Abstract
Ocrelizumab (Ocrevus®) is a humanized anti-CD20 monoclonal antibody approved for the treatment of adults with relapsing multiple sclerosis (RMS) or primary progressive multiple sclerosis (PPMS). In the two identically designed, 96-week OPERA I and II trials in patients with RMS, ocrelizumab significantly reduced annualized relapse rates versus interferon β-1a. In the ≥ 120-week ORATORIO trial in patients with PPMS, ocrelizumab significantly reduced the risk of ≥ 12-week confirmed disability progression relative to placebo. These primary endpoint results were supported by a number of secondary outcomes, including disease activity in the brain assessed by magnetic resonance imaging. Ocrelizumab was generally well tolerated in these studies, with infusion-related reactions and infections being the most common adverse events, which were mostly mild to moderate in severity. In summary, ocrelizumab is a novel high-efficacy disease-modifying therapy for RMS that is more effective than interferon β-1a and also a valuable new treatment option for delaying progression in early PPMS. It offers a convenient once every 6 months treatment regimen, with no need for routine monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Selectively depletes CD20-positive B cells |
Significantly reduced annualized relapse rate relative to interferon β-1a in patients with RMS |
Significantly reduced the risk of 12-week confirmed disability progression relative to placebo in patients with PPMS |
Generally well tolerated; the most common adverse events were infusion-related reactions and infections |
1 Introduction
Multiple sclerosis (MS) is an immune-mediated CNS disease that involves inflammation, demyelination and axonal damage [1]. Relapsing MS (RMS) is the most common form, characterized by recurrent relapses and remissions of neurological symptoms; over the course of time, untreated RMS often transitions to secondary progressive MS [2]. Primary progressive MS (PPMS) is a less common form, characterized by steady worsening of symptoms from the onset of the disease [2]. Disease-modifying therapies (DMTs) are the cornerstone of long-term MS management (Sect. 7). In general, DMTs act via suppression or modulation of immune and inflammatory responses [1].
In addition to T cells, B cells play an important role in the pathogenesis of MS through autoantibody production, antigen presentation, pathogenic cytokine production and formation of meningeal ectopic lymphoid tissues [3]. Therefore, B cell depletion is an effective treatment strategy for MS and several B cell-depleting anti-CD20 monoclonal antibodies (mAbs) have been investigated [4]. A chimeric anti-CD20 mAb (not approved for MS) was associated with a high incidence of anti-drug antibodies in MS [5], although the clinical relevance of such antibodies is not fully known. Therefore, efforts have been made to develop humanized or fully human anti-CD20 mAbs.
Ocrelizumab (Ocrevus®), an intravenously administered glycosylated immunoglobulin (Ig) G1, is a recombinant, humanized anti-CD20 mAb that is approved in the EU (Sect. 6) [6], USA and elsewhere for the treatment of RMS and PPMS. It is the first anti-CD20 mAb approved for RMS and the first ever pharmacotherapy approved for PPMS. This article reviews the efficacy and tolerability of ocrelizumab in patients with RMS and PPMS from the EU perspective and summarizes relevant pharmacological data.
2 Pharmacodynamic Properties of Ocrelizumab
The precise mechanism by which ocrelizumab exerts its clinical benefits in MS is not fully understood, but is thought to involve immunomodulation through a reduction in the number and function of CD20-expressing B cells [6]. Ocrelizumab binds to CD20 and selectively depletes CD20-expressing B cells through antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity, and apoptosis [6, 7]. CD20 is expressed on pre-, mature and memory B cells, but not on lymphoid stem cells and plasma cells [6, 7]. Thus, B cell reconstitution by lymphoid stem cells and pre-existing humoral immunity due to plasma cells are preserved during ocrelizumab therapy [6]. Furthermore, in patients with MS, innate [6] and adaptive [8] immunity was intact after ocrelizumab therapy, and ocrelizumab did not appear to modulate peripheral T cell number or functions [9].
