Abstract
Introduction
Adverse drug reactions (ADRs) have been commonly cited as a major cause of hospital admissions in older individuals. However, despite the apparent magnitude of this problem, there are limited prospective data on ADRs as a cause of hospitalization in elderly medical patients.
Objectives
The objective of this study was to evaluate the proportion, clinical characteristics, causality, severity, preventability, and outcome of ADR-related admissions in older patients admitted to two Tasmanian hospitals.
Methods
We conducted a prospective cross-sectional study at the Royal Hobart and Launceston General Hospitals in Tasmania, Australia. A convenience sample of patients, aged 65 years and older, undergoing unplanned overnight medical admissions was screened. ADR-related admissions were determined through expert consensus from detailed review of medical records and patient interviews. The causality, preventability and severity of each ADR-related admission were assessed.
Results
Of 1008 admissions, the proportion of potential ADR-related medical admissions was 18.9%. Most (88.5%) ADR-related admissions were considered preventable. Cardiovascular complaints (29.3%) represented the most common ADRs, followed by neuropsychiatric (20.0%) and renal and genitourinary disorders (15.2%). The most frequently implicated drug classes were diuretics (23.9%), agents acting on the renin angiotensin system (16.4%), β-blocking agents (7.1%), antidepressants (6.9%), and antithrombotic agents (6.9%). Application of the Naranjo algorithm found 5.8% definite, 70.1% probable, and 24.1% possible ADRs. ADR severity was rated moderate and severe in 97.9% and 2.1% of admissions, respectively. For most (93.2%) ADR-related admissions the ADR resolved and the patient recovered.
Conclusion
Hospitalization due to an ADR is a common occurrence in this older population. There is need for future studies to implement and evaluate interventions to reduce the risk of ADR-related admissions in elderly populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Almost one in five unplanned overnight hospital admissions to medical wards in elderly Australian patients were related to ADRs. |
Most ADRs were preventable, and cardiovascular medications were commonly implicated. |
In the majority of patients, ADR-related admissions were caused as a result of a combination of two or more drugs sharing a similar ADR profile (e.g., hypotension). |
1 Introduction
Adverse drug reaction (ADR) rates in the USA increased between 1999 and 2006, with higher ADR death rates observed among elderly individuals [1]. It has also been estimated that ADRs cause 100,800–197,000 deaths annually in the European Union [2], while a considerable proportion (5.6%) of all unplanned admissions were medication-related based on a multicenter prospective study in The Netherlands [3]. An Australian study found that hospital admissions due to ADRs in elderly patients had increased despite programs to promote rational and safer use of medicines [4]. Elderly people are particularly vulnerable to ADRs due to an increased chronic disease burden, polypharmacy (concomitant prescription of five or more drugs [5]), and age-related physiological changes affecting the pharmacokinetics and pharmacodynamics of drugs [6–8]. Although one of the more serious outcomes of ADRs in elderly people living in the community is hospitalization, data on the occurrence of these events are often not well-documented and difficult to obtain [9, 10].
Prospective studies allow more accurate recording of both drug history and symptoms to assess the causality of ADRs [11]. Pirmohamed et al. [11] conducted a large prospective analysis of ADR-related hospital admissions in two large general hospitals in the UK (2001–2002) and found that patients admitted with ADRs (median age 76 years) were significantly older than hospitalized patients without ADRs (median age 66 years). The majority of the other prospective studies [8] on ADR-related admissions in the elderly were conducted in specialist settings (geriatric/emergency departments), rather than general medical settings. Furthermore, few studies have utilized patient interviews to complement ‘intensive monitoring’ [12] in identification of ADRs and assessment of ADR preventability. Franceschi et al. [13] and Conforti et al. [14] conducted prospective studies in Italy (2004–2005 and 2009, respectively) and found 6–11% of admissions to a geriatric unit were due to ADRs. De Paepe et al. [15], Olivier et al. [16], and Ma et al. [17] conducted prospective studies in Belgium (2007), France (2002–2003), and China (2008–2011), respectively, and found 7–30% of admissions to an emergency department were due to ADRs. From an Australian perspective, Chan et al. [18] conducted a prospective cross-sectional survey in 1998 at the Royal Hobart Hospital (RHH) and found that 13.3% of admissions to medical wards were ADR-related, although this study was small relative to other studies.
Given the scarcity of ADR-related hospital admissions data in the elderly identified using intensive monitoring, and the lack of recent data from Australia, additional prospective studies on elderly ADR-related admissions are needed. Hence, our aim was to ascertain the proportion of ADR-related medical admissions in older patients admitted to Tasmanian hospitals, identify the commonly implicated drugs, describe the clinical manifestations and outcomes of these ADRs, and determine their causality, preventability, and severity.
