Abstract
Central post-stroke pain is a chronic neuropathic pain syndrome following a cerebrovascular accident. The development of central post-stroke pain is estimated to occur in 8 to 55% of stroke patients and is described as constant or intermittent neuropathic pain accompanied by dysesthesia of temperature and/or pressure sensations. These pain and sensory deficits are within the area of the body corresponding to the stroke lesion. The onset of pain is usually gradual, though it can develop either immediately after stroke or years after. Given the diversity in its clinical presentation, central post-stroke pain is a challenging diagnosis of exclusion. Furthermore, central post-stroke pain is often resistant to pharmacological treatment options and a clear therapeutic algorithm has not been established. Based on current evidence, amitriptyline, lamotrigine, and gabapentinoids should be used as first-line pharmacotherapy options when central post-stroke pain is suspected. Other drugs, such as fluvoxamine, steroids, and Intravenous infusions of lidocaine, ketamine, or even propofol, can be considered in intractable cases. In addition, interventional therapies such as motor cortex stimulation or transcranial magnetic stimulation have been shown to provide relief in difficult-to-treat patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The exact pathophysiology of central post-stroke pain is still not clearly elucidated; although previously believed that the pain was entirely generated in the central nervous system, newer theories suggest that signals from the peripheral nervous system may be perceived by newly sensitized central nervous system cells as painful. |
Amitriptyline and lamotrigine should be considered as first- and second-line drugs for central post-stroke pain. Gabapentinoids are acceptable alternative second-line choices as well. |
Alternative drugs, such as low-dose naltrexone and memantine, which are increasingly used to alleviate centralized pain, should be further evaluated in treating central post-stroke pain. |
1 Pathogenesis
Central post-stroke pain (CPSP) was originally described by Dejerine and Roussey in 1906 as a pain syndrome following a stroke with thalamic involvement. With subsequent studies, it has been shown that extra-thalamic lesions in the spinothalamic pathway and its cortical projections can contribute to CPSP [1]. Central post-stroke pain itself is a chronic neuropathic syndrome characterized by pain and sensory abnormalities in a body part occurring after a stroke-related lesion in the central nervous system (CNS). It is postulated that a CNS injury can lead to neurochemical changes that can affect central sensitization, disinhibition, alterations in the spinothalamic tract, and thalamic changes [1,2,3]. Newer literature has shown that CPSP may be a result of sensitization of CNS neurons to afferent input rather than autonomous ectopic CNS activity [2]. To demonstrate this concept, Haroutounian et al. conducted a prospective study where an ultrasound-guided blockade of peripheral sensory input from a painful extremity with lidocaine in eight patients with CPSP resulted in greater than 50% pain relief within 30 min. This finding suggests that pain may not be spontaneously generated within the CNS itself as previously hypothesized. Instead, pain may be secondary to CNS misinterpretation of peripheral sensory signals [2]. Given the complexity of the proposed mechanisms in the literature, it would be appropriate to say that CPSP is a multifactorial dysfunction of a network of interrelated neurons without any one mechanistic explanation.
The leading types of post-stroke pain are shoulder pain, spasticity, headaches, and CPSP [3]. Thus, the diagnosis of CPSP is one of exclusion because there are no pathognomonic features for this syndrome. The visual analog scale or numeric rating scale (NRS) is useful in the evaluation of pain intensity, but no scale exists specifically for CPSP. Onset is typically within 1–2 months of stroke though some may develop pain as late as 1–6 years post-stroke [4]. Central post-stroke pain can be described as either continuous or intermittent, and nearly all patients with CPSP have deficits of temperature and/or pain sensation [5]. Symptoms are usually described as some combination of burning, cold, lancinating, aching, pressing, stinging, and pins-and-needles-like pain. The distribution of pain can range from a small focal area to larger areas, more commonly affecting the trunk and face. The impact of CPSP can be profound and can affect a patient’s ability to carry out their activities of daily living, hinder effective rehabilitation, cause emotional disturbances, and decrease the patients’ quality of life [6]. The diagnosis remains difficult given not only the longitudinal nature needed for symptoms to develop, but also the wide range of pain symptoms that overlap with other chronic pain disorders. In addition, cognitive insults as a result of the stroke make diagnosis more difficult to obtain.
2 Epidemiology/Incidence
Stroke is the leading cause of disability and the second leading cause of death worldwide, primarily affecting older individuals [7]. Age is considered a major risk factor for both ischemic and hemorrhagic stroke, as the risk of stroke approximately doubles for each decade after age of 55 years [8]. Even though there is an ever-growing interest in the treatment for stroke, there are only few epidemiologic studies of CPSP. Estimates of CPSP in stroke patients vary widely from 8 to 55% [4]. In one prospective study of stroke patients (n = 15,754) with a mean age of 65.8 years, it was found that 2.7% of patients developed CPSP at 1 year after stroke [9]. In another study, 8.4% of the 191 stroke patients that remained in the study demonstrated CPSP [4]. The condition is particularly high after lateral medullary infarction in the ventroposterior part of the thalamus. Age, sex, and side of the lesion are not considered predictors of CPSP [10]. Nevertheless, given that post-stroke pain is one of the most frequent complications after a stroke, it is important for healthcare providers caring for these patients to understand effective treatment modalities for CPSP.