Ocrelizumab 600 mg every 24 weeks decreased CD19-positive peripheral cell counts (a surrogate marker for CD20-positive B cell depletion) to negligible levels by week 2, which was sustained over 96 weeks of treatment, in pivotal trials in patients with RMS [10] or PPMS [11]. In a phase 2 study [12] in patients with relapsing-remitting MS receiving four cycles of ocrelizumab 600 mg every 24 weeks, the median time to B cell repletion was 72 weeks after the last infusion [13].
3 Pharmacokinetic Properties of Ocrelizumab
The pharmacokinetics of the approved dosage of ocrelizumab (Sect. 6) in patients with RMS or PPMS in clinical studies were described by a two compartment model with time-dependent clearance [6]. The overall exposure to ocrelizumab following a single 600 mg intravenous infusion in patients with RMS was similar to that after two 300 mg infusions in patients with PPMS. Ocrelizumab has an estimated central volume of distribution of 2.78 L, with an estimated peripheral volume of 2.68 L and an intercompartment clearance of 0.297 L/day. Ocrelizumab is expected to be cleared primarily via catabolism, with an estimated constant clearance of 0.17 L/day. The terminal half-life of ocrelizumab was 26 days [6].
The pharmacokinetics of ocrelizumab have not been formally investigated in patients aged < 18 or ≥ 55 years, or in those with renal or hepatic impairment [6]. Mild renal or hepatic impairment did not affect the pharmacokinetics of ocrelizumab in clinical trials; however, pharmacokinetic data are not available for patients with moderate or severe renal or hepatic impairment [6].
Concomitant use of ocrelizumab with other immunosuppressive therapy is not recommended due to an increased risk of infection [6]. When initiating ocrelizumab and immunosuppressive therapy one after the other, the potential for overlapping pharmacodynamic effects should be considered. Vaccination with live-attenuated or live vaccines is not recommended during ocrelizumab therapy and not until B cell repletion. No formal drug interaction studies have been conducted for ocrelizumab, as no drug interactions with cytochrome P450 enzymes, metabolic enzymes or transporters are expected [6].
4 Therapeutic Efficacy of Ocrelizumab
4.1 Relapsing Multiple Sclerosis
The efficacy of ocrelizumab in patients aged 18–55 years with RMS (2010 McDonald criteria) was evaluated in two identically designed, randomized, double-blind, double-dummy, active-controlled, multinational, pivotal phase 3 trials (OPERA I and OPERA II) [10]; some data are available as abstracts [14,15,16,17,18,19,20,21,22,23,24]. At screening, patients had documented clinical relapses (at least two in the previous 2 years or one in the previous year), an Expanded Disability Status Scale (EDSS) score of 0–5.5 (scores range from 0 to 10, with higher scores indicating greater disability) and no neurological worsening for ≥ 30 days. Exclusion criteria included RMS for > 10 years with an EDSS score of ≤ 2, PPMS and previous B cell-targeted or other immunosuppressive therapy [10].
Patients were randomized to receive ocrelizumab 600 mg every 24 weeks (two 300 mg infusions 14 days apart for the first dose and a single 600 mg infusion thereafter) or subcutaneous interferon β-1a 44 µg thrice weekly for 96 weeks [10]. Management of infusion-related reactions (IRRs) by premedications, infusion rate adjustment and symptomatic treatment during infusion was permitted [10].
The primary endpoint was annualized relapse rate (ARR) at 96 weeks [10]. The ten secondary endpoints were assessed following a statistical hierarchy. Confirmed disability progression (CDP) and confirmed disability improvement (CDI; assessed in patients with a baseline EDSS score of ≥ 2) rates were assessed in a prespecified pooled analysis of OPERA I and II, and all other endpoints were assessed in the individual trials. Efficacy analyses were conducted in the intent-to-treat (ITT) population, with one secondary endpoint [no evidence of disease activity (NEDA)] analysed in a modified ITT population. NEDA was defined as no relapse, no 12- or 24-week CDP, no new or newly enlarged lesions on T2-weighted MRI and no gadolinium-enhancing (Gd+) lesions on T1-weighted MRI [10].