2 Methods
This prospective cross-sectional study was carried out at two tertiary care hospitals in Tasmania, Australia: the Royal Hobart Hospital (RHH) and Launceston General Hospital (LGH). The RHH is Tasmania’s largest public acute care hospital for Southern Tasmanians (500-bed capacity in a population of approximately 250,000). The LGH provides acute care for the northern region of Tasmania (300-bed capacity in a population of approximately 87,000). Both hospitals are within the Tasmanian Health Service, and provide a comprehensive range of general and specialty medical and surgical services. The majority of patients in both hospitals are seen by clinical pharmacists on the wards, who undertake an admission medication history and reconciliation as per national standards [19]. The best possible medication history is collected from the patients and their relatives and/or caregivers, their general practitioner (GP), and/or community pharmacy. This information is entered into a state-wide hospital medication management system available for all staff to access across the state. The patients’ previous admission/discharge details are also stored as an electronic patient file or digital medical record, which could also be accessed during the study for any missing information.
The study was approved by the Tasmanian Health and Medical Human Research Ethics Committee, and study participants provided written informed consent. The data presented here were collected as part of the PADR-EC (Prediction of Hospitalization due to Adverse Drug Reactions in Elderly Community-Dwelling Patients) study, which has been published elsewhere [20]. The previous paper reported on the derivation of the prediction score from the RHH cohort and the validation of the dataset in the LGH cohort. In the present analysis, we pooled the available data to create a larger dataset to allow us to report on the proportion of ADR-related hospital admissions in older patients admitted to Tasmanian hospitals, identify commonly implicated drugs, and describe the clinical manifestations and outcomes of ADRs.
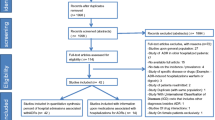
A convenience sample of community-dwelling patients aged ≥65 years with acute, unplanned admissions to the medical wards of the RHH and LGH was included in the study. Data were collected from March 2014 to March 2015 at the RHH and from September 2015 to December 2015 at the LGH. Exclusion criteria included an inability to be interviewed due to their medical condition (e.g., patients with infections in isolation, a terminal illness, or a hearing impairment or low vision), refusal to participate, or unavailability of medical records.
We assessed each patient to determine whether the admission was potentially due to an ADR. An ADR was defined as “a response to a drug that is noxious and unintended and occurs at doses normally used in humans for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function” [12, 21]. This definition excludes therapeutic failures, under-treatment, intentional and accidental poisoning (i.e., overdose), and drug abuse. ADRs that were observed during the hospital stay were excluded. All elderly admissions to the medical wards between Monday and Friday (9 am–5 pm) were identified by the primary clinical pharmacist researcher (NPN) using computerized admission entry details. Patients were followed until discharge to collect sufficient information for the final assessment of the ADR. All data were collected manually using a data collection form and later transferred into a Microsoft Access® database (Microsoft Corporation, Redmond, WA, USA). Each ADR contributing to the patient admission was assessed if the symptoms of admission were consistent with the known adverse effect profile of the drug/drugs (according to the Australian Medicines Handbook [22] or UpToDate database [23]) and, after investigation, other causes were excluded [11]. The assessment of whether a certain drug/drugs may have caused or contributed to an acute admission was determined through expert consensus from comprehensive review of medical records and patient interviews. The patient interviews were conducted in the presence of their family members using a pre-tested structured questionnaire (Table 1 of the Electronic Supplementary Material). During the recruitment process, we initially tested the questionnaire in a small sample of patients and structured it to suit the patients’ understanding of specific questions. Questions that patients found difficult to answer (e.g., recent drug changes, previous history of ADRs) were confirmed with family members. These data were verified using the detailed medication reconciliation notes from clinical pharmacists, GPs’ medical records if available, and other patient history notes by nurses and doctors. Patients who could not be interviewed due to their illness at the time of their admission were interviewed at a later stage of their hospital stay. All patients initially categorized as having an ADR-related admission by the clinical pharmacist researcher (NPN) and a random selection of 10% of cases without a suspected ADR-related admission were independently and blindly assessed by a senior clinical pharmacist (MC). The clinical pharmacists met to reach a consensus decision regarding the presence of an ADR-related admission, causality, and preventability, and subsequently excluded the doubtful cases. This method of ADR assessment had been reported in previous studies [3, 9, 24]. During expert consensus, the average time spent for assessment of ADR cases was 15–30 min per case. The causality of the relation between drug use and ADRs were determined using the Naranjo algorithm [25]. ADRs were classified as definite (score from 9 to 12), probable (score from 5 to 8), possible (score from 1 to 4), or doubtful (score from 0 to −2) and only definite, probable, and possible ADRs were considered for this study. We assessed the causality of each suspected ADR [26, 27] and of the ADR-related admission [11, 13]. When a patient had multiple ADRs, we used the ADR with the highest score using the Naranjo algorithm for further analysis [28].