3 Pharmacological Treatment Options
A primary challenge for CPSP treatment is that it is vastly understudied compared to peripheral pain syndromes. The European Journal of Neurology and American Academy of Neurology have provided evidence-based guidelines comparing first-line agents for peripheral pain syndromes and stating strong evidence for anticonvulsants such as pregabalin and moderate evidence for antidepressants [11]. A prospective observational study by Staudt et al. showed that at the 12-month-follow-up patients with CPSP had less pain reduction but higher functional improvement compared with patients with peripheral pain [12]. This is most likely because of peripheral pain syndrome guidelines commonly adapted and applied to neuropathic pain of all etiologies. The true efficacies of these drugs are not well established and this likely results in poorly optimized care for CPSP. The problem remains for CPSP because of difficulties in patient recruitment and retention, appropriately powered studies are difficult to obtain outside of a few case reports and small-sized controlled trials (Table 1).
3.1 Antidepressants
Within the antidepressant category, tricyclic antidepressants (TCAs) are considered as first-line drugs [13]. More specifically, amitriptyline at a dose of 75 mg daily has been shown to be most efficacious [13]. A double-blind placebo-controlled crossover study by Leijon and Boivie showed pain-relieving effects of amitriptyline in patients with CPSP without depression [5]. Responders to amitriptyline had clinical improvement through the 4 weeks of the trial. Nevertheless, amitriptyline has been accepted as a first-line therapy for CPSP in the literature [3, 10, 11, 13, 14]. Other TCAs such as nortriptyline, imipramine, and desipramine, or serotonin/norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine, duloxetine, and milnacipran may be used, though efficacies have not been individually established for these medications [5]. Furthermore, a study of 31 patients with CPSP taking fluvoxamine, a selective serotonin reuptake inhibitor, showed a 1.7-point reduction in the visual analog scale for pain as well [15]. However, for selective serotonin reuptake inhibitors, it is important to note that there was no improvement in patients who had their stroke greater than 1 year. It should be noted however that TCA use in an elderly patient who had a cerebrovascular accident with concomitant comorbidities should be carefully monitored for anticholinergic as well as cardiac side effects [16]. Additionally, SNRIs and selective serotonin reuptake inhibitors have been linked to various side effects, including electrolyte abnormalities, suicidal ideation, drug–drug interaction, and serotonin syndrome in elderly patients [16,17,18].
3.2 Anticonvulsants
Gabapentin and pregabalin have well-documented efficacies in central neuropathic pain syndromes [19,20,21]. They have been regarded as first- or second-line agents for a spectrum of neuropathic pain conditions, including CPSP [3, 14]. Gabapentin (900–2400 mg/day) has been reported to be effective in reducing continuous and paroxysmal pain in studies of patients with both peripheral and central neuropathic pain, including CPSP [20,21,22]. Furthermore, Kim et al. conducted a double-blinded placebo-controlled trial of pregabalin (150–600 mg/day) vs placebo with a total of 219 patients [23]. Although the primary result was negative during the study period of 13 weeks, the pregabalin group had significant pain relief up to 8 weeks (decrease of approximately 1.5 points on the NRS pain scale). Secondary outcomes, such as quality of sleep, anxiety, and global impression of change, had significant improvements as well. Both gabapentin and pregabalin are generally considered to be safe with the most common side effects being dizziness, somnolence, diminished cognitive performance, and nausea. Therefore, they can be especially useful in treating neuropathic pain when patients are not able to tolerate higher dose of TCAs as their first-line drug. Although gabapentinoids have a better safety profile when compared to TCAs, they should still be started at a minimal dose and titrated gradually in elderly patients [16].
As neuropathic pain is transmitted via several pathways, monotherapy treatment alone may fail to provide complete relief. Combination drug therapy, pursuing multiple drug targets as well as achieving a synergistic effect, has been used successfully in other medical conditions. To enhance efficacy, similar strategies have been used for neuropathic pain. A combination therapy consisting of a gabapentinoid and an antidepressant, can provide additional pain relief while using lower doses of each to minimize side effects [24,25,26]. However, patients with CPSP often experience comorbid musculoskeletal pain, spasticity, and depression, which necessitates a combination pharmacotherapy, as well as a multimodal treatment approach including physical therapy and psychosocial counseling.
