Abstract
Aspirin has been the cornerstone of therapy for the secondary prevention treatment of patients with cardiovascular disease since landmark trials were completed in the late 1970s and early 1980s that demonstrated the efficacy of aspirin for reducing the risk of ischemic events. Notwithstanding the consistent benefits demonstrated with aspirin for both acute and chronic cardiovascular disease, there are a number of toxicities associated with aspirin that have been showcased by recent long-term clinical trials that have included an aspirin monotherapy arm. As an inhibitor of cyclooxygenase (COX), aspirin impairs gastric mucosal protective mechanisms. Previous trials have shown that up to 15–20 % of patients developed gastrointestinal symptoms with aspirin monotherapy, and approximately 1 % of patients per year had a clinically significant bleeding event, including 1 in 1000 patients who suffered an intracranial or fatal bleed. These risks have been shown to be compounded for patients with acute coronary syndromes (ACS) and those undergoing percutaneous coronary intervention (PCI) who are also treated with other antithrombotic agents during the acute care/procedural period, as well as for an extended time period afterwards. Given observations of substantial increases in bleeding rates from many prior long-term clinical trials that have evaluated aspirin together with other oral platelet inhibitors or oral anticoagulants, the focus of contemporary research has pivoted towards tailored antithrombotic regimens that attempt to either shorten the duration of exposure to aspirin or replace aspirin with an alternative antithrombotic agent. While these shifts are occurring, the safety profile of aspirin when used for the secondary prevention treatment of patients with established cardiovascular disease deserves further consideration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aspirin is the mainstay of secondary prevention for patients with coronary artery disease but has a number of toxicities, including gastrointestinal discomfort, urticaria, and bleeding. |
In clinical trials enrolling patients treated with aspirin monotherapy for secondary prevention, up to 1 in 20 patients will develop gastrointestinal symptoms, 1 in 100 will have a clinically significant bleed, and 1 in 1000 will have an intracranial or fatal bleeding event. |
Ongoing clinical trials will help define the optimal duration and composition of antithrombotic therapy for secondary prevention. |
1 Introduction
Cardiovascular disease afflicts nearly 27 million people in the US, with more than 30 million predicted to be affected by the year 2030 [1, 2]. For more than 30 years, aspirin has been the cornerstone of secondary prevention strategies designed to reduce the risk of ischemic events among patients with cardiovascular disease, including patients with coronary artery disease (CAD) [3]. Meta-analyses of secondary prevention trials demonstrated a 19 % reduction in serious vascular events for patients taking aspirin compared with placebo, and a 25 % reduction in recurrent cardiovascular events in the subgroup of patients with prior myocardial infarction (MI) [4, 5]. Consequently, practice guidelines in the US and Europe strongly recommend the use of aspirin for the secondary prevention treatment of patients with stable, established CAD [6, 7].
However, alongside its beneficial effects, aspirin does have side effects, most frequently gastrointestinal toxicity and bleeding, including gastrointestinal, mucosal, and intracranial bleeding. Critical appraisals of aspirin’s toxicity by the US Preventive Services Task Force (USPSTF) and the European Society of Cardiology led to cautious recommendations for the use of aspirin in primary prevention, with the 2015 USPFTF guideline noting that aspirin should be used for patients without established cardiovascular disease only in cases where the patient has a 10-year risk of cardiovascular events ≥10 % and is at a low risk of bleeding [8–10]. Although the benefits of aspirin for the reduction of recurrent ischemic events in patients with established cardiovascular disease have been determined to outweigh the risks, aspirin is a relatively modest antiplatelet agent that has been shown to be associated with both a number of serious toxicities and a degree of residual risk of long-term ischemic events.
For the past 25 years, a variety of new antithrombotic agents have been developed (oral glycoprotein IIb/IIIa inhibitors, first- and second-generation P2Y12 inhibitors, novel oral anticoagulants, and protease-activated receptor antagonists) and pivotal clinical trials have sought to combine these new agents with background aspirin therapy to reduce recurrent cardiovascular events. Although many of these studies have demonstrated a reduction in ischemic events with the addition of a second (or third) antithrombotic agent to aspirin, all combinations have demonstrated an increased risk of bleeding. In patients with acute coronary syndromes (ACS) and those undergoing percutaneous coronary intervention (PCI), the increased risk of bleeding with dual antiplatelet therapy (DAPT) is offset by considerable reductions in ischemic events, and DAPT for 12 months is the guideline-endorsed standard of care for these patients [11–13]. In recent clinical trials enrolling patients with stable CAD and PCI more than 12 months prior, the relatively modest benefit of extended-duration DAPT for the reduction of ischemic events has been offset by concerns about increases in bleeding complications with more aggressive antithrombotic therapy [14, 15].
For this reason, several ongoing clinical trials are investigating alternative antithrombotic strategies: replacing aspirin monotherapy with an oral anticoagulant in patients with stable CAD [16], stopping aspirin after 30 days but continuing a P2Y12 inhibitor in stable patients undergoing PCI [17], replacing aspirin with an oral anticoagulant as part of dual therapy in patients with recent ACS [18], and dropping aspirin in favor of a P2Y12 inhibitor plus an oral anticoagulant in patients undergoing PCI who have an indication (such as atrial fibrillation) for anticoagulation [19–21]. As these trials enroll patients, it is important to review aspirin’s toxicity and side effects to establish a baseline by which alternative regimens should be judged. Thus, this review focuses on the safety profile of aspirin when used in the long-term setting to prevent recurrent cardiovascular events in patients with established cardiovascular disease, and does not specifically address the safety profile of aspirin when initiated acutely for the treatment of MI. We first discuss aspirin’s mechanism of action and its implications for aspirin’s toxicities and side effects, and then review data regarding side effects from pivotal and representative clinical trials.
2 Pharmacology of Aspirin and Mechanisms of Toxicity
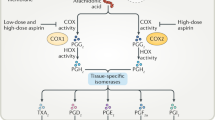
Aspirin’s mechanism of action remained unknown until 1971, when John Vane described the inhibition of prostaglandin synthesis by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs), and identified this as their mechanism of action [22]. Later work identified aspirin’s target as COX, an enzyme that converts arachidonic acid to prostaglandin G2. Prostaglandin G2 is upstream of several other important molecules, including thromboxane A2, which is a vasoconstrictor that also acts to increase platelet aggregation, and prostaglandins D2, E2, and I2, all of which act to increase renal blood flow and inhibit gastric acid production [3]. Prostaglandin I2, in contrast to thromboxane A2, is also a vasodilator that reduces platelet aggregation. The effects of NSAIDs, including aspirin, on platelet inhibition, gastric acid production, and renal blood flow are largely determined by their ability to inhibit the production of prostaglandins and thromboxane via COX inhibition (Fig. 1).