The Anatomical Therapeutic and Chemical (ATC) classification was used to code the medications taken before hospital admission [29]. We determined the preventability of the ADRs using the modified Schumock and Thornton criteria [30, 31], as follows:
-
The drugs were not appropriate for the patient’s condition;
-
The dose, frequency, and route of administration were inappropriate for the patient’s age, weight, or disease state;
-
Therapeutic drug monitoring or other necessary laboratory tests were not performed;
-
The patient had a history of allergy or previous reaction to the administered drug;
-
A documented drug interaction was involved in the ADR;
-
A serum concentration above the therapeutic range was documented;
-
Non-compliance was involved in the ADR; or
-
A medication error was the cause of the reaction.
We assessed the preventability of each suspected ADR [26] and the preventability of the ADR-related admission [11]. When a combination of drugs was involved but the preventability varied for each drug, the preventability of the ADR was assessed as that for the drug scoring the highest grade of preventability [18].
The severity of ADRs was determined using the Hartwig et al. scale [27]. Severe reactions were defined as those that were life-threatening, caused permanent damage, or required intensive care. Moderate reactions were those requiring hospital admission, change in therapy, or specific treatment. Although this classification also includes a definition for mild ADRs, all ADRs in this study at least resulted in hospital admission and were therefore only classified as either moderate or severe.
We grouped ADRs as Type A and Type B reactions based on the Rawlins and Thompson [32] classification and whether it was due to any drug–drug interactions (DDIs), including with any ‘over-the-counter’ (OTC) medications, as evaluated according to the UpToDate database (Lexi-Interact™ Online) [23]. Type A reactions were defined as dose-dependent reactions as an exaggeration of a drug’s normal pharmacological actions and Type B reactions were dose-independent and unpredictable. A drug interaction was defined as the modification of one drug by the prior administration of another, producing loss of therapeutic effect or toxicity [18]. The UpToDate database assigns each DDI a risk rating of A (no known interaction), B (no action required), C (monitor therapy), D (consider therapy modification), or X (avoid combination). The outcome of the ADR-related admission was categorized as recovery (i.e., patients were clinically stable at discharge), death, or unknown.
Data were analyzed descriptively and presented as median (interquartile range [IQR]) or number (%) based on type and distribution of data. Analyses were performed using SPSS® version 20.0 (SPSS Inc., Chicago, IL, USA).
3 Results
A total of 1789 elderly patients were screened and 781 (43.7%) were excluded either due to their unwillingness to consent (253 patients) or inability to be interviewed due to the severity of their medical condition (528 patients). The remaining 1008 patients were enrolled in the study (RHH, 768 patients; LGH, 240 patients). The characteristics of the study population are summarized in Table 1 and Electronic Supplementary Material Table 2. The median age of the participants was 81 years (IQR 74–86) and the median number of medications (including OTC medications) taken at the time of admission was ten (IQR 7–14). Most (89.0%) were taking five or more medications. Males and females were almost equally distributed. The patients’ characteristics were comparable in both hospitals [20].
Of the 1008 patients examined, ADRs potentially caused or contributed to 191 (18.9%) acute medical admissions. Participants with an ADR had a median age of 82 years (IQR 73–86) and 54.5% were females. The median number of medications taken at the time of admission was 11 (IQR 8–15), and the median length of hospital stay was 6 days (IQR 3–12). Among the 191 patients with ADRs, 83 (43.5%) were using OTC medications and 43 (22.5%) were using herbal medications; no ADRs to these medications were identified. Of the 191 patients, 108 (56.5%) had a history of previous ADRs, and in 102 (94.4%) of these patients the medications were altered after the last ADR was experienced. Only two (1.1%) patients were admitted with the same ADR (rash induced by furosemide and dizziness induced by pregabalin) as previously reported.