Baseline demographic and disease characteristics were generally similar between the treatment groups in both OPERA I and II [10]. Across the groups, the mean time since RMS diagnosis was 3.71–4.15 years (mean time since symptom onset 6.25–6.74 years), the mean number of relapses in the previous year was 1.31–1.34 and the mean EDSS score at baseline was 2.75–2.86. Approximately three-quarter of patients were not treated with any DMT in the 2 years prior to screening. Overall, ≈ 60% of patients had no Gd+ lesion on T1-weighted MRI, and the mean number/volume of lesions on T2-weighted MRI was ≈ 51/≈ 10.5 cm3 per treatment group. Overall, the mean normalized brain volume was ≈ 1501 cm3 [10].
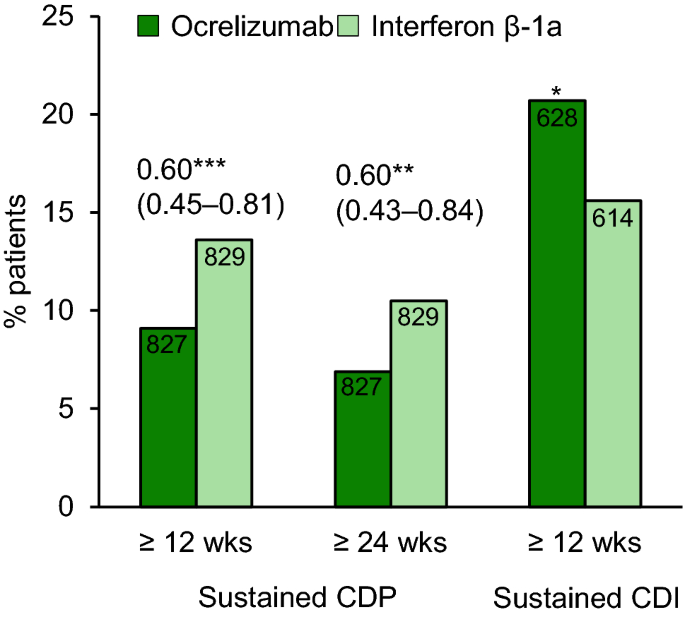
In OPERA I and II, ocrelizumab significantly reduced the ARR at 96 weeks by 46 and 47%, respectively, compared with interferon β-1a (primary endpoint; Table 1) [10]. This finding was supported by the first six of the ten prespecified secondary endpoints. In the prespecified pooled analysis, ocrelizumab was associated with significantly better CDP and CDI outcomes than interferon β-1a (Fig. 1) [10]. Furthermore, in both OPERA I and II, ocrelizumab was associated with significantly fewer Gd+ T1 lesions (an indicator of inflammation), new or newly enlarged hyperintense T2 lesions (an indicator of plaque formation) and new hypointense T1 lesions (an indicator of more severe damage) than interferon β-1a (Table 1) [10].
Efficacy of ocrelizumab in patients with relapsing multiple sclerosis in a prespecified pooled analysis of the OPERA I and II trials [10]. Confirmed disability progression (CDP) or confirmed disability improvement (CDI, assessed in patients with a baseline EDSS score of ≥ 2) were defined as sustained increase or decrease from the baseline EDSS score of ≥ 1.0 (0.5 if the baseline score was > 5.5). Values above the bars are hazard ratio (95% CI). Values inside the bars are patient numbers. *p = 0.02, **p < 0.003, ***p < 0.001 ocrelizumab vs. interferon β1-a
Based on the hierarchical testing, results for the remaining secondary endpoints were considered to be nonconfirmatory, although ocrelizumab produced favourable results in terms of Multiple Sclerosis Functional Composite score, brain volume loss, NEDA and physical-component summary (PCS) score of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) in at least one of the trials [10]. NEDA improved by 33–75% with ocrelizumab relative to interferon β-1a in all epochs assessed (weeks 0–24, 0–48, 24-48, 24–96, 48–96 and 0–96) based on a pooled analysis of the OPERA trials [25].