Aspirin exerts its physiologic effects via inhibition of prostaglandin synthesis. Adapted from Fuster et al. [3], with permission. COX cyclooxygenase, TXA 2 thromboxane A2, PGI 2 prostacyclin 2, PGD2 prostaglandin D2, PGE 2 prostaglandin E2
The effect of COX inhibition on various cell types is determined by how the cells process the prostaglandin G2 that COX produces. Moreover, there are two separate subtypes of COX that are important to human health (COX-1 and COX-2), which are expressed in different cell types. COX-1 is expressed constitutively by platelets and gastric mucosal cells, while COX-2 is expressed in renal cells, vascular endothelial cells, and neutrophils [23–25]. Downstream of COX-1, platelets convert prostaglandin G2/H2 primarily into thromboxane A2 via platelet-specific thromboxane synthase, and gastric mucosal cells process it into prostaglandins I2 and D2 [26]. Downstream of COX-2, vascular endothelial cells, renal cells, and inflammatory cells process prostaglandin G2 into prostaglandins I2 and E2 [23–25].
Because aspirin acts to irreversibly inhibit COX, and platelets are unable to resynthesize COX, aspirin’s platelet inhibitory effect lasts for the lifetime of the platelet [27, 28]. In nucleated cells, inhibition of COX at the cellular level is temporary since cells are able to resynthesize COX. Moreover, although aspirin inhibits both COX-1 and COX-2, it is nearly 100-fold less efficient as an inhibitor of COX-2 [29, 30].
Thus, at low doses (100 mg/day), aspirin completely inhibits platelet COX-1 activity and partially inhibits gastrointestinal mucosal COX-1 activity [24, 25, 31]. Inhibition of platelet thromboxane A2 mediates aspirin’s primary antithrombotic activity and bleeding side effects, and inhibition of gastrointestinal mucosal prostaglandins could lead to gastrointestinal toxicity. Mechanistically, it is difficult to untangle aspirin’s antithrombotic effect from its effects on gastric toxicity, and impossible to separate its antithrombotic effect from its bleeding effects. By contrast, aspirin only has the potential to inhibit vascular, inflammatory, and renal COX-2 activity at higher daily doses [26]. Thus, it would not be expected that successful aspirin treatment for secondary prevention would obligatorily be associated with cardiovascular or renal toxicity in the same way that it should be with gastric and bleeding toxicities. However, a detailed discussion of the clinical risks versus benefits of aspirin dose is beyond the scope of this review.
2.1 Renal and Cardiovascular Toxicity
Given its ability to inhibit both COX isoforms, aspirin could theoretically have nephrotoxic and cardiotoxic effects similar to the other NSAIDs, since inhibition of COX-2-mediated production of prostaglandin I2 by vascular endothelial cells could cause vasoconstrictive and prothrombotic effects, and inhibition of COX-2-mediated production of prostaglandins I2 and E2 could reduce renal blood flow [32, 33]. Indeed, in healthy volunteers, exposure to aspirin dosages as low as 325 mg/day has been shown to have a paradoxical increase in platelet aggregation despite total thromboxane A2 suppression [34]. Moreover, the COX-2 selective inhibitors (celecoxib, rofecoxib, and meloxicam) prevent vascular cell production of prostaglandin I2 without preventing platelet production of thromboxane A2, and have been shown to be associated with an increased risk of ischemic cardiovascular events [35, 36]. Thus, while inhibition of the COX-2 pathway may contribute to improved anti-inflammatory effects, the adverse cardiovascular consequences of selective COX-2 inhibitors highlight the careful balance between the anti-inflammatory and antiplatelet effects that are unique to aspirin.
2.2 Gastric Toxicity
Aspirin’s gastric toxicity arises from both direct gastric mucosal injury and reduced prostaglandin synthesis. Aspirin is acidic, which may cause direct topical injury to gastric mucosa [37]. As it is a weak acid, aspirin remains non-ionized and lipophilic within the strongly acidic gastric lumen, allowing it to penetrate the gastric mucous layer to the surface endothelial cells, where the environment is less acidic and allows for dissociation of aspirin and trapping of hydrogen ions. However, aspirin’s systemic effects on gastrointestinal toxicity are more important than its local, topical effects, which may explain the failure of enteric-coated aspirin to reduce gastrointestinal complications [38]. Inhibition of prostaglandin synthesis by aspirin causes reductions in bicarbonate secretion, epithelial mucous production, epithelial cell proliferation, and mucosal blood flow [37]. The overall effect is to make the gastric lining more susceptible to injury and less able to heal itself following injury, whether caused by endogenous acid, pepsin or bile salts, or by exogenous factors such as aspirin itself, other NSAIDs, alcohol, or Helicobacter pylori infection. Although mucosal injury is most often superficial and self-limited, aspirin’s antiplatelet effects may predispose these patients to gastrointestinal hemorrhage, especially when they are concurrently treated with anticoagulants or other potent antiplatelet agents such as P2Y12 inhibitors [39].
2.3 Bleeding
Since aspirin’s major mechanism of action in the prevention of cardiovascular events is the inhibition of platelet activity, bleeding is an expected side effect. In a recent observational cohort study conducted in Italy, 186,425 patients taking aspirin (<300 mg daily) were matched with 186,425 controls [40]. The risks of gastrointestinal bleeding requiring hospitalization and intracranial hemorrhage were approximately 50 % higher in those patients taking aspirin. Importantly, despite the increased relative risk (RR) of major bleeding with aspirin, the absolute risk only increased from 3.60 bleeds per 1000 patient years in the control arm to 5.58 bleeds per 1000 patient years in the cohort treated with aspirin. A meta-analysis of observational studies including patients taking high- and low-dose aspirin demonstrated a twofold increase in the RR of gastrointestinal hemorrhage in patients taking aspirin; this RR increase corresponded to an additional one to two gastrointestinal bleeds per 1000 patient-years [41]. A meta-analysis of clinical trials that compared aspirin with placebo for multiple indications demonstrated a 22 % increase in the incidence of hemorrhagic strokes, corresponding to one to two hemorrhagic strokes per 10,000 patient-years [5, 31].