Of the 191 patients with potential ADRs, 100 (52.4%) had a single ADR; 62 (32.5%) had two ADRs; 18 (9.4%) had three ADRs; six (3.1%) had four ADRs; four (2.1%) had five ADRs, and one (0.5%) had six ADRs. In 58 (30.4%) cases the ADRs were caused by a single drug and in 133 (69.6%) cases the ADRs were caused by a combination of drugs. Also, in the majority (123 [64.4%]) of cases, a combination of two or more drugs sharing a similar ADR profile (e.g., hypotension) caused the ADR-related admissions. Thus, a total of 328 ADRs caused by 452 drugs contributed to all ADR-related admissions. Applying the Naranjo algorithm to the 328 ADRs, there were 27 (8.2%) definite ADRs, 208 (63.4%) probable ADRs, and 93 (28.4%) possible ADRs. When only the one highest scoring ADR per patient was considered, 11 (5.8%) patients had definite ADRs, 134 (70.1%) had a probable ADR, and 46 (24.1%) had a possible ADR.
The most frequently involved drug classes were cardiovascular drugs (269 [59.5%]), followed by drugs acting on the nervous system (100 [22.1%]) and antithrombotic agents (31 [6.9%]). Among the cardiovascular drugs, diuretics (108 [23.9%]), agents acting on the renin–angiotensin system (74 [16.4%]), and β-blockers (β-adrenoceptor antagonists) (32 [7.1%]) were frequently implicated in causing ADRs (Table 3 of the Electronic Supplementary Material). Antidepressants (31 [6.9%]) and opioids (22 [4.9%]) were the most frequently implicated centrally acting drugs. Considering individual drugs, furosemide was the most common drug responsible for ADRs (61 [13.5%]), followed by perindopril (19 [4.2%]), metoprolol (15 [3.3%]), candesartan (15 [3.3%]), and amitriptyline (14 [3.1%]).
The type of ADRs observed in the study cohort and the most frequently implicated drugs are presented in Table 2. The most common manifestations of ADRs were cardiovascular (96 [29.3%]), neuropsychiatric (72 [20.0%]), renal and genitourinary (50 [15.2%]), and hematological (35 [10.7%]) in nature (Table 4 of the Electronic Supplementary Material).
Of the 328 ADRs, 286 (87.2%) were assessed as preventable. When the preventability of ADR-related admissions was assessed based on the highest grade of preventability, 169 (88.5%) ADR-related admissions were considered preventable. Overall, 187 (97.9%) of ADR-related admissions were classified as moderately severe, while only 4 (2.1%) were severe. Of the 191 ADR-related admissions, the ADR resolved in 178 (93.2%) and the patient recovered, while in four cases (2.1%) the outcome was fatal and in nine cases (4.7%) the outcome was unknown due to the patient’s transfer to another hospital. The severe ADRs that contributed to the four deaths were ‘probable’ ADRs and these included digoxin toxicity, pancytopenia related to antiplatelets (aspirin) in combination with other drugs (methotrexate, hydroxychloroquine), hypotension caused by a combination of diuretic (furosemide), ACE inhibitor (ACEI) (perindopril) and a β-blocker (carvedilol), and acute renal failure related to the combination of an ACEI (fosinopril) and a diuretic (indapamide) (glomerular filtration rate on admission was 7 mL/min). The patients who were admitted with digoxin toxicity and pancytopenia died due to hospital-acquired pneumonia and multiple organ failure, respectively. The patient admitted due to severe hypotension eventually died because of arrhythmia. Multiple organ failure was the reason of death in the patient admitted with acute renal failure.
A total of 181 DDIs were potentially involved in 82 (42.9%) of the ADR-related admissions. Of 181 DDIs observed, 131 (72.4%) were assigned a risk rating of C, 48 (26.5%) a risk rating of D, and two (1.1%) a risk rating of B. Examples of DDIs included confusion caused by multiple nervous system depressants, hypotension caused by multiple blood pressure-lowering agents, bleeding caused by clopidogrel and aspirin, and renal failure associated with concomitant use of diuretics and ACEIs. All ADRs were classified as Type A reactions except one ADR (rash induced by furosemide), which was considered a Type B reaction.
4 Discussion
We conducted a prospective analysis of ADR-related hospital admissions in an elderly Tasmanian population. Our study found that approximately one in five unplanned admissions to medical wards were potentially due to ADRs in patients aged ≥65 years. A similarly high rate (17%) of ADR-related hospitalizations in the elderly was also found in a meta-analysis of 17 observational studies [33]. Determining the number of ADR-related admissions depends primarily on the methods used in their detection [34]. Prospective and intensive monitoring usually have the highest detection rate and can provide data not otherwise available [12, 35]. In other studies, the proportion of all hospital admissions due to ADRs has ranged from 3 to 20% [9, 24, 28, 36–38]. Differences in definitions of ADR, method of data collection, and target populations may account for the difference in these proportions [13]. In our study, the inclusion of all definite, probable, and possible ADRs, together with a thorough review of ADR cases by the two expert clinical pharmacists, might have contributed to the identification of more ADRs. We interviewed all patients included in the study, in addition to reviewing medical records, to identify ADR-related admissions. Patient interviews by pharmacists identified more ADRs than spontaneous reporting by physicians and nurses in a previous prospective study [36]. There is also strong evidence that pharmacists report higher rate of adverse drug events than non-pharmacists [39].