Lamotrigine was found to be moderately effective at a dose of 200 mg/day in a randomized, double-blind, placebo-controlled trial of 27 patients with CPSP [27]. These patients were followed for 8 weeks and 44% had response to the treatment with a significant reduction in pain by more than two points in NRS pain scores. Lamotrigine at lower than 200 mg/day did not produce significant pain relief. There were only a few cases of minor transient side effects consisting of mild rashes and headache. More serious, yet rare complications of lamotrigine include Stevens–Johnson syndrome and toxic epidermal necrolysis. In addition, several case reports support the efficacy of lamotrigine in treating CPSP [28, 29]. Even though further studies are warranted in evaluating lamotrigine specifically for CPSP, the drug possesses as robust evidence as any other medication to support its role early in the CPSP treatment algorithm.
Carbamazepine 800 mg/day was evaluated by Leijon and Boivie in a trial that also studied amitriptyline in CPSP. However, unlike amitriptyline, its pain-relieving effects did not reach any statistical significance [5]. Topiramate 200 mg three times daily, another anticonvulsant that was studied for CPSP treatment, did not show any efficacy in a study of seven patients with CPSP [30]. Last, a randomized, double-blind, placebo-controlled trial by Jungehulsing et al. concluded that levetiracetam does not have any analgesic effect in chronic CPSP [31].
3.3 Opioids
Opioids are generally considered ineffective in treating CPSP. In a placebo-controlled study, both the intravenous (IV) and the oral formulation of morphine failed to provide any meaningful benefit in treating patients with centralized pain [32]. Nevertheless, morphine was shown to alter the significant aspect of pain perception, thereby increasing thresholds for allodynia and thermal-induced pain. This phenomenon may be explained by the fact that one study demonstrated that morphine causes a reduction in concurrent nociceptive and psychogenic influence in CPSP [13]. Overall, opioids should be avoided in treating CPSP as high doses are necessary for clinical benefit, which often leads to a variety of harmful side effects, such as physiological dependence, addiction, overdose, and ultimately treatment failures. A notable exception could be made for tramadol, as it also possesses SNRI properties that could explain its efficacy in treating central neuropathic pain conditions [33, 34]. Furthermore, tramadol has been recommended as a second-line agent for treating a wide range of neuropathic pain conditions by the Neuropathic Pain SIG and the European Federation of Neurological Societies [11, 35]. As an opioid with a unique SNRI mechanism of action, tramadol carries additional side effects of decreased seizure thresholds, confusion in elderly patients, and serotonin syndrome when combined with other serotonergic drugs [11]. Last, IV naloxone, up to 8 mg, was studied in a double-blind trial in patients with CPSP without any clinical significance in providing pain relief [36].
3.4 IV Medications
Several IV infusions such as lidocaine, thiopental, propofol, and ketamine have been evaluated in treating CPSP with variable short-term relief [37,38,39,40,41,42,43]. For instance, a small double-blinded, placebo-controlled, cross-over study of systemic lidocaine (5 mg/kg over 30 min) had significant pain reductions in ten out of 16 patients with either CPSP (n = 6) or spinal cord injury pain (n = 10) [37]. However, pain relief lasted only up to 45 min in this study, and other IV agents that have been examined to date also appeared to provide only a very short period of efficacy. Furthermore, the evidence for IV infusion in treating CPSP is limited to case reports or prospective studies with small sample sizes with mixed results [38]. Last, each IV agent has associated serious side effects, such as cardiovascular toxicity with lidocaine and hallucinations with ketamine, which require close monitoring of patients during the treatment period [44, 45]. Although IV agents are not often mentioned as part of a CPSP treatment algorithm, IV medications may be reserved as a last resort for temporarily alleviating unrelenting pain in an inpatient setting administered by a qualified provider, as the search for a more sustainable long-lasting medication regimen continues.
3.5 Other Promising Pharmacotherapies
In a recent small retrospective study, a course of oral methylprednisolone reduced pain scores and frequency of as-needed pain medication when compared with patients treated with varying combinations amitriptyline, fluvoxamine, gabapentin, pregabalin, and lamotrigine [46]. The pain reduction was significant at 1 day after treatment initiation and 1 day prior to discharge from the rehabilitation center. The effectiveness of methylprednisolone in CPSP has not yet been replicated in any other studies.
Microglial and macrophage activation in the setting of neuroinflammation associated with strokes have been implicated as potential therapeutic targets [47]. In a rat model study by Anttila et al., intranasal (+)-naloxone administration for 7 days starting on day 1 post-stroke decreased microglia/macrophage activation in the striatum and thalamus, promoting behavioral recovery. Whether the suppression of microglia/macrophage activation after stroke can reduce CPSP is yet to be determined. Furthermore, low-dose naltrexone, which has been utilized successfully in patients with centralized pain such as chronic regional pain syndrome and fibromyalgia, is also hypothesized to reduce pain by suppressing microglial cell activation [48]. The side effects are generally considered as being very mild, and the most common are vivid dreams and headaches [48]. Therefore, both low-dose naltrexone and intranasal naloxone warrant further investigation in their potential microglial-remodeling mechanism in CPSP.