2.4 Anaphylaxis and Upper Respiratory Symptoms
In addition to prostaglandin G2, arachidonic acid is converted into the leukotrienes—potent inducers of airway swelling, bronchoconstriction, and mucous secretion. Prostaglandin E2, production of which is inhibited by aspirin, is also a bronchodilator. The combination of these effects can produce the syndrome of aspirin-exacerbated respiratory disease, characterized by chronic rhinosinusitis with nasal polyps, asthma, and acute upper and lower respiratory tract reactions in response to aspirin ingestion. This syndrome is present in 7 % of patients with asthma, but is far less common in the broader population of patients with CAD [42, 43]. COX inhibition also appears to be responsible for urticarial reactions to aspirin, which develop in patients with and without chronic urticaria. The prevalence of aspirin hypersensitivity—either aspirin-exacerbated respiratory disease or aspirin-induced urticaria—ranges from 0.6 to 2.5 % in studies of the general population [44]. However, multiple protocols exist to safely and rapidly desensitize patients to aspirin. In a cohort of 1306 patients with ACS admitted to a Spanish coronary care unit, 24 (1.8 %) had a history of aspirin hypersensitivity. All were safely treated with an aspirin desensitization strategy involving pretreatment with antihistamines and corticosteroids followed by eight escalating doses of aspirin administered every 15 min; one patient developed hives during the desensitization protocol but was nevertheless safely desensitized to aspirin [45].
3 Safety Profile of Aspirin When Compared with Placebo for Patients with a Prior Myocardial Infarction
For the secondary prevention treatment of patients with cardiovascular disease, aspirin was initially compared with placebo in multiple trials that included a long-term exposure to blinded study drug. Six trials enrolling a total of 10,859 patients treated for a mean duration of 27 months were completed in the late 1970s and early 1980s, and compared aspirin with placebo in patients with a prior MI, randomizing them to high-dose aspirin (300 mg to 1 g daily) versus placebo [4, 5]. These trials reported on aspirin’s side effects compared with placebo in considerable detail, and provided much of the data for aspirin’s tolerability in the population of patients with CAD.
The earliest trial examining the performance of aspirin for secondary prevention in patients with prior MI (Cardiff-1), enrolled only men, who were screened immediately after hospital discharge following MI and randomized to 300 mg aspirin daily versus placebo [46]. Specific side effects were not reported, but 3.6 % of enrolled patients withdrew from the trial due to side effects, which the authors describe as ‘occasionally gastrointestinal’. Cardiff-2 was a larger trial, and enrolled both men and women who were screened immediately following hospital discharge for MI, and who were randomized to 300 mg of aspirin three times daily versus placebo [47]. Of 832 patients in the aspirin arm, 98 withdrew from the trial due to side effects that were not specified, but only 8 patients were reported to have a gastrointestinal bleed. Notably, however, 89 patients in the placebo arm also withdrew from the trial due to side effects, suggesting that aspirin was nearly as well-tolerated as placebo at the high doses tested.
Further observations on the safety and tolerability of aspirin were developed from a group of blinded, placebo-controlled trials that also evaluated aspirin as a secondary prevention treatment for prior MI patients. The Coronary Drug Project Aspirin (CDP–A) trial, Aspirin Myocardial Infarction Study (AMIS), and Persantine–Aspirin Reinfarction Study (PARIS) enrolled patients with a recent or remote prior MI, randomized patients to aspirin versus placebo, and reported details of the side effects observed with aspirin, whereas the the German–Austrian Aspirin Trial (GAMIS) did not report details on the side effects (other than those that led to the discontinuation of blinded study drug) observed with aspirin versus placebo (Table 1) [48–51]. All four trials randomized patients to high-dose aspirin versus placebo; the aspirin dose in CDP–A was 324 mg daily, 500 mg twice daily in AMIS, 324 mg three times daily in PARIS, and 500 mg three times daily in GAMIS. The most commonly reported symptoms were related to the gastrointestinal tract, with up to 18 % of patients developing a composite of symptoms suggestive of peptic ulcer, gastritis, or gastric mucosal erosion that occurred significantly more frequently in the aspirin group. For both stomach pain and heartburn, there did appear to be a dose–response relationship, with both reported more frequently in AMIS and PARIS than CDP–A. The trials also noted higher rates of nausea, vomiting, and melena in aspirin-treated patients. In many cases, these gastrointestinal symptoms were severe; in PARIS, up to 25 % of patients randomized to aspirin temporarily or permanently discontinued study drug due to gastrointestinal complaints compared with 10.3 % of patients randomized to placebo.
CDP–A demonstrated a slightly higher incidence of urticaria and pruritus in the aspirin arm, but neither occurred in more than 1.1 % of patients, while AMIS demonstrated opposite findings. Due to concern that aspirin-mediated prostaglandin I2 suppression could contribute to renal insufficiency, both AMIS and PARIS monitored patients’ serum creatinine concentration, with no difference being observed between the aspirin and placebo groups. None of these trials rigorously reported bleeding complications, but there is a suggestion of excess bleeding in the aspirin arm in AMIS. In GAMIS, nine patients (2.8 %) treated with aspirin stopped their study drug due to hemorrhage; no patients randomized to placebo had a hemorrhage resulting in cessation of study drug.
Data from these trials have been included in several meta-analyses comparing aspirin with placebo for the prevention of cardiovascular events [4, 5]. In the meta-analyses, a 20 % reduction was observed in major coronary events (death or non-fatal MI) with the use of aspirin compared with placebo (RR 0.80, 95 % CI 0.73–0.88), corresponding to an absolute risk reduction of 1 % per year. When used in patients with known vascular disease, aspirin reduced the risk of major coronary events to a similar degree in both men (RR 0.81, 95 % CI 0.72–0.92) and women (RR 0.73, 95 % CI 0.51–1.03), although with only 272 total coronary events in women, the reduction in coronary events for women was not significant. Although there is insufficient data in the post-MI trials to draw conclusions regarding bleeding rate in aspirin and placebo-treated patients, data pooled from all secondary prevention trials comparing aspirin with placebo (including those enrolling patients with prior stroke) demonstrated increased risks of hemorrhagic stroke (RR 1.67, 95 % CI 0.97–2.90) and major extracranial bleed (RR 2.69, 95 % CI 1.25–5.76) with aspirin treatment compared with placebo. In another meta-analysis, which included only trials of low-dose aspirin, aspirin again increased the risk of all major bleeding (RR 1.71, 95 % CI 1.41–2.08) (Fig. 2) [52].