Almost 90% of ADR-related hospitalizations were preventable, which is consistent with a subgroup meta-analysis demonstrating that 88% of ADR-related hospitalizations in the elderly were preventable [33]. In other studies, the preventability varied from 37 to 77% [9, 13, 40]. Even though the preventability estimates vary across studies, it is evident that more than 50% of ADRs are preventable [41]. We found a predominance of ADRs due to Type A reactions resulting from known pharmacological actions, consistent with other studies [11, 18, 27, 37]. This study found 2.1% of patients had fatal outcomes due to ADRs. Fatal outcomes and increased length of stay in older patients due to ADRs have been observed in some studies [11, 13] and the proportion of severe ADRs was found to be as high as 18.6% in a prospective study [40]. A recent study found the crude in-hospital mortality rate was 10.2% in elderly patients with an ADR-related admission [38].
Our data showed that cardiovascular complaints, such as hypotension/orthostatic hypotension/syncope, were the most common ADRs resulting in hospital admission, and these results are consistent with other studies [17, 18, 37, 42]. This proportion may have been even higher if cases of dizziness associated with antihypertensives were also included. Some studies have reported gastrointestinal complaints as frequent ADRs causing admission [13, 27, 38], while hematological complaints were reported in other studies [16, 43]. Patients with cardiovascular disease are particularly vulnerable to ADRs due to their advanced age, polypharmacy, and the influence of heart disease on drug pharmacokinetics, such as a reduction in the volume of distribution and impairment of clearance, as seen in patients with congestive heart failure [44, 45]. Antihypertensive agents were the most frequent class of drugs responsible for ADR-related admissions, as found in other studies [9, 14, 17, 18, 24, 27, 37, 42]. Additionally, renal impairment (25.7% of admissions) was impacted by antihypertensive medications such as diuretics and agents acting on the renin angiotensin system. In other studies, the most frequent therapeutic classes implicated in ADR-related admissions in the elderly were NSAIDs [4, 13, 27], antithrombotic agents [4, 13, 14, 16], or antidiabetic agents [16, 43]. The prevalence of orthostatic hypotension was very high (35–65%) in one international study in the elderly and significantly related to the number of concurrent causative medications [46]; elderly patients are also more prone to diuretic-induced dehydration and resulting orthostatic hypotension [47]. Our findings highlight the importance of cautious prescribing of antihypertensive agents, especially combinations of diuretics and agents acting on the renin–angiotensin system, in the elderly to prevent hypotension/orthostatic hypotension/syncope and renal impairment.
More than 50% of the ADRs identified in the present study were caused by a combination of drugs and most of our study participants were exposed to polypharmacy (five or more medications). Polypharmacy has been identified as an important potential risk factor for ADRs [9, 27, 28]. We also evaluated one important factor that has not been explicitly investigated in previous prospective studies, i.e., almost 65% of ADR-related admissions were caused by two or more drugs that share the same ADRs. Since some ADRs (e.g., hypotension) were particularly associated with simultaneous use of multiple medications with synergistic therapeutic and adverse effects, such as antihypertensives, prescribers need to be sure that the benefit of prescribing multiple similar medications is justified, to outweigh the risk of additive adverse effects of these agents. DDIs might have played a role in over 40% of ADR-related admissions, which is consistent with another prospective study in the elderly (32.3%) [13] and a cross-sectional study in which DDIs were suspected in 49% of cases [38]. These findings highlight the importance of obtaining an accurate medication history at each stage of a patient’s medication journey so that potential DDIs are not overlooked by their healthcare team.
Decreasing the medication burden in community-dwelling elderly patients will lead to reduced adverse events and improvement in health [48]. There is an increasing body of research demonstrating that deprescribing inappropriate or unnecessary medications is feasible, safe, and can improve older patient’s quality of life and decrease mortality [49, 50]. From our results, an obvious focus of deprescribing would be to reduce the number of different drugs with similar modes of action and/or adverse effects. Communication between health professionals such as a physician (geriatrician), nurse, and pharmacist enables optimal pharmacotherapy in elderly patients [6]. Clinical pharmacists can play a vital role, particularly at the point of discharge of elderly patients, in preventing ADR-related readmissions. In a randomized trial, pharmacist medication review, patient counselling, and telephone follow-up were associated with a lower rate of preventable adverse drug events after hospital discharge [51]. We also suggest implementing a comprehensive medication reconciliation at every transition point (admission, discharge, transfer) for the elderly, as suggested by the US Joint Commission on Accreditation of Healthcare Organizations [52].