NLRP3 inflammasome, a multimeric protein complex that triggers the release of proinflammatory cytokines interleukin-1B and interleukin-18, has been implicated in the pathogenesis of CPSP [49,50,51]. Currently, drugs targeting interleukin-1B, such as canakinumab and anakinra, are clinically used for NLRP3-related pathologies including cryopyrin-associated periodic syndrome, rheumatoid arthritis, and neonatal-onset multisystem inflammatory disease [50]. Furthermore, there are several other inhibitors of NLRP3, such as MCC950, MNS, Bay 11-70802, and OLT1177 depansutrile, under investigation currently and could potentially become useful therapies in treating CPSP [50].
Memantine is an N-methyl-d-aspartate (NMDA) antagonist that is most commonly used to treat dementia in patients with Alzheimer’s disease. It has also been studied in various types of centralized pain, including fibromyalgia, phantom limb pain, complex regional pain syndrome, postoperative pain, and postherpetic neuralgia [52,53,54,55,56]. Dizziness has been reported as the most common side effect, affecting 8 out of 63 patients diagnosed with fibromyalgia [56]. Therefore, a special precaution should be taken when prescribed for elderly patients. Although memantine has not been firmly established within a centralized pain syndrome treatment algorithm, its NMDA antagonist property, along with a structurally similar drug, amantadine, makes it an interesting drug to study in treating CPSP as well. Another weak NMDA antagonist, dextromethorphan, at a daily dose of 81 mg was investigated in patients with CPSP, and was found to be non-superior to placebo [57]. Additionally, methadone is a unique opioid with NMDA antagonism. However, because of its opioid-related side effects, QT prolongation effect, and unpredictable metabolism, we advise against utilizing methadone for treating CPSP.
Cannabinoids have recently garnered a significant amount of attention as an opioid-sparing treatment option for various types of pain conditions. Cannabinoid formulations under investigations include nabilone (synthetic tetrahydrocannabinol), nabiximols (extract oral spray containing tetrahydrocannabinol and cannabidiol), dronabinol (tetrahydrocannabinol plant extract), and inhaled dried cannabis. The Canadian Pain Society has even recommended cannabinoids as a third-line treatment option for chronic neuropathic pain [58]. However, the consensus from systemic reviews of available studies to date is that the quality of evidence for its potential benefit in decreasing pain is low and that the risks (including but not limited to somnolence, confusion, and dizziness) may outweigh the benefit [35, 59,60,61].
4 Non-Pharmacological Therapies
Various non-pharmacological therapies have been explored in treating CPSP, although the majority of the evidence to date are based on case reports or small-sized comparison studies. In a recent case report by Corbetta et al., mirror physiotherapy was successfully applied in a patient with CPSP, providing a 4.5-point reduction in the visual analog scale pain score that lasted through a 1-year follow-up [62]. Although historically used for central pain, motor cortex stimulation has failed to produce an acceptable long-term benefit [63]. Repetitive transcranial magnetic stimulation with motor cortex stimulation was shown to provide sustained pain relief, lasting up to 2–3 weeks [64,65,66]. In addition, repetitive transcranial magnetic stimulation is considered extremely safe because of its non-invasive nature. A small case series by McGeoch et al. demonstrated immediate pain reduction of 2.58 NRS points via vestibular caloric stimulation in CPSP [67]. The author proposed that stimulation of the parieto-insular vestibular cortex cross-activates and modulates the adjacent thermosensory cortex, which has been hypothesized to play a role in CPSP pathogenesis. Deep brain stimulation (DBS) has been considered for intractable pain syndromes with two recent trials showing symptom improvement by targeting the affective sphere of the brain [68, 69]. There have also been literature reviews addressing the efficacy of DBS for CPSP, phantom limb as well as brachial plexus pain; however, more randomized, double-blind, placebo-controlled trials focusing on well-defined diagnoses are needed to substantiate the benefit of DBS therapy [70]. Last, there have been successful case reports for spinal cord stimulation for CPSP, in addition to a renewed interest in identifying predictive factors associated with pain relief [71,72,73].
5 Preventive Treatments
Amitriptyline has been evaluated as a prophylactic measure in patients with acute thalamic stroke in preventing the development of CPSP [74]. Out of 39 patients in the study, 21% of patients taking the placebo developed CPSP vs 17% of patients taking 75 mg of amitriptyline 1 year after the diagnosis of thalamic stroke. However, the result did not reach meaningful significance, and there is no further evidence to support its routine use in preventing CPSP. Unfortunately, there are no other studies examining pharmacological prophylaxis for CPSP.