Aspirin increases the risk of major bleeding compared with placebo. The authors included studies of adults assigned to low-dose aspirin (75–325 mg/day) for secondary prevention. Summary statistics were calculated using a random effects model; heterogeneity was calculated using the Chi-square test, and significant heterogeneity was defined as p < 0.1. For this analysis, p = 0.47, indicating no significant heterogeneity. Reproduced from McQuaid and Laine [52], with permission. CREDO Clopidogrel for the Reduction of Events During Observation, SPAF Stroke Prevention in Atrial Fibrillation, SALT Swedish Aspirin Low-Dose Trial, EAFT European Atrial Fibrillation Trial, TPT Thromobosis Prevention Trial, PPP Primary Prevention Project, HOT Hypertension Optimal Treatment, SAPAT Swedish Angina Pectoris Aspirin Trial, APRICOT Aspirin Versus Coumadin in the Prevention of Reocclusion and Recurrent Ischemia After Successful Thrombolysis
In patients with documented CAD, but not necessarily with a prior MI, data from the Swedish Angina Pectoris trial (SAPAT), which randomized 2035 patients with stable angina and treated them with aspirin 75 mg daily, or placebo for 50 months, demonstrated a 34 % reduction in MI and sudden death, the trial’s primary outcome [53]. However, a total of 20 patients (2.0 %) randomized to aspirin suffered a major bleeding episode, which was defined as any bleed requiring transfusion, causing death, or having serious implications for the patient, compared with 13 (1.2 %) in the placebo arm. Of the 20 major bleeding episodes, 11 were gastrointestinal bleeds, of which two were fatal, and six were intracranial bleeds, of which 4 were fatal.
On the strength of evidence from SAPAT and the meta-analyses of aspirin’s effect on preventing recurrent cardiovascular events in patients with prior MI, aspirin became established as the standard of care for secondary prevention in patients with CAD, and subsequent trials included aspirin as the active comparator in the control arm (Table 2). Aspirin therefore transitioned to becoming a benchmark therapy for the chronic secondary prevention treatment of cardiovascular disease.
4 Safety Profile of Aspirin as an Active Comparator Compared with Other Antithrombotic Regimens
The Coumadin Aspirin Reinfarction Study (CARS), Combination Hemotherapy and Mortality Prevention (CHAMP) study, Warfarin Aspirin Reinfarction Study (WARIS II), and Aspirin and Coumadin After Acute Coronary Syndromes (ASPECT-2) study were the first studies to evaluate alternative antithrombotic regimens in patients with CAD. Each study enrolled only patients stabilized after MI, and randomized them to aspirin alone (160 mg daily, except in ASPECT-2, where the dose was 80 mg daily) or the combination of warfarin plus aspirin [54–57]. In each case, follow-up was at least 12 months, and no trial demonstrated a reduction in ischemic events with the addition of warfarin. CARS and CHAMP both defined major bleeding as intracranial bleeding, bleeding requiring surgical intervention or transfusion, bleeding contributing to death, or a drop of hemoglobin by 2 g/dl or more; in both trials, the rate of major bleeding was approximately 0.7 % per year [54, 55]. WARIS II and ASPECT-2 had a more restrictive definition of major bleeding, including only bleeds that were fatal or required transfusion or surgery. Nevertheless, major bleeding rates were similar. In all trials, less than 0.1 % of patients randomized to aspirin suffered a fatal bleeding event. None of the trials reported specific side effects, but 12.6 % of patients randomized to aspirin alone in CHAMP stopped treatment with the study drug [55]. In each case, treatment with warfarin plus aspirin or warfarin alone was associated with a higher rate of bleeding than aspirin monotherapy.
The development of oral glycoprotein IIb/IIIa inhibitors provided another opportunity to evaluate novel antithrombotic strategies in comparison to aspirin. The Sibrafiban Versus Aspirin to Yield Maximum Protection from Ischemic Heart Events Post Acute Coronary Syndromes (SYMPHONY), Evaluation of Oral Xemilofiban in Controlling Thrombotic Events (EXCITE), and the Oral Glycoprotein IIb/IIIa Inhibition with Orofiban in Patients with Unstable Coronary Syndromes (OPUS) trials randomized patients with CAD, most or all of whom with ACS, to either aspirin alone (80–162 mg) or aspirin plus an oral glycoprotein IIb/IIIa inhibitor [58–61]. Unlike the warfarin secondary prevention trials, these trials enrolled patients either very shortly after presentation with ACS or very shortly after PCI with stent placement and, as a result, patients in the aspirin-only arms of these trials were exposed to a variety of other antithrombotic agents along with aspirin.
EXCITE enrolled patients undergoing PCI; 71 % of the patients enrolled underwent stent placement and received open-label ticlopidine for 14–28 days, such that the aspirin-only arm of the trial truly reflects DAPT for this duration, followed by aspirin alone up to 182 days [58]. In this group, 41 % of patients had any bleeding, including 1.8 % of patients with moderate or severe bleeding. One (of 2442) patient had an intracranial bleed and four had a moderate or severe gastrointestinal bleed; the study drug was stopped in 36 patients (1.5 %) due to bleeding.
SYMPHONY and OPUS enrolled patients with ACS within 3–5 days of presentation; 10 % of patients in SYMPHONY and 28 % of patients in OPUS underwent PCI with stenting. In SYMPHONY and OPUS, patients undergoing coronary stent placement assigned to the aspirin-only group received open-label ticlopidine in addition to aspirin for 14–28 days before returning to aspirin alone for the remainder of follow-up. In both trials, nearly all patients were exposed to heparin or low-molecular-weight heparin around the time of trial enrollment. In OPUS, 11.4 % of patients in the aspirin-only arm had a thrombolysis in myocardial infarction (TIMI) major or minor bleeding event at 10 months follow-up, and 0.12 % (n = 4) had a fatal bleeding event [61]. Most of these bleeding events (7.2 %) came within the first 30 days after presentation, when patients were treated with multiple anticoagulants in the acute ACS setting. In SYMPHONY’s aspirin-only arm, 401 patients (of 3075) had major or minor bleeding (13.0 %), with 3.9 % of patients suffering a major bleeding event [59]. The overall early rate of aspirin discontinuation was 19.2 %, although many patients later reported open-label use; 44 patients (1.4 %) stopped treatment with aspirin due to bleeding.