The major strength of the study is that patients were prospectively included on admission and were followed up until discharge. We have also interviewed all the patients included in the study in addition to reviewing their medical records, which is different from a recent Spanish study [38] in which ADRs were identified either from the medical record or by direct patient interview. We also used a large sample from two major Tasmanian public hospitals which allowed us to characterize the ADRs in a detailed manner, including their preventability. To our knowledge, many prospective studies on ADRs have focused on patients admitted in a single hospital and many studies did not assess the preventability of ADRs.
Our study has some limitations. The main limitation included the difficulty in determining the contribution of a certain drug/drugs to an acute admission due to ADRs. Some of the parameters for assessing the causality of ADRs, such as the inclusion of a re-challenge and use of placebo, could not be performed since they were not routine clinical practice. In addition, measurement of drug concentrations was not performed in most suspected ADRs. Another limitation was the collection of the data using convenience sampling. With limited resources, the study team relied on recruiting elderly patients whose availability coinciding with that of the primary clinical pharmacist researcher. The degree of generalizability of the study is restrained by this study design. In addition, we could not recruit some patients due to the severity of their medical conditions and these patients were perhaps at higher risk of admissions due to ADRs. A retrospective study could have included all patients, although such a study would lose the ability to obtain information through interview. While we believe that the study results might be generalizable to the Tasmanian elderly population, as well as in other states of Australia that have similar standards of healthcare delivery to the elderly, there are inherent limitations of convenience sampling. We suggest that further studies explore the burden of ADRs in elderly residing in other states of Australia. We could only review by consensus 10% of patients who were not admitted due to an ADR (controls), which may be another limitation of the study. This might have caused an underestimation of the ADR-related admissions even though the primary clinical pharmacist researcher assessed the cases and controls comprehensively and thoroughly. Participation of a physician might have provided a more comprehensive perspective to the assessment of ADRs [24], although clinical pharmacists can play a major role in recognizing drug-related problems in the elderly [53]. Finally, with limited resources, we could not assess whether patients had any sustained disability because of an ADR, despite being clinically stable at discharge.
Given the fact that the majority of the ADRs that resulted in hospitalization were preventable in the present study, prevention of ADRs represents an important aim for physicians treating older patients [54]. Some strategies have been mentioned here and these include medication review, avoiding use of inappropriate medications, and comprehensive geriatric assessment and management [55]. In order to ensure the cost effectiveness of such strategies, it would be necessary to target them to elderly patients who are at highest risk of ADR-related admission [56]. The recently developed PADR-EC score could facilitate identification of community-dwelling elderly people at risk of ADRs and subsequent ADR-related admission [20]. The PADR-EC score could potentially be integrated into a prescribing software at the point of patient discharge as well as in primary care to alert healthcare providers (primary care physicians, pharmacists, and nurses) to their patients’ risk of ADRs and execute preventive strategies such as deprescribing.
5 Conclusion
Our research supports the findings from previous studies and further strengthens the evidence of ADRs as a cause of admissions in the elderly, along with updating the available information with respect to their proportion, preventability, outcome, and clinical characteristics. Cardiovascular medications prescribed to elderly patients need thorough and regular scrutiny as these medications were frequently implicated in ADRs. Improved medication management services in primary care are required to address the high rate of unnecessary hospitalization due to preventable ADRs. Further research is needed to address the effectiveness of some interventions, such as deprescribing, in reducing the risk of ADR-related admissions in elderly populations.
References
Shepherd G, Mohorn P, Yacoub K, May DW. Adverse drug reaction deaths reported in United States vital statistics, 1999–2006. Ann Pharmacother. 2012;46(2):169–75. doi:10.1345/aph.1P592.
European Commission. Proposal for a regulation of the European Parliament and of the Council amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. Regulation (EC) No 726/2004. Impact assessment. 2008. http://ec.europa.eu/health/files/pharmacos/pharmpack_12_2008/pharmacovigilance-ia-vol1_en.pdf. Accessed 3 June 2016.
Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890–6. doi:10.1001/archinternmed.2008.3.
Burgess CL, Holman CD, Satti AG. Adverse drug reactions in older Australians, 1981–2002. Med J Aust. 2005;182(6):267–70.
Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74. doi:10.1186/s12916-015-0322-7.
Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007;370(9582):185–91. doi:10.1016/s0140-6736(07)61092-7.