6 Conclusions
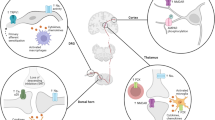
Central post-stroke pain is a subset of post-stroke pain that necessitates a prompt diagnosis and a distinct pharmacotherapy plan as outlined in Fig. 1. A systemic review of randomized controlled trials for various treatment modalities, including amitriptyline and anticonvulsants, for CPSP failed to replicate any benefit [75]. Nevertheless, it is important to be aware of various therapeutic options to formulate a strategy. Based on available evidence and consensus opinions mentioned above, amitriptyline is considered as the first-line drug of choice. This should be weighed carefully in patients who are either elderly or frail, placing them at a higher risk of having side effects. In cases where amitriptyline fails, lamotrigine has demonstrated potential as a second-line of pharmacotherapy. Gabapentin and pregabalin can be alternative second-line choices given their proven efficacies in wide ranges of central neuropathic pain with relatively well-tolerated side effects, especially when combined with lower doses of antidepressants. Non-opioid medications, such as low-dose naltrexone and memantine, that are commonly used in central pain syndromes have yet to be studied extensively in CPSP. However, given their high safety profile, these medications should also be considered when amitriptyline, lamotrigine, and gabapentinoids are inadequate or intolerable in patients with CPSP. In intractable CPSP cases, short-term analgesia with IV agents, including lidocaine, propofol, or ketamine infusions may be attempted with caution, until more long-term feasible solutions are established. Table 2 summarizes pharmacologic agents that could be useful in treating CPSP. Finally, non-pharmacological modalities such as mirror physiotherapy, motor cortex stimulation, repetitive transcranial magnetic stimulation, vestibular caloric stimulation, DBS, and spinal cord stimulation may be options in patients with recalcitrant CPSP.
Recommended pharmacological treatment algorithm for central post-stroke pain. Amitriptyline and lamotrigine have shown efficacy in the literature at the dosing of 75 mg daily and 200 mg daily, respectively. Gabapentinoids may be utilized as an alternative second-line choice given their relatively safe side-effect profile. First- and second-line agents may be combined when a single-agent therapy is inadequate or causes intolerable side effects at higher doses. Several intravenous infusion medications have been reported to provide a short duration of pain relief in intractable cases of central post-stroke pain. Non-pharmacological treatments with varying degrees of invasiveness can be considered when conservative pharmacological options do not provide adequate relief. SNRIs serotonin/norepinephrine reuptake inhibitors, TCAs tricyclic antidepressants
References
Henry JL, Lalloo C, Yashpal K. Central poststroke pain: an abstruse outcome. Pain Res Manag. 2008;13(1):41–9. https://doi.org/10.1155/2008/754260.
Haroutounian S, Ford AL, Frey K, Nikolajsen L, Finnerup NB, Neiner A, et al. How central is central poststroke pain? The role of afferent input in poststroke neuropathic pain: a prospective, open-label pilot study. Pain. 2018;159(7):1317–24. https://doi.org/10.1097/j.pain.0000000000001213.
Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8(9):857–68. https://doi.org/10.1016/S1474-4422(09)70176-0.
Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995;61(2):187–93. https://doi.org/10.1016/0304-3959(94)00144-4.
Leijon G, Boivie J. Central post-stroke pain: a controlled trial of amitriptyline and carbamazepine. Pain. 1989;36(1):27–36. https://doi.org/10.1016/0304-3959(89)90108-5.
Bashir AH, Abdullahi A, Abba MA, Mukhtar NB. Central poststroke pain: its profile among stroke survivors in Kano, Nigeria. Behav Neurol. 2017;2017:9318597. https://doi.org/10.1155/2017/9318597.
Habibi-Koolaee M, Shahmoradi L, Niakan Kalhori SR, Ghannadan H, Younesi E. Prevalence of stroke risk factors and their distribution based on stroke subtypes in Gorgan: a retrospective hospital-based study-2015-2016. Neurol Res Int. 2018;2018:2709654. https://doi.org/10.1155/2018/2709654.
Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871–95, vii. https://doi.org/10.1016/j.ncl.2008.07.003.
O’Donnell MJ, Diener HC, Sacco RL, Panju AA, Vinisko R, Yusuf S, et al. Chronic pain syndromes after ischemic stroke: PRoFESS trial. Stroke. 2013;44(5):1238–43. https://doi.org/10.1161/STROKEAHA.111.671008.
Bowsher D. The management of central post-stroke pain. Postgrad Med J. 1995;71(840):598–604. https://doi.org/10.1136/pgmj.71.840.598.
Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. https://doi.org/10.1111/j.1468-1331.2010.02999.x.
Staudt MD, Clark AJ, Gordon AS, Lynch ME, Morley-Forster PK, Nathan H, et al. Long-term outcomes in the management of central neuropathic pain syndromes: a prospective observational cohort study. Can J Neurol Sci. 2018;45(5):545–52. https://doi.org/10.1017/cjn.2018.55.
Oh H, Seo W. A comprehensive review of central post-stroke pain. Pain Manag Nurs. 2015;16(5):804–18. https://doi.org/10.1016/j.pmn.2015.03.002.