Ocrelizumab significantly reduced the ARR versus interferon β-1a as early as week 8 [17]. The 96-week ARR benefit with ocrelizumab was seen in the overall pooled population and in all pooled subgroups by age, gender, use of DMT in the previous 2 years, baseline EDSS score, relapses in the last 12 months and Gd+ T1 lesions at baseline [16]. Similarly, 12- and 24-week CDP benefits were seen in all subgroups, with the exception of the subgroup with two or more relapses in the last 12 months [24].
Ocrelizumab significantly (p < 0.05 vs. interferon β-1a) reduced 12- and 24-week composite CDP [defined as an increase in EDSS score of ≥ 1.0 (0.5 if baseline score was > 5.5), or ≥ 20% progression in timed 25-foot walk test or in 9-hole peg test] independent of relapse activity in patients with RMS, including those at higher risk of secondary progressive MS [15]. Ocrelizumab significantly (p < 0.05 vs. interferon β-1a) improved visual outcomes [14] and cognition in patients at increased risk of progressive disease [23], including those with visual [14] and cognition impairment [23] at baseline.
Compared with interferon β-1a, ocrelizumab significantly (p < 0.0001) reduced Gd + T1 lesions and new/enlarging T2 lesions in patients with early RMS [18] and was associated with significantly (p < 0.001) smaller losses of cerebral white and grey matter in the overall population in at least one trial [20]. Of note, myelin-related MRI findings (which are thought to be more sensitive than those of conventional MRI) favoured ocrelizumab over interferon β-1a in a small substudy [19].
During a 2-year follow-up of an open-label extension (OLE) of OPERA I and II, the benefits of ocrelizumab therapy in terms of ARR [21], 24-week CDP [21], MRI disease activity [22] and brain atrophy measures [22] were maintained in patients who had received ocrelizumab in the core studies. Patients who switched from interferon β-1a to ocrelizumab at the start of the OLE had rapid and robust reductions in ARR [21] and MRI disease activity [22].
4.2 Primary Progressive Multiple Sclerosis
The efficacy of ocrelizumab in patients aged 18–55 years with PPMS (2005 McDonald criteria) was evaluated in the randomized, double-blind, placebo-controlled, phase 3 ORATORIO trial [11]. Some data are available as abstracts [26,27,28,29,30,31,32]. At screening, patients had to have an EDSS score of 3.0–6.5, a pyramidal functions component score of ≥ 2, MS symptoms for < 15 years (if EDSS score was > 5) or < 10 years (if EDSS score was ≤ 5) and an elevated IgG index or at least one IgG oligoclonal band in the cerebrospinal fluid. Patients with relapsing-remitting, secondary progressive or progressive relapsing forms of MS were excluded, as were patients previously treated with B cell-targeted or other immunosuppressive therapy [11].
Patients were randomized to receive ocrelizumab 600 mg (two 300 mg infusions 14 days apart) every 24 weeks or placebo [11]. Management of IRRs was similar to that in the OPERA trials (Sect. 4.1). ORATORIO was event-driven, where the double-blind treatment was administered for a minimum of 120 weeks (five doses) and was continued until 253 events of ≥ 12-week CDP (primary endpoint) had occurred. The primary and five secondary endpoints were assessed in the ITT population, following a statistical hierarchy. The median trial duration was 2.9 and 2.8 years in the ocrelizumab and placebo groups. Patients continued the double-blind treatment beyond the clinical cut-off date (i.e. extended control period) until transition to an OLE [11].
Baseline demographic and disease characteristics were generally similar between the treatment groups [11]. Across the groups, the mean time since PPMS diagnosis was ≈ 3 years (mean time since symptom onset ≈ 6 to 7 years) and the mean EDSS score at baseline was 4.7. Approximately 88% of patients had not received any DMT in the 2 years before study entry. Overall, ≈ 73% of patients had no Gd+ T1 lesions; per treatment group, the mean number of T2 lesions was 48–49 (mean total volume of T2 lesions 10.9–12.7 cm3). Overall, the mean normalized brain volume was ≈ 1466 cm3 [11].