Neither SYMPHONY, EXCITE, nor OPUS demonstrated a reduction in ischemic endpoints with the combination of a glycoprotein IIb/IIIa inhibitor and aspirin compared with aspirin alone, and the addition of a glycoprotein IIb/IIIa inhibitor to aspirin increased bleeding rates. For this reason, glycoprotein IIb/IIIa inhibition was abandoned as a therapeutic target for long-term prevention of ischemic events in patients with CAD.
The subsequent development of the P2Y12 inhibitors ticlopidine and, later, clopidogrel, offered investigators another target for the prevention of ischemic events. The Randomized, Blinded Trial of Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) investigated the efficacy of replacing aspirin with clopidogrel in a population of patients with atherosclerotic disease in any vascular bed. In CAPRIE, 19,185 patients with MI within 35 days of randomization, ischemic stroke within 6 months of randomization, or ongoing intermittent claudication were randomized to aspirin 325 mg daily, or clopidogrel 75 mg daily, and treated for a mean follow-up period of 1.91 years [62]. Treatment with clopidogrel reduced the RR of vascular death, MI, or stroke by 8.7 % (95 % CI 0.3–16.5) without significant differences in safety. In the aspirin arm, 890 patients (9.3 %) reported any bleeding complication, including 149 (1.55 %) judged by the investigators to be severe. These severe bleeding events included 68 gastrointestinal bleeding events (0.71 %) and 41 intracranial hemorrhages (0.43 %). The rate of bleeding complications in the aspirin arm was numerically higher than the rate in the clopidogrel arm, in which 47 (0.49 %) suffered a severe gastrointestinal bleed and 30 (0.31 %) suffered a severe intracranial hemorrhage. In addition, 1686 patients in the aspirin arm (17.59 %) reported upper gastrointestinal discomfort, which was judged to be severe in 118 (1.23 %) patients. Overall, 11 % of patients randomized to aspirin alone in CAPRIE stopped the drug due to bleeding, indigestion, or abnormal liver function tests; considerably larger proportions of patients reported minor adverse events that did not lead to treatment discontinuation [62].
After CAPRIE demonstrated the long-term safety and efficacy of clopidogrel in patients with stable atherosclerosis, the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial tested the performance of clopidogrel in addition to aspirin, compared with aspirin alone, in patients with non-ST segment elevation ACS (NSTE-ACS). CURE demonstrated a significant reduction in recurrent ischemic events with the addition of clopidogrel to aspirin in patients with NSTE-ACS, although this came at a cost of increased bleeding. In CURE’s aspirin-only arm, 8.5 % of patients had a bleeding complication during 12-months of follow-up, with 3.7 % suffering a major bleed, defined by study investigators as any life-threatening bleed or one requiring transfusion of two or more units of blood [60]. Notably, more than one-third of major bleeds were clearly procedurally related, with bleeding either at a retroperitoneal, surgical, or arterial puncture site; however, the authors did not specify whether bleeding was procedural or non-procedural.
The rate of non-procedural bleeding in contemporary post-PCI patients taking aspirin was better elucidated in the Clopidogrel for the Reduction of Events During Observation (CREDO) study [63], which randomized 2116 patients undergoing PCI (67 % for an ACS indication) to one of two regimens: (i) clopidogrel 300 mg prior to PCI, followed by 75 mg daily, plus aspirin 325 mg daily, for 12 months; or (ii) clopidogrel 75 mg daily for 28 days following PCI, plus aspirin 81–325 mg daily for 12 months. The long-term results of CREDO can thus be conceptualized as a trial of aspirin plus clopidogrel versus aspirin alone. Overall, the rates of bleeding in CREDO’s aspirin-only arm were comparable to those in CURE, OPUS, and EXCITE, with 12.2 % of patients having TIMI major or minor bleeding over 12 months; however, the rate of TIMI major or minor non-procedural bleeding was 1.5 %, with 1 % of patients suffering a bleeding complication after the first month.
Although CREDO attempted to control for procedural bleeding, its results remain complicated by the fact that most of its patients presented with ACS and were thus exposed to numerous antiplatelet and anticoagulant medications. The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization study (CHARISMA) investigated the efficacy of adding clopidogrel to aspirin in a population of patients with stable atherosclerotic disease or risk factors for atherosclerosis. CHARISMA randomized patients to aspirin alone (75–162 mg daily) or aspirin plus clopidogrel [64]. In a prespecified subgroup analysis of 9478 patients with documented atherosclerotic disease, the cohort of patients treated with aspirin alone had an incidence of Global Utilization of streptokinase and tPA for Occluded Arteries (GUSTO) moderate or severe bleeding of 2.8 % over 28 months, and 0.4 % of patients developed intracranial bleeding. The addition of clopidogrel to aspirin increased the rate of major bleeding in these patients. Although patients with established atherosclerotic disease had a reduction in ischemic events with DAPT, the effect size was small (absolute risk reduction 1 %), and the overall trial did not demonstrate a reduction in ischemic events.
Based on the results of CAPRIE, CURE, CREDO, and CHARISMA, 12 months of DAPT with aspirin and clopidogrel became the standard of care for patients with ACS, but not for patients with stable atherosclerotic disease, for whom aspirin alone was standard of care. However, neither CREDO nor CURE enrolled patients undergoing PCI with drug-eluting stents, and the duration of therapy with DAPT following drug-eluting stent placement was not well-established. In the Basel Stent KostenEffektivitats Trial–Late Thrombotic Events (BASKET–LATE) a series of patients treated with drug-eluting or bare metal stents were treated for 6 months with DAPT; clopidogrel was then stopped and patients were followed for an additional year. Over that year, 2.6 % of patients who had undergone drug-eluting stent placement had a stent thrombosis event resulting in death or MI [65]. Although guidelines recommended 6–12 months of DAPT following PCI with drug-eluting stent [66, 67], the optimal duration was unknown.
