. Some skills can, for example, be learned via training courses or web seminars, others may need a more individual PV centre approach. For future studies, it is important to evaluate PV centres’ performance in monitoring drug safety during pregnancy, such as use of PV indicators to identify gaps and by monitoring performance before and after interventions.
5 Conclusion
This study demonstrated that current activities of national PV centres in the field of drug safety during pregnancy are limited. The majority of PV centres are, however, willing to improve or implement activities. Programmes should be set up in order to support and encourage PV centres with these activities. The lack of signal detection in many of the PV centres shows that there is a need to increase capacity of analysis. The additional information needed for analysis of safety in pregnancy can be incorporated in general capacity-building activities. The aim of all these activities is to increase knowledge about the safety of drugs during pregnancy; knowledge that is needed by patients and physicians in order to make prescribing decisions concerning the safety of the woman and the unborn child.
References
Mitchell AA. Systematic identification of drugs that cause birth defects–a new opportunity. N Engl J Med. 2003;349(26):2556–9.
World Health Organization. The importance of pharmacovigilance: safety monotoring of medicinal products. 2002. http://apps.who.int/medicinedocs/en/d/Js4893e/ (cited 1 Dec 2016).
Coscia LA, Armenti DP, King RW, Sifontis NM, Constantinescu S, Moritz MJ. Update on the teratogenicity of maternal Mycophenolate Mofetil. J Pediatr Genet. 2015;4(2):42–55.
Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology. 1995;51(6):435–45.
World Health Organization. Potential safety issue affecting women living with HIV using dolutegravir ar the time of coenception. 2018. http://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf (cited 13 Jun 2018).
World Health Organization. The WHO Programme for International Drug Monitoring. 2018. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/National_PV_Centres_Map/en/ (cited 28 Aug 2018).
Kasliwal R. Spontaneous reporting in pharmacovigilance: strengths, weaknesses and recent methods of analysis. JCPC. 2012;1:20–3.
Carey JC, Martinez L, Balken E, Leen-Mitchell M, Robertson J. Determination of human teratology by the astute clinical method: review of illustrative agents and a proposal of guidelines. Birth Defects Res (Part A). 2009;85:63–8.
Ishiguro C, Hall M, Neyarapally GA, Dal PG. Post-market drug safety evidence sources: an analysis of FDA drug safety communications. Pharmacoepidemiol Drug Saf. 2012;21(10):1134–6.
Pacurariu AC, Coloma PM, van Haren A, Genov G, Sturkenboom MC, Straus SM. A description of signals during the first 18 months of the EMA pharmacovigilance risk assessment committee. Drug Saf. 2014;37(12):1059–66.
Uppsala Monitoring Centre. VigiBase. 2017. http://www.who-umc.org/ (cited 28 Aug 2017).
MedDRA®. MedDRA®: Medical Dictionary for Regulatory Activities. 2018. https://www.meddra.org/ (cited 13 Jun 2018).
Survey Monkey. 2016. http://www.surveymonckey.com (cited 2016 Sep 1).
World Bank. World Bank list of economies. 2018. http://cmsdata.iucn.org/downloads/world_bank_list_of_economies_january_2015.pdfpdf (cited 8 Mar 2018).
Widnes SF, Schjott J. Risk perception regarding drug use in pregnancy. Am J Obstet Gynecol. 2017;216(4):375–8.
Polika JE, Faustman EM, Neil N. Weighting the risk and the benefits: a call for the empirical assessment of perceived teratogenic risk. Reprod Toxicol. 1997;11(4):633–40.
Härmark L. Web-based intensive monitoring: a patient based pharmacovigilance tool. Thesis, University of Groningen; 2012.
Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36(2):75–81.
Suku CK, Hill G, Sabblah G, Darko M, Muthuri G, Abwao E, et al. Experiences and lessons from implementing cohort event monitoring programmes for antimalarials in four African countries: results of a questionnaire-based survey. Drug Saf. 2015;38(11):1115–26.
Wallberg M. Tools for pharmacovigilance and cohort event monitoring. 2009. http://www.who.int/hiv/topics/pharmacovigilance/4_pharmacovigilance_cem.pdf (cited 2018 Jun 13).
Sinclair SM, Miller RK, Chambers C, Cooper EM. Medication safety during pregnancy: improving evidence-based practice. J Midwifery Womens Health. 2016;61(1):52–67.
Charlton RA, Neville AJ, Jordan S, Pierini A, Damase-Michel C, et al. Healthcare databases in Europe for studying medicine use and safety during pregnancy. Pharmacoepidemiol Drug Saf. 2014;23(6):586–94. https://doi.org/10.1002/pds.3613.
Montastruc F, Salvo F, Arnaud M, Begaud B, Pariente A. Signal of gastrointestinal congenital malformations with antipsychotics after minimising competition bias: a disproportionality analysis using data from Vigibase. Drug Saf. 2016;39(7):689–96.
Acknowledgements
The authors would like to thank Dr. N. Iessa and Dr. S. Pal from the WHO for reviewing this article. The authors are indebted to the national centres that make up the WHO Programme for International Drug Monitoring and contribute reports to VigiBase. However, the opinions and conclusions of this study are not necessarily those of the various centres, nor of the WHO.
Author information
Authors and Affiliations
Contributions
AK is responsible for the questionnaire setup, planning and critical revision of the manuscript. LV is responsible for the questionnaire setup, planning, analysis of the data and the drafting and revision of the manuscript. LR is responsible for analysis of the data and the drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
Agnes Kant, Loes de Vries and Leàn Rolfes declare that they have no conflicts of interest that are directly relevant to the content of this study.
Funding/Support
Writing this article was one of the activities of Lareb as World Health Organization (WHO) Collaborating Centre for Pharmacovigilance in Education and Patient Reporting (http://www.who.int/medicines/regulation/medicines-safety/about/collab-centres-netherlands/en/). Lareb received support from the WHO Programme for International Drug Monitoring.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Kant, A., de Vries, L. & Rolfes, L. Surveillance of Drug Safety During Pregnancy: Insight in Current International Activities, Future Intentions and Need for Support of National Pharmacovigilance Centres. Drug Saf 42, 35–43 (2019). https://doi.org/10.1007/s40264-018-0729-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0729-0