In the ITT population, significantly fewer ocrelizumab than placebo recipients had ≥ 12-week CDP, corresponding to a 24% reduction in the risk (primary endpoint; Fig. 2) [11]. Prespecified (but not statistically powered) subgroup analyses of the primary endpoint suggest that patients who are younger (≤ 45 years) or those with T1 Gd+ lesions at baseline achieve a greater treatment benefit than patients who are older or without T1 Gd+ lesions [6, 11]. In an exploratory subgroup analysis, the proportion of female patients with ≥ 12-week CDP was similar between ocrelizumab and placebo groups (≈ 36% in each group) [33], although the clinical significance of this finding is currently unclear.
The ≥ 12-week CDP benefit with ocrelizumab was supported by ≥ 24-week CDP, the first secondary endpoint in the statistical hierarchy (Fig. 2). Ocrelizumab also reduced walking impairment versus placebo, as assessed by the mean percent change from baseline in timed 25-foot walk test at week 120 (i.e. worsening in the performance of 38.9 vs. 55.1%; relative reduction 29.3%, 95% CI – 1.6 to 51.5, p = 0.04). However, there was no between-group difference in physical-health-related quality of life, as assessed by SF-36 PCS score [11].
MRI outcomes also favoured ocrelizumab over placebo [11]. The total volume of T2 lesions decreased in ocrelizumab recipients, whereas it increased in placebo recipients (adjusted geometric mean percent change from baseline at 120 weeks –3.37 vs. 7.43%; p < 0.001). Moreover, brain volume loss from week 24 to 120 was significantly smaller with ocrelizumab than with placebo (mean percent change – 0.90 vs. – 1.09%, p = 0.02). [11].
In addition, prespecified exploratory clinical (12- and 24-week composite CDP) and MRI (new or enlarging T2 lesions per scan) endpoints significantly (p < 0.001) favoured ocrelizumab over placebo [11].
The CDP benefit with ocrelizumab was sustained during the extended treatment period [26]. Ocrelizumab was associated favourable outcomes relative to placebo in terms of severe disability progression [29], risk of becoming wheelchair-bound [28], upper extremity disability progression [27], no evidence of progression [32], no evidence of progression or active disease [30], and mental well-being (assessed by SF-36 mental component summary) and fatigue (assessed by Modified Fatigue Impact Scale) [31].
5 Tolerability of Ocrelizumab
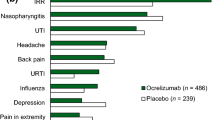
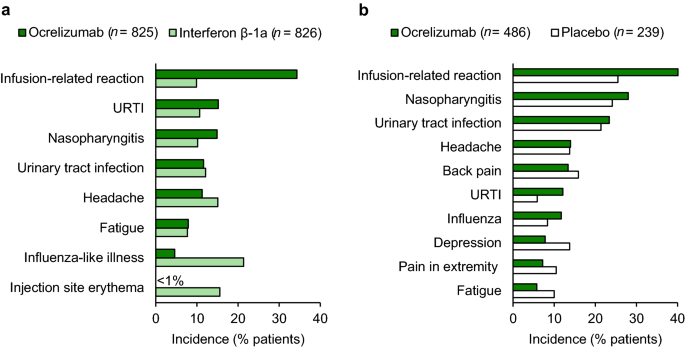
Ocrelizumab 600 mg every 24 weeks was generally well tolerated in patients with RMS in OPERA I and II [10] and PPMS in ORATORIO [11]. In a pooled analysis of the OPERA trials, ocrelizumab was associated with the same incidence of any adverse event (AE) versus interferon β-1a (83.4 vs. 83.4%) and numerically lower incidences of serious AEs (7.0 vs. 8.8%) and withdrawal because of AEs (3.5 vs. 6.0%) [10]. In ORATORIO, although the incidences of any AE (95.1 vs. 90.0%) and withdrawal because of AEs (4.1 vs. 3.3%) were numerically higher with ocrelizumab than with placebo, the incidence of serious AE was lower with ocrelizumab (21.0 vs. 23.4%) [11].
The very common (≥ 1/10) adverse reactions associated with the use of ocrelizumab in patients with RMS or PPMS [n = 1311; 3054 patient-years (PY)] during the controlled treatment period in clinical trials were IRRs, infection and infestations, and decreased levels of blood IgM [6]. The safety profile of ocrelizumab in an updated analysis (n = 3778; 9474 PY) was generally consistent with that seen during the controlled treatment period [34].