Kim JS. Pharmacological management of central post-stroke pain: a practical guide. CNS Drugs. 2014;28(9):787–97. https://doi.org/10.1007/s40263-014-0194-y.
Shimodozono M, Kawahira K, Kamishita T, Ogata A, Tohgo S, Tanaka N. Reduction of central poststroke pain with the selective serotonin reuptake inhibitor fluvoxamine. Int J Neurosci. 2002;112(10):1173–81. https://doi.org/10.1080/00207450290026139.
Pickering G, Marcoux M, Chapiro S, David L, Rat P, Michel M, et al. An algorithm for neuropathic pain management in older people. Drugs Aging. 2016;33(8):575–83. https://doi.org/10.1007/s40266-016-0389-7.
Greenblatt HK, Greenblatt DJ. Antidepressant-associated hyponatremia in the elderly. J Clin Psychopharmacol. 2016;36(6):545–9. https://doi.org/10.1097/JCP.0000000000000608.
Mihanovic M, Restek-Petrovic B, Bodor D, Molnar S, Oreskovic A, Presecki P. Suicidality and side effects of antidepressants and antipsychotics. Psychiatr Danub. 2010;22(1):79–84.
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67(10):1792–800. https://doi.org/10.1212/01.wnl.0000244422.45278.ff.
Attal N, Brasseur L, Parker F, Chauvin M, Bouhassira D. Effects of gabapentin on the different components of peripheral and central neuropathic pain syndromes: a pilot study. Eur Neurol. 1998;40(4):191–200. https://doi.org/10.1159/000007979.
Serpell MG, Neuropathic pain study G. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99(3):557–66. https://doi.org/10.1016/s0304-3959(02)00255-5.
Holtom N. Gabapentin for treatment of thalamic pain syndrome. Palliat Med. 2000;14(2):167. https://doi.org/10.1177/026921630001400216.
Kim JS, Bashford G, Murphy TK, Martin A, Dror V, Cheung R. Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain. 2011;152(5):1018–23. https://doi.org/10.1016/j.pain.2010.12.023.
Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374(9697):1252–61. https://doi.org/10.1016/S0140-6736(09)61081-3.
Holbech JV, Bach FW, Finnerup NB, Brosen K, Jensen TS, Sindrup SH. Imipramine and pregabalin combination for painful polyneuropathy: a randomized controlled trial. Pain. 2015;156(5):958–66. https://doi.org/10.1097/j.pain.0000000000000143.
Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tolle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”: a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154(12):2616–25. https://doi.org/10.1016/j.pain.2013.05.043.
Vestergaard K, Andersen G, Gottrup H, Kristensen BT, Jensen TS. Lamotrigine for central poststroke pain: a randomized controlled trial. Neurology. 2001;56(2):184–90. https://doi.org/10.1212/wnl.56.2.184.
Carrieri PB, Provitera VV, Lavorgna L, Bruno R. Response of thalamic pain syndrome to lamotrigine. Eur J Neurol. 1998;5(6):625–6. https://doi.org/10.1046/j.1468-1331.1998.560625.x.
Canavero S, Bonicalzi V. Lamotrigine control of central pain. Pain. 1996;68(1):179–81. https://doi.org/10.1016/s0304-3959(96)03168-5.
Canavero S, Bonicalzi V, Paolotti R. Lack of effect of topiramate for central pain. Neurology. 2002;58(5):831–2. https://doi.org/10.1212/wnl.58.5.831-a.
Jungehulsing GJ, Israel H, Safar N, Taskin B, Nolte CH, Brunecker P, et al. Levetiracetam in patients with central neuropathic post-stroke pain: a randomized, double-blind, placebo-controlled trial. Eur J Neurol. 2013;20(2):331–7. https://doi.org/10.1111/j.1468-1331.2012.03857.x.
Attal N, Guirimand F, Brasseur L, Gaude V, Chauvin M, Bouhassira D. Effects of IV morphine in central pain: a randomized placebo-controlled study. Neurology. 2002;58(4):554–63. https://doi.org/10.1212/wnl.58.4.554.
Tanei T, Kajita Y, Noda H, Takebayashi S, Hirano M, Nakahara N, et al. Efficacy of tramadol/acetaminophen medication for central post-stroke pain. No Shinkei Geka. 2013;41(8):679–85.
Norrbrink C, Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin J Pain. 2009;25(3):177–84. https://doi.org/10.1097/AJP.0b013e31818a744d.
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. https://doi.org/10.1016/S1474-4422(14)70251-0.
Bainton T, Fox M, Bowsher D, Wells C. A double-blind trial of naloxone in central post-stroke pain. Pain. 1992;48(2):159–62. https://doi.org/10.1016/0304-3959(92)90052-d.
Attal N, Gaude V, Brasseur L, Dupuy M, Guirimand F, Parker F, et al. Intravenous lidocaine in central pain: a double-blind, placebo-controlled, psychophysical study. Neurology. 2000;54(3):564–74. https://doi.org/10.1212/wnl.54.3.564.