The Dual Antiplatelet (DAPT) study was designed to answer this question. DAPT enrolled 25,682 patients who had undergone placement of a drug-eluting stent 1 year prior, and had tolerated (without a moderate or severe bleeding event) 1 year of treatment with aspirin plus a P2Y12 inhibitor [15]. Patients were randomized to continuation of their P2Y12 inhibitor or to continuation of aspirin only, at a dose of 75–162 mg, and were followed for 18 months. Continued treatment with a P2Y12 inhibitor significantly reduced the rate of major adverse cerebrovascular and cardiovascular events by 29 % (hazard ratio [HR] 0.71, 95 % CI 0.59–0.89). In the aspirin-only arm (n = 4476), 84 patients suffered a GUSTO severe or moderate bleed (1.88 %), and 85 patients suffered a Bleeding Academic Research Consortium (BARC) 3 or 5 bleed (1.90 %). Prolonged DAPT reduced the risk of recurrent ischemic events (4.3 % with DAPT vs. 5.9 % with aspirin alone; p < 0.001), but came at a cost of increased moderate and severe bleeding (2.5 vs. 1.6 %; p < 0.001). The effect of DAPT on clinical practice and guideline recommendations remains to be seen.
The Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin (PEGASUS) study also evaluated the performance of aspirin versus aspirin plus a P2Y12 inhibitor in stable patients who underwent stent placement at least 1 year prior to randomization. In PEGASUS, 21,162 patients with prior MI (83 % with PCI) within 1–3 years, and who were currently tolerating aspirin, were randomized to a strategy of usual care or ticagrelor plus usual care and followed for a median of 33 months [14]. In the trial, 99.9 % of patients received aspirin, and 97.3 % received a dose between 75 and 100 mg daily. Therefore, PEGASUS represents a contemporary trial of low-dose aspirin versus DAPT in stable post-MI patients. Treatment with ticagrelor reduced the primary endpoint (a composite of death, MI, or stroke) by 16 % (HR 0.84, 95 % CI 0.76–0.94). In the aspirin-only arm, 1 % of patients suffered a TIMI major or minor bleeding complication, 0.47 % of patients suffered an intracranial bleed, and 1.5 % of patients had bleeding that led to study drug discontinuation. The risk of bleeding was higher in the aspirin plus ticagrelor arm.
Importantly, both DAPT and PEGASUS explicitly required patients to be tolerating aspirin at the time of trial enrollment; DAPT required that patients tolerate a year of treatment with aspirin plus a P2Y12 inhibitor prior to enrollment. Both trials also excluded patients with indications for anticoagulation. Despite the fact that their features would tend to prevent patients at risk of bleeding from enrolling, the incidence of bleeding events in the aspirin-only arms of these trials was comparable to prior trials. Thus, even in a contemporary population of patients known to tolerate aspirin, residual risk of bleeding exists.
Overall, randomized clinical trials have validated several of aspirin’s theoretical toxicities, but disproved others. Despite a putative prothrombotic effect mediated by inhibition of vascular endothelial cell production of prostaglandin I2 and a putative nephrotoxic effect mediated by inhibition of renal cell production of prostaglandins E2 and D2, aspirin has no documented nephrotoxic or cardiotoxic effects at doses ranging from 75 mg to 1 g daily. Aspirin-exacerbated respiratory disease was not described at all in these trials, and hives were no more common in aspirin-treated patients than in placebo-treated patients. By contrast, aspirin approximately doubled the risk of acute gout flares, with an absolute increase in incidence of these events by approximately 0.5 % per year. Compared with placebo, aspirin treatment was also associated with a two- or threefold increase in the risk of gastrointestinal intolerance, depending on the dose of aspirin used and the gastrointestinal side effect of interest. Aspirin increased the absolute risk of developing stomach pain by 6–7 %, nausea by 2–6 %, vomiting by 1–2 %, and melena by 1 %. Although the tolerability of these side effects was not well-reported in the pivotal aspirin versus placebo trials, other studies showed many of these gastrointestinal intolerances to be transient and able to be overcome without cessation of treatment.
The pivotal trials comparing aspirin with placebo did not report bleeding complications in a standard manner, but numerous other trials comparing aspirin with alternative antithrombotic trials did. In stable secondary prevention patients, the risk of clinically significant bleeding (GUSTO moderate/severe, TIMI minor/major, or any bleeding complication defined as fatal, life-threatening, or requiring surgery or transfusion) generally ranged from 0.5 to 1 % per year of treatment, depending on the bleeding definitions used and the dose of aspirin. Even in the DAPT and PEGASUS cohorts of patients who were known to tolerate antiplatelet treatment, this risk persisted. Intracranial and fatal bleeds were rare, generally occurring in approximately 0.1 % of patients per year of treatment, in line with estimates of the frequency of this complication in observational studies [68]. In patients with recent ACS exposed to multiple anticoagulant and antiplatelet agents in addition to aspirin, bleeding complications were more frequent, with a rate of bleeding often exceeding 10 % annually, although much of this bleeding was procedurally-related, and still more was concentrated in the first month after ACS diagnosis, when patients were more likely to be exposed to multiple antithrombotic agents and may be critically ill.
5 Conclusions
Despite over 30 years of clinical trial and observational evidence demonstrating its efficacy for the prevention of recurrent cardiovascular events in patients with established CAD and its availability in the US and UK without a prescription, aspirin does have side effects and toxicities. In the doses tested for secondary prevention of cardiovascular events, aspirin does not share the cardiovascular and renal toxicities of the other NSAIDs, largely due to its COX-1 selectivity. However, up to 20 % of patients with established CAD will have gastrointestinal symptoms when taking aspirin, and serious bleeding will affect between 1 in 200 and 1 in 100 patients treated with aspirin, depending on the dose used and the definition of serious bleeding. Based on aspirin’s mechanism of action as an inhibitor of COX-1, with downstream inhibition of the production of platelet thromboxane A2 and gastrointestinal mucosal prostaglandins, uncoupling aspirin’s gastrointestinal and bleeding side effects from its therapeutic benefit is unlikely to be possible, especially in light of the failure of specific thromboxane A2 receptor antagonists to prevent cardiovascular events [69, 70]. Moreover, aspirin’s direct and indirect effects on gastrointestinal mucosa are likely to potentiate the bleeding toxicities of both aspirin and other antiplatelet and anticoagulant medications, a particular concern for patients in need of DAPT who have a concurrent indication for anticoagulation, in whom the annual incidence of bleeding may exceed 15 %, with a incidence of intracranial bleeding approaching 2 % [39, 71].