IRRs were the most common AE in ocrelizumab recipients (Fig. 3) [10, 11], with the most common symptoms being pruritus, rash, throat irritation and flushing [10]. The majority of IRRs with ocrelizumab were mild or moderate in severity, with ≤ 2.4% of ocrelizumab recipients experiencing severe IRRs [6, 10, 11]. There were no life threatening (apart from one case of bronchospasm in OPERA I) or fatal IRRs with ocrelizumab [10, 11]. IRRs were reported most commonly after the first dose and decreased with subsequent administration, and were treated with premedication and infusion adjustments [10, 12].
Although infections occurred numerically more frequently with ocrelizumab versus interferon β-1a (58.5 vs. 52.5%) [10] or placebo (71.4 vs. 69.9%) [11], serious infections were numerically less frequent with ocrelizumab (1.3 vs. 2.9% [10] and 6.2 vs. 6.7% [11], respectively). The most common infections with ocrelizumab were upper respiratory tract infection, nasopharyngitis, urinary tract infection and influenza (Fig. 3) [10, 11]. In addition, herpes virus-related infection was numerically more frequent with ocrelizumab than with interferon β-1a (5.9 vs. 3.4%) [10] or placebo (4.7 vs. 3.3%) [11], with oral herpes being the most common (3.0 vs. 2.2% [10] and 2.3 vs. 0.4% [11], respectively). The majority of respiratory [6] and herpes-virus-related [10, 11] infections were mild or moderate in severity, with the latter resolving with treatment.
Ocrelizumab treatment was associated with a decrease in total Ig (most notably IgM) levels in some patients [6, 10, 11]. The proportion of ocrelizumab recipients with IgG, IgA and IgM levels below the lower level of normal (LLN) at 96 weeks was 1.5, 2.4 and 16.5%, respectively, in the pooled OPERA trials [10]. The corresponding proportions at 120 weeks were 1.1, 0.5 and 15.5% (vs. 1.2, 0.6 and 1.2% in the placebo group) in ORATORIO [11]. Where reported, there was no apparent relationship between decreased IgM levels and serious infections [11].
Ocrelizumab treatment was associated with lymphocytopenia and neutropenia in some patients [6]. In patients with RMS, numerically fewer ocrelizumab than interferon β-1a recipients had lymphocytes (20.7 vs. 32.6%) and neutrophils (14.7 vs. 40.9%) below the LLN. However, in patients with PPMS, numerically more ocrelizumab than placebo recipients had lymphocytes (26.3 vs. 11.7%) and neutrophils (12.9 vs. 10.0%) below the LLN, although the majority of these decreases in ocrelizumab recipients were of grade 1 or 2 severity.
Across all ocrelizumab studies in patients with MS, there was a numerical imbalance in malignancies between the ocrelizumab (exposure 6467 PY) and comparator (interferon β-1a or placebo; 2053 PY) arms [11]. The overall incidence rate of malignancy per 100 PY was 0.402 (95% CI 0.263–0.589) with ocrelizumab versus 0.195 (0.053–0.499) with comparators. The malignancies included breast cancer in women [0.230 (0.105–0.437) vs. 0 (0–0.293)] and non-melanoma skin cancer [0.108 (0.044–0.223) vs. 0.097 (0.012–0.352)] [11]. However, the rate of malignancies in MS patients treated with ocrelizumab was within the range reported in epidemiological surveys [34].
Of 1311 patients treated with ocrelizumab, 12 (0.9%) patients developed treatment-emergent anti-drug antibodies, including two patients developing neutralizing antibodies [6].
6 Dosage and Administration of Ocrelizumab
In the EU, ocrelizumab is indicated for adult patients with active RMS (as defined by clinical or imaging features), or early PPMS in terms of disease duration and level of disability and with imaging features characteristic of inflammatory activity [6]. The recommended dosage is 600 mg every 6 months administered intravenously (two 300 mg infusions 2 weeks apart for the first dose, then single 600 mg infusions) [6]. Consult local prescribing information for details of premedications for IRRs, management of IRRs during therapy, contraindications, use in special populations and special warnings and precautions.