Frese A, Husstedt IW, Ringelstein EB, Evers S. Pharmacologic treatment of central post-stroke pain. Clin J Pain. 2006;22(3):252–60. https://doi.org/10.1097/01.ajp.0000173020.10483.13.
Awerbuch GI, Sandyk R. Mexiletine for thalamic pain syndrome. Int J Neurosci. 1990;55(2–4):129–33. https://doi.org/10.3109/00207459008985960.
Canavero S, Bonicalzi V, Pagni CA, Castellano G, Merante R, Gentile S, et al. Propofol analgesia in central pain: preliminary clinical observations. J Neurol. 1995;242(9):561–7. https://doi.org/10.1007/bf00868808.
Canavero S, Bonicalzi V. Intravenous subhypnotic propofol in central pain: a double-blind, placebo-controlled, crossover study. Clin Neuropharmacol. 2004;27(4):182–6. https://doi.org/10.1097/01.wnf.0000138635.42121.9e.
Vick PG, Lamer TJ. Treatment of central post-stroke pain with oral ketamine. Pain. 2001;92(1–2):311–3. https://doi.org/10.1016/s0304-3959(00)00488-7.
Yamamoto T, Katayama Y, Hirayama T, Tsubokawa T. Pharmacological classification of central post-stroke pain: comparison with the results of chronic motor cortex stimulation therapy. Pain. 1997;72(1–2):5–12. https://doi.org/10.1016/s0304-3959(97)00028-6.
Rosenbaum SB, Gupta V, Palacios JL. Ketamine. Treasure Island (FL): StatPearls; 2020.
Torp KD, Simon LV. Lidocaine toxicity. Treasure Island (FL): StatPearls; 2020.
Pellicane AJ, Millis SR. Efficacy of methylprednisolone versus other pharmacologic interventions for the treatment of central post-stroke pain: a retrospective analysis. J Pain Res. 2013;6:557–63. https://doi.org/10.2147/JPR.S46530.
Anttila JE, Albert K, Wires ES, Matlik K, Loram LC, Watkins LR, et al. Post-stroke intranasal (+)-naloxone delivery reduces microglial activation and improves behavioral recovery from ischemic injury. eNeuro. 2018. https://doi.org/10.1523/ENEURO.0395-17.2018.
Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33(4):451–9. https://doi.org/10.1007/s10067-014-2517-2.
Li SJ, Zhang YF, Ma SH, Yi Y, Yu HY, Pei L, et al. The role of NLRP3 inflammasome in stroke and central poststroke pain. Medicine (Baltimore). 2018;97(33):e11861. https://doi.org/10.1097/MD.0000000000011861.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. https://doi.org/10.1038/s41577-019-0165-0.
Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. https://doi.org/10.1038/s41419-019-1413-8.
Pickering G, Morel V. Memantine for the treatment of general neuropathic pain: a narrative review. Fundam Clin Pharmacol. 2018;32(1):4–13. https://doi.org/10.1111/fcp.12316.
Kurian R, Raza K, Shanthanna H. A systematic review and meta-analysis of memantine for the prevention or treatment of chronic pain. Eur J Pain. 2019;23(7):1234–50. https://doi.org/10.1002/ejp.1393.
Martin E, Sorel M, Morel V, Marcaillou F, Picard P, Delage N, et al. Dextromethorphan and memantine after ketamine analgesia: a randomized control trial. Drug Des Devel Ther. 2019;13:2677–88. https://doi.org/10.2147/DDDT.S207350.
Buvanendran A, Kroin JS. Early use of memantine for neuropathic pain. Anesth Analg. 2008;107(4):1093–4. https://doi.org/10.1213/ane.0b013e318180ebfe.
Olivan-Blazquez B, Herrera-Mercadal P, Puebla-Guedea M, Perez-Yus MC, Andres E, Fayed N, et al. Efficacy of memantine in the treatment of fibromyalgia: a double-blind, randomised, controlled trial with 6-month follow-up. Pain. 2014;155(12):2517–25. https://doi.org/10.1016/j.pain.2014.09.004.
McQuay HJ, Carroll D, Jadad AR, Glynn CJ, Jack T, Moore RA, et al. Dextromethorphan for the treatment of neuropathic pain: a double-blind randomised controlled crossover trial with integral n-of-1 design. Pain. 1994;59(1):127–33. https://doi.org/10.1016/0304-3959(94)90056-6.
Mu A, Weinberg E, Moulin DE, Clarke H. Pharmacologic management of chronic neuropathic pain: review of the Canadian Pain Society consensus statement. Can Fam Phys. 2017;63(11):844–52.
Lee G, Grovey B, Furnish T, Wallace M. Medical cannabis for neuropathic pain. Curr Pain Headache Rep. 2018;22(1):8. https://doi.org/10.1007/s11916-018-0658-8.