Onder G, Petrovic M, Tangiisuran B, Meinardi MC, Markito-Notenboom WP, Somers A, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med. 2010;170(13):1142–8. doi:10.1001/archinternmed.2010.153.
Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–86. doi:10.2147/cia.s71178.
Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc. 2012;60(1):34–41. doi:10.1111/j.1532-5415.2011.03772.x.
Wu C, Bell CM, Wodchis WP. Incidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments: a retrospective study. Drug Saf. 2012;35(9):769–81. doi:10.2165/11599540-000000000-00000.
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–9. doi:10.1136/bmj.329.7456.15.
World Health Organization. International drug monitoring: the role of the hospital. Geneva: World Health Organization; 1969. Technical Report Series no. 425. http://apps.who.int/iris/handle/10665/40747. Accessed 1 Mar 2014.
Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients. Drug Saf. 2008;31(6):545–56.
Conforti A, Costantini D, Zanetti F, Moretti U, Grezzana M, Leone R. Adverse drug reactions in older patients: an Italian observational prospective hospital study. Drug Healthc Patient Saf. 2012;4:75–80. doi:10.2147/dhps.s29287.
De Paepe P, Petrovic M, Outtier L, Van Maele G, Buylaert W. Drug interactions and adverse drug reactions in the older patients admitted to the emergency department. Acta Clin Belg. 2013;68(1):15–21. doi:10.2143/acb.68.1.2062714.
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging. 2009;26(6):475–82. doi:10.2165/00002512-200926060-00004.
Ma J, Wang Y, Gao M, Meng Q, Liu J. Adverse drug reactions as the cause of emergency department admission of patients aged 80 years and older. Eur J Intern Med. 2012;23(6):e162–3. doi:10.1016/j.ejim.2012.05.004.
Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J. 2001;31(4):199–205.
The Society of Hospital Pharmacy of Australia Committee of Specialty Practice in Clinical Pharmacy. SHPA standards of practice for clinical pharmacy. J Pharm Pract Res. 2005;35(2):122–46.
Parameswaran Nair N, Chalmers L, Connolly M, Bereznicki BJ, Peterson GM, Curtain C, et al. Prediction of Hospitalization due to Adverse Drug Reactions in Elderly Community-Dwelling Patients (The PADR-EC Score). PLoS One. 2016;11(10):e0165757. doi:10.1371/journal.pone.0165757.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5.
Australian Medicines Handbook 2015. Adelaide: Australian Medicines Handbook Pty Ltd; 2015 Jan.http://amhonline.amh.net.au/. Accessed 1 Mar 2015.
UpToDate. A comprehensive clinical database. Alphen aan den Rijn: Wolters Kluwer. http://www.uptodate.com. Accessed 2014–2015.
Gustafsson M, Sjolander M, Pfister B, Jonsson J, Schneede J, Lovheim H. Drug-related hospital admissions among old people with dementia. Eur J Clin Pharmacol. 2016;72(9):1143–53. doi:10.1007/s00228-016-2084-3.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Gholami K, Shalviri G. Factors associated with preventability, predictability, and severity of adverse drug reactions. Ann Pharmacother. 1999;33(2):236–40.
Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–32.
Mannesse CK, Derkx FH, de Ridder MA, Man in ’t Veld AJ, van der Cammen TJ. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing. 2000;29(1):35–9.
Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–11.
Calderon-Ospina C, Bustamante-Rojas C. The DoTS classification is a useful way to classify adverse drug reactions: a preliminary study in hospitalized patients. Int J Pharm Pract. 2010;18(4):230–5. doi:10.1111/j.2042-7174.2010.00039.x.
Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6):538.
Rawlins MD, Thompson JW. Mechanisms of adverse drug reactions. In: Davies DM, editor. Textbook of adverse drug reactions. Oxford: Oxford University Press; 1991. p. 18–45.
Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24(2):46–54.
Brvar M, Fokter N, Bunc M, Mozina M. The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol. 2009;9:8. doi:10.1186/1472-6904-9-8.
Miguel A, Bernardo M, Freitas A, Lopes F, Azevedo L, Pereira AC. Detection of adverse drug reactions using hospital databases—a nationwide study in Portugal. Pharmacoepidemiol Drug Saf. 2013;22(8):907–13. doi:10.1002/pds.3468.
Somers A, Petrovic M, Robays H, Bogaert M. Reporting adverse drug reactions on a geriatric ward: a pilot project. Eur J Clin Pharmacol. 2003;58(10):707–14. doi:10.1007/s00228-002-0535-5.