7 Place of Ocrelizumab in the Management of Multiple Sclerosis
There is no curative therapy for MS, and the goal of the current therapeutic strategy is to reduce the risk of relapses and disability progression [2]. A wide range of DMTs have been approved for RMS in the EU, including subcutaneous β-interferons (interferon β-1a, interferon β-1b, peginterferon β-1a), subcutaneous glatiramer acetate, small-molecule oral agents (fingolimod, dimethyl fumarate, teriflunomide, cladribine), intravenous mAbs (alemtuzumab, natalizumab, ocrelizumab) and an intravenous chemotherapeutic agent (mitoxantrone) [2]. PPMS is a challenging form of MS to treat and a number of DMTs have failed to demonstrate clinical efficacy in this indication [35]. Currently, ocrelizumab is the only DMT approved for PPMS.
The cellular target and the mechanism of action of ocrelizumab in MS are different from those of existing DMTs [1]. Ocrelizumab selectively depletes CD20-expressing B cells while preserving normal immune function (Sect. 2). The EU approval of ocrelizumab was based on results from pivotal phase 3 trials in patients with RMS (OPERA I and II) and in those with PPMS (ORATORIO) [Sect. 4]. In these trials, ocrelizumab significantly reduced ARR (as well as ≥ 12- and ≥ 24-week CDP) relative to high-dose interferon β-1a in patients with RMS over 96 weeks (Sect. 4.1) and significantly reduced the risk of ≥ 12-week CDP (as well as progression to walking impairment) relative to placebo in patients with PPMS over ≥ 120 weeks (Sect. 4.2). In both populations, ocrelizumab significantly reduced signs of MS activity in the brain as assessed by MRI. Ocrelizumab was generally well tolerated in clinical trials, with the most common adverse events being IRRs and infections, which were mostly mild or moderate in severity (Sect. 5). IRRs were reported most commonly after the first infusion and decreased over subsequent infusions, and were managed well with premedication and infusion adjustments. Consistent with humanized mAbs, ocrelizumab has low immunogenicity (Sect. 5).
According to the recent ECTRIMS/EAN guidelines, ocrelizumab has high quality of evidence for ARR reduction in patients with RMS and for ≥ 12-week CDP risk reduction in those with PPMS [2]. For active relapsing-remitting MS, these guidelines recommend choosing among the wide range of available DMTs, from the modestly effective to the highly effective, depending on patient characteristics and comorbidities, disease severity and activity, drug safety profile and accessibility of the drug [2]. ECTRIMS/EAN guidelines recommend ocrelizumab for PPMS [2]. In the UK, NICE recommends ocrelizumab as an option for treating relapsing-remitting MS in adults with active disease (defined by clinical or imaging features) if alemtuzumab is contraindicated or not suitable and if the manufacturer of ocrelizumab provides the drug at the discounted price agreed in the confidential patient access scheme [36].
Although pivotal studies support the use of ocrelizumab in RMS and PPMS, additional comparative and longer-term benefit-risk data will be useful to more clearly define the relative place of ocrelizumab in the management of MS. Furthermore, the small numerical imbalance in malignancies in ocrelizumab clinical trials (Sect. 5) warrants ongoing evaluation within the context of malignancies in the MS population and the long-term safety of anti-CD20 therapies, including ocrelizumab [10, 11]. In terms of patient convenience, ocrelizumab therapy may be minimally disruptive to patient’s personal and professional life, as it is administered once every 6 months [36]; moreover, routine monitoring of patients taking ocrelizumab is not required [6].
In conclusion, ocrelizumab is a novel high-efficacy DMT for RMS that is more effective than interferon β-1a and also a valuable new treatment option for delaying the progression in early PPMS. Ocrelizumab offers a convenient once every 6 months treatment regimen, with no need for routine monitoring.