It was for this reason that the What is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting (WOEST) study evaluated treating patients with an indication for triple therapy with either aspirin, clopidogrel, and warfarin or clopidogrel plus warfarin [72]. The trial demonstrated that eliminating aspirin from the triple-therapy regimen substantially reduced the risk of bleeding, and although the trial was not powered to detect a difference in ischemic outcomes, it did not show an excess in death, MI, stroke, or stent thrombosis in the group randomized to take clopidogrel and warfarin alone. As a result of WOEST, PEGASUS, DAPT, and multiple other studies, the optimal duration and composition of antiplatelet therapy for secondary prevention are under debate. A number of other trials are evaluating other aspirin-excluding regimens in patients with an indication for triple therapy [19–21]; the currently enrolling COMPASS and GEMINI ACS trials will evaluate the efficacy of replacing aspirin with rivaroxaban in patients with stable CAD and ACS, respectively [16, 18], and the GLOBAL LEADERS trial will replace a traditional 12-month DAPT regimen with 1 month of DAPT followed by 23 months of ticagrelor monotherapy [17]. The results of these studies and others will help determine aspirin’s role in secondary prevention, balancing efficacy and side effects.
References
Blackwell DL, Lucas J, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. Vital Health Stat. 2014;10(260):1–161.
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44.
Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123(7):768–78.
Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–60.
Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J. 2013;34(38):2949–3003.
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Douglas PS, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–164.
Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012;33(13):1635–701.
Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2009;150(6):405–10.
U.S. Preventive Service Task Force. Draft recommendation statement: aspirin to prevent cardiovascular disease and cancer. September 2015. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed 3 Sept 2016.
Amsterdam E, Wenger N. 2014 ACC/AHA guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: executive summary. J Am Coll Cardiol. 2014;64:2645–87.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78–140.
Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–800.
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–66.
Bayer. Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS). [Clinicaltrials.gov identifier NCT01776424]. US National Institutes of Health, ClinicalTrials.gov. Available at: https://clinicaltrials.gov/show/NCT01776424. Accessed 10 Mar 2016.
Vranckx P, Valgimigli M, Windecker S, Steg P, Hamm C, Jüni P, et al. Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation: rationale and design of the GLOBAL LEADERS trial. EuroIntervention. 2015;11(7).
Janssen Research & Development LLC. A study to compare the safety of rivaroxaban versus acetylsalicylic acid in addition to either clopidogrel or ticagrelor therapy in participants with acute coronary syndrome (GEMINI ACS 1). [ClinicalTrials.gov identifier NCT02293395]. US National Institutes of Health, Clinicaltrials.gov. Available at: https://clinicaltrials.gov/show/NCT02293395. Accessed 10 Mar 2016.
Gibson CM, Mehran R, Bode C, Halperin J, Verheugt F, Wildgoose P, et al. An open-label, randomized, controlled, multicenter study exploring two treatment strategies of rivaroxaban and a dose-adjusted oral vitamin k antagonist treatment strategy in subjects with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI). Amn Heart J. 2015;169(4):472–8.e5.
Boehringer Ingelheim. Evaluation of dual therapy with dabigatran vs. triple therapy with warfarin in patients with AF that undergo a PCI with stenting (REDUAL-PCI). [ClinicalTrials.gov identifier NCT02164864]. US National Institutes of Health, ClinicalTrials.gov. Available at: https://clinicaltrials.gov/show/NCT02164864. Accessed 10 Mar 2016.
Bristol-Myers Squibb. Study apixaban to vitamin K antagonist for the prevention of stroke or systemic embolism and bleeding in patients with non-valvular atrial fibrillation and acute coronary syndrome/percutaneous coronary intervention [ClinicalTrials.gov identifier NCT02415400. US National Institutes of Health, Clinicaltrials.gov. Available at: https://clinicaltrials.gov/show/NCT02415400. Accessed 10 Mar 2016.
Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231(25):232–5.
Vane J, Botting R. The mechanism of action of aspirin. Thromb Res. 2003;110(5):255–8.
Topper JN, Cai J, Falb D, Gimbrone MA. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci. 1996;93(19):10417–22.
Pierucci A, Simonetti BM, Pecci G, Mavrikakis G, Feriozzi S, Cinotti GA, et al. Improvement of renal function with selective thromboxane antagonism in lupus nephritis. N Engl J Med. 1989;320(7):421–5.
Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2008;133(6 Suppl):199S–233S.
Roth G, Majerus PW. The mechanism of the effect of aspirin on human platelets. I: acetylation of a particulate fraction protein. J Clin Invest. 1975;56(3):624.
Patrono C, Ciabattoni G, Patrignani P, Pugliese F, Filabozzi P, Catella F, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985;72(6):1177–84.
Cipollone F, Patrignani P, Greco A, Panara MR, Padovano R, Cuccurullo F, et al. Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation. 1997;96(4):1109–16.
Bala M, Chin CN, Logan AT, Amin T, Marnett LJ, Boutaud O, et al. Acetylation of prostaglandin H2 synthases by aspirin is inhibited by redox cycling of the peroxidase. Biochem Pharmacol. 2008;75(7):1472–81.
Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373–83.
Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26(4):285–91.
Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.
Voora D, Ortel TL, Lucas JE, Chi J-T, Becker RC, Ginsburg GS. Time-dependent changes in non-COX-1-dependent platelet function with daily aspirin therapy. J Thromb Thrombolysis. 2012;33(3):246–57.
Gislason GH, Jacobsen S, Rasmussen JN, Rasmussen S, Buch P, Friberg J, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113(25):2906–13.
FitzGerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351(17):1709–11.
Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340(24):1888–99.
Lanza FL, Royer GL Jr, Nelson RS. Endoscopic evaluation of the effects of aspirin, buffered aspirin, and enteric-coated aspirin on gastric and duodenal mucosa. N Engl J Med. 1980;303(3):136–8.