Mucke M, Phillips T, Radbruch L, Petzke F, Hauser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. https://doi.org/10.1002/14651858.CD012182.pub2.
Amato L, Minozzi S, Mitrova Z, Parmelli E, Saulle R, Cruciani F, et al. Systematic review of safeness and therapeutic efficacy of cannabis in patients with multiple sclerosis, neuropathic pain, and in oncological patients treated with chemotherapy. Epidemiol Prev. 2017;41(5–6):279–93. https://doi.org/10.19191/EP17.5-6.AD01.069.
Corbetta D, Sarasso E, Agosta F, Filippi M, Gatti R. Mirror therapy for an adult with central post-stroke pain: a case report. Arch Physiother. 2018;8:4. https://doi.org/10.1186/s40945-018-0047-y.
Sachs AJ, Babu H, Su YF, Miller KJ, Henderson JM. Lack of efficacy of motor cortex stimulation for the treatment of neuropathic pain in 14 patients. Neuromodulation. 2014;17(4):303–10. https://doi.org/10.1111/ner.12181 (discussion 310–1).
Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76(6):833–8. https://doi.org/10.1136/jnnp.2004.055806.
Ramger BC, Bader KA, Davies SP, Stewart DA, Ledbetter LS, Simon CB, et al. Effects of non-invasive brain stimulation on clinical pain intensity and experimental pain sensitivity among individuals with central post-stroke pain: a systematic review. J Pain Res. 2019;12:3319–29. https://doi.org/10.2147/JPR.S216081.
Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, et al. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain. 2009;10(12):1205–16. https://doi.org/10.1016/j.jpain.2009.03.010.
McGeoch PD, Williams LE, Lee RR, Ramachandran VS. Behavioural evidence for vestibular stimulation as a treatment for central post-stroke pain. J Neurol Neurosurg Psychiatry. 2008;79(11):1298–301. https://doi.org/10.1136/jnnp.2008.146738.
Lempka SF, Malone DA Jr, Hu B, Baker KB, Wyant A, Ozinga JGT, et al. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol. 2017;81(5):653–63. https://doi.org/10.1002/ana.24927.
Boccard SGJ, Prangnell SJ, Pycroft L, Cheeran B, Moir L, Pereira EAC, et al. Long-term results of deep brain stimulation of the anterior cingulate cortex for neuropathic pain. World Neurosurg. 2017;106:625–37. https://doi.org/10.1016/j.wneu.2017.06.173.
Frizon LA, Yamamoto EA, Nagel SJ, Simonson MT, Hogue O, Machado AG. Deep brain stimulation for pain in the modern era: a systematic review. Neurosurgery. 2020;86(2):191–202. https://doi.org/10.1093/neuros/nyy552.
Pickering AE, Thornton SR, Love-Jones SJ, Steeds C, Patel NK. Analgesia in conjunction with normalisation of thermal sensation following deep brain stimulation for central post-stroke pain. Pain. 2009;147(1–3):299–304. https://doi.org/10.1016/j.pain.2009.09.011.
Tanei T, Kajita Y, Takebayashi S, Aoki K, Nakahara N, Wakabayashi T. Predictive factors associated with pain relief of spinal cord stimulation for central post-stroke pain. Neurol Med Chir (Tokyo). 2019;59(6):213–21. https://doi.org/10.2176/nmc.oa.2018-0292.
Owen SL, Green AL, Nandi DD, Bittar RG, Wang S, Aziz TZ. Deep brain stimulation for neuropathic pain. Acta Neurochir Suppl. 2007;97(Pt 2):111–6. https://doi.org/10.1007/978-3-211-33081-4_13.
Lampl C, Yazdi K, Roper C. Amitriptyline in the prophylaxis of central poststroke pain: preliminary results of 39 patients in a placebo-controlled, long-term study. Stroke. 2002;33(12):3030–2. https://doi.org/10.1161/01.str.0000037674.95228.86.
Mulla SM, Wang L, Khokhar R, Izhar Z, Agarwal A, Couban R, et al. Management of central poststroke pain: systematic review of randomized controlled trials. Stroke. 2015;46(10):2853–60. https://doi.org/10.1161/STROKEAHA.115.010259.
Author information
Authors and Affiliations
Contributions
Hanwool Choi contributed to the content of sections 3, 4, 5, 6, Figure 1, and Table 2 . Adem Aktas contributed to the content of sections 1, 2, 3, Figure 1, and Table 2. Michael Bottros contributed the outline and editing of this manuscript, and content of sections 1–6.
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of Interest
Hanwool Ryan Choi, Adem Aktas, and Michael Bottros have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Rights and permissions
About this article
Cite this article
Choi, H.R., Aktas, A. & Bottros, M.M. Pharmacotherapy to Manage Central Post-Stroke Pain. CNS Drugs 35, 151–160 (2021). https://doi.org/10.1007/s40263-021-00791-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00791-3