Wawruch M, Zikavska M, Wsolova L, Kuzelova M, Kahayova K, Strateny K, et al. Adverse drug reactions related to hospital admission in Slovak elderly patients. Arch Gerontol Geriatr. 2009;48(2):186–90. doi:10.1016/j.archger.2008.01.004.
Pedros C, Formiga F, Corbella X, Arnau JM. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016;72(2):219–26. doi:10.1007/s00228-015-1974-0.
Phansalkar S, Hoffman JM, Nebeker JR, Hurdle JF. Pharmacists versus nonpharmacists in adverse drug event detection: a meta-analysis and systematic review. Am J Health Syst Pharm. 2007;64(8):842–9. doi:10.2146/ajhp060335.
Alexopoulou A, Dourakis SP, Mantzoukis D, Pitsariotis T, Kandyli A, Deutsch M, et al. Adverse drug reactions as a cause of hospital admissions: a 6-month experience in a single center in Greece. Eur J Intern Med. 2008;19(7):505–10. doi:10.1016/j.ejim.2007.06.030.
Hakkarainen KM, Hedna K, Petzold M, Hagg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions–a meta-analysis. PLoS One. 2012;7(3):e33236. doi:10.1371/journal.pone.0033236.
Laroche ML, Charmes JP, Nouaille Y, Picard N, Merle L. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol. 2007;63(2):177–86. doi:10.1111/j.1365-2125.2006.02831.x.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–12. doi:10.1056/NEJMsa1103053.
Shammas FV, Dickstein K. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet. 1988;15(2):94–113. doi:10.2165/00003088-198815020-00002.
Faulx MD, Francis GS. Adverse drug reactions in patients with cardiovascular disease. Curr Probl Cardiol. 2008;33(12):703–68. doi:10.1016/j.cpcardiol.2008.08.002.
Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther. 2005;30(2):173–8. doi:10.1111/j.1365-2710.2005.00629.x.
Dickerson LM, Gibson MV. Management of hypertension in older persons. Am Fam Physician. 2005;71(3):469–76.
Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–54. doi:10.1001/archinternmed.2010.355.
Turner JP, Shakib S, Bell JS. Is my older cancer patient on too many medications? J Geriatr Oncol. doi:10.1016/j.jgo.2016.10.003. (Epub 2016 Nov 11).
Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583–623. doi:10.1111/bcp.12975.
Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–71. doi:10.1001/archinte.166.5.565.
National Patient Safety Goal on Reconciling Medication Information (NPSG.03.06.01). The Joint Commission; 2017. https://www.jointcommission.org/assets/1/6/NPSG_Chapter_HAP_Jan2017.pdf. Accessed 10 Jan 2017.
Glassman P. Chapter 4: clinical pharmacist’s role in preventing adverse drug events: brief update review. In: Making health care safer II: an updated critical analysis of the evidence for patient safety practices. Rockville: Agency for Healthcare Research and Quality (US), 2013 Mar. (Evidence Reports/Technology Assessments, no. 211). https://www.ncbi.nlm.nih.gov/books/NBK133380/. Accessed 5 Dec 2016.
Cherubini A, Ruggiero C, Gasperini B, Dell’Aquila G, Cupido MG, Zampi E, et al. The prevention of adverse drug reactions in older subjects. Curr Drug Metab. 2011;12(7):652–7.
Onder G, van der Cammen TJ, Petrovic M, Somers A, Rajkumar C. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing. 2013;42(3):284–91. doi:10.1093/ageing/aft038.
Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Castelino RL, Bereznicki LR. Hospitalization in older patients due to adverse drug reactions—the need for a prediction tool. Clin Interv Aging. 2016;11:497–505. doi:10.2147/cia.s99097.
Australian guidelines to reduce health risks from drinking alcohol. 2009. https://www.nhmrc.gov.au/guidelines-publications/ds10. Accessed 1 April 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Luke R. Bereznicki reports receiving personal fees for the provision of expert advice from Boehringer Ingelheim Pty Ltd outside this study. Nibu Parameswaran Nair, Leanne Chalmers, Bonnie J. Bereznicki, Colin Curtain, Gregory M. Peterson, and Michael Connolly declare that they have no conflicts of interest that are directly relevant to the content of this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval for the study was obtained from the Tasmanian Health and Medical Human Research Ethics Committee of the University of Tasmania and informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parameswaran Nair, N., Chalmers, L., Bereznicki, B.J. et al. Adverse Drug Reaction-Related Hospitalizations in Elderly Australians: A Prospective Cross-Sectional Study in Two Tasmanian Hospitals. Drug Saf 40, 597–606 (2017). https://doi.org/10.1007/s40264-017-0528-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0528-z