Hansen ML, Sorensen R, Clausen MT, Fog-Petersen ML, Raunso J, Gadsboll N, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170(16):1433–41.
De Berardis G, Lucisano G, D’Ettorre A, Pellegrini F, Lepore V, Tognoni G, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307(21):2286–94.
De Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol. 2001;52(5):563–71.
Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135(3):676–81.e1.
LaPointe NMA, Kramer JM, DeLong ER, Ostbye T, Hammill BG, Muhlbaier LH, et al. Patient-reported frequency of taking aspirin in a population with coronary artery disease. Am J Cardiol. 2002;89(9):1042–6.
Niżankowska-Mogilnicka E, Bochenek G, Mastalerz L, Świerczyńska M, Picado C, Scadding G, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007;62(10):1111–8.
Córdoba-Soriano JG, Corbí-Pascual M, López-Neyra I, Navarro-Cuartero J, Hidalgo-Olivares V, Barrionuevo-Sánchez MI, et al. Early aspirin desensitization in unstable patients with acute coronary syndrome: short and long-term efficacy and safety. Eur Heart J Acute Cardiovasc Care 2015 (Epub 20 Nov 2015:2048872615618509).
Elwood PC, Cochrane AL, Burr ML, Sweetnam P, Williams G, Welsby E, et al. A randomized controlled trial of acetyl salicyclic acid in the secondary prevention of mortality from myocardial infarction. Br Med J. 1974;1(5905):436.
Elwood PC, Sweetnam P. Aspirin and secondary mortality after myocardial infarction. Lancet. 1979;314(8156):1313–5.
Aspirin in coronary heart disease. The Coronary Drug Project Research Group. J Chronic Dis. 1976;29(10):625–42.
Schoenberger M. A randomized, controlled trialof aspirin in persons recovered from myocardial infarction. JAMA. 1980;243:661–9.
Krol W. Persantine and aspirin in coronary heart disease. Circulation. 1980;62(3):449–61.
Breddin K, Loew D, Lechner K, Oberla K, Walter E. The German-Austrian aspirin trial: a comparison of acetylsalicylic acid, placebo and phenprocoumon in secondary prevention of myocardial infarction. On behalf of the German-Austrian Study Group. Circulation. 1980;62(6 Pt 2):V63–72.
McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119(8):624–38.
Juul-Moller S, Edvardsson N, Sorensen S, Jahnmatz B, Rosén A, Omblus R. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. Lancet. 1992;340(8833):1421–5.
Randomised double-blind trial of fixed low-dose warfarin with aspirin after myocardial infarction. Coumadin Aspirin Reinfarction Study (CARS) Investigators. Lancet. 1997;350(9075):389–96.
Fiore LD, Ezekowitz MD, Brophy MT, Lu D, Sacco J, Peduzzi P. Department of Veterans Affairs Cooperative Studies Program clinical trial comparing combined warfarin and aspirin with aspirin alone in survivors of acute myocardial infarction: primary results of the CHAMP study. Circulation. 2002;105(5):557–63.
Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969–74.
van Es RF, Jonker JJ, Verheugt FW, Deckers JW, Grobbee DE. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002;360(9327):109–13.
O’Neill WW, Serruys P, Knudtson M, van Es G-A, Timmis GC, van der Zwaan C, et al. Long-term treatment with a platelet glycoprotein-receptor antagonist after percutaneous coronary revascularization. N Engl J Med. 2000;342(18):1316–24.
Comparison of sibrafiban with aspirin for prevention of cardiovascular events after acute coronary syndromes: a randomised trial. The SYMPHONY Investigators. Sibrafiban versus Aspirin to Yield Maximum Protection from Ischemic Heart Events Post-acute Coronary Syndromes. Lancet. 2000;355(9201):337–45.
Yusuf S, Fox K, Tognoni G, Mehta S, Chrolavicius S, Keltai M, et al. Effects of clopidogrel in addition to aspirin in patients with acutecoronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502.
Cannon CP, McCabe CH, Wilcox RG, Langer A, Caspi A, Berink P, et al. Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI 16) trial. Circulation. 2000;102(2):149–56.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329–39.
Steinhubl SR, Berger PB, Mann JT III, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–20.
Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17.
Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584–91.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–122.
Windecker S, Kolh P, Alfonso F, Collet J-P, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619.
Hennekens C. Final report on the aspirin component of the ongoing Physicians Health Study. N Engl J Med. 1989;321(3):129–35.
Savage MP, Goldberg S, Bove AA, Deutsch E, Vetrovec G, Macdonald RG, et al. Effect of thromboxane A2 blockade on clinical outcome and restenosis after successful coronary angioplasty: multi-hospital eastern atlantic restenosis trial (M-HEART II). Circulation. 1995;92(11):3194–200.
Serruys PW, Rutsch W, Heyndrickx GR, Danchin N, Mast E, Wijns W, et al. Prevention of restenosis after percutaneous transluminal coronary angioplasty with thromboxane A2-receptor blockade. A randomized, double-blind, placebo-controlled trial. Coronary Artery Restenosis Prevention on Repeated Thromboxane-Antagonism Study (CARPORT). Circulation. 1991;84(4):1568–80.
Hess CN, Peterson ED, Peng SA, de Lemos JA, Fosbol EL, Thomas L, et al. Use and outcomes of triple therapy among older patients with acute myocardial infarction and atrial fibrillation. J Am Coll Cardiol. 2015;66(6):616–27.
Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman J-P, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Alexander Fanaroff has no conflicts of interest that are directly related to the content of this manuscript. Matthew Roe reports receiving research funding from Eli Lilly, Sanofi–Aventis, Daiichi Sanko, Janssen Pharmaceuticals, Ferring Pharmaceuticals, American College of Cardiology, American Heart Association, and the Familial Hypercholesterolemia Foundation; and reports receiving consulting fees or honoraria from PriMed, Astra Zeneca, Boehringer–Ingelheim, Merck, Amgen, Myokardia, Eli Lilly, and Elsevier Publishers.
Rights and permissions
About this article
Cite this article
Fanaroff, A.C., Roe, M.T. Contemporary Reflections on the Safety of Long-Term Aspirin Treatment for the Secondary Prevention of Cardiovascular Disease. Drug Saf 39, 715–727 (2016). https://doi.org/10.1007/s40264-016-0421-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0421-1