Data Selection Ocrelizumab: 376 records identified
Duplicates removed | 46 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 216 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 78 |
Cited efficacy/tolerability articles | 23 |
Cited articles not efficacy/tolerability | 13 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Ocrelizumab, Ocrevus, multiple sclerosis, PPMS, RRMS, RMS. Records were limited to those in English language. Searches last updated 3 Aug 2018 | |
References
Dargahi N, Katsara M, Tselios T, et al. Multiple sclerosis: immunopathology and treatment update. Brain Sci. 2017;7(7):78.
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25(2):215–37.
Lehmann-Horn K, Kinzel S, Weber MS. Deciphering the role of B cells in multiple sclerosis—towards specific targeting of pathogenic function. Int J Mol Sci. 2017;18(10):2048.
Gelfand JM, Cree BAC, Hauser SL. Ocrelizumab and other CD20+ B-cell-depleting therapies in multiple sclerosis. Neurotherapeutics. 2017;14(4):835–41.
Dunn N, Juto A, Ryner M, et al. Rituximab in multiple sclerosis: frequency and clinical relevance of anti-drug antibodies. Mult Scler. 2017;9(3):409–16.
Ocrevus 300 mg concentrate for solution for infusion: EU summary of product characteristics. 2018. http://www.ema.europa.eu. Accessed 3 Aug 2018.
Sorensen PS, Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord. 2016;9(1):44–52.
Laurent S, Michel B, Wu H, et al. Effect of ocrelizumab on B and T cell immune repertoires in patients with relapsing multiple sclerosis [abstract no. P693]. Mult Scler J. 2017;23(Suppl 3):337.
von Buedingen H-C, Shon Nguyen Q, Harp C, et al. Ocrelizumab does not modulate peripheral T cell functionality or prevalence in a small subset of relapsing MS patients enrolled in OPERA I, a phase III double-blind double-dummy interferon beta-1a-controlled study [abstract no. P659]. Mult Scler J. 2017;23(Suppl 3):312.
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34.
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20.
Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–87.
Ocrevus: assessment report. 2018. http://www.ema.europa.eu. Accessed 3 Aug 2018.
Balcer L, Hauser SL, Kappos L, et al. Effect of ocrelizumab vs that of interferon beta-1a on visual outcomes in patients with relapsing multiple sclerosis in the OPERA studies [abstract no. 192]. Mult Scler J. 2017;23(Suppl 3):56–7.
Kappos L, Wolinsky JS, Giovannoni G, et al. Ocrelizumab reduces disability progression independent of relapse activity in patients with relapsing multiple sclerosis [abstract no. P654]. Mult Scler J. 2017;23(Suppl 3):309–10.
Papeix C, Cree B, Turner B, et al. Subgroup analyses of annualised relapse rates in patients with relapsing multiple sclerosis who received ocrelizumab or interferon beta-1a in the phase III OPERA I and OPERA II studies [abstract no. P687]. Mult Scler J. 2017;23(Suppl 3):332–3.
Hauser S, Kappos L, Bar-Or A, et al. Rapidity of onset of ocrelizumab clinical efficacy in relapsing multiple sclerosis [abstract no. S31.002]. Neurology. 2017;88(16 Suppl 1).
Traboulsee A, Hauser S, Havrdova E, et al. Efficacy of ocrelizumab on brain MRI outcomes in patients with early relapsing multiple sclerosis: pooled analysis of the OPERA studies [abstract no. P6.338]. Neurology. 2017;88(16 Suppl 1).
Kolind S, Vavasour I, Tang L, et al. Advanced myelin-related MRI measures in relapsing multiple sclerosis patients treated with ocrelizumab or interferon beta-1a over 96 weeks [abstract no. P6.371]. Neurology. 2017;88(16 Suppl 1).
Arnold DL, Bar-Or A, Comi G, et al. Effect of ocrelizumab on magnetic resonance imaging markers of neurodegeneration in patients with relapsing multiple sclerosis: analysis of the phase III, double-blind, double-dummy, interferon beta-1a-controlled OPERA I and OPERA II studies [abstract no. P1011]. Mult Scler J. 2016;22(Suppl 3):514–5.
Hauser S, Brochet B, Montalban X, et al. Annualized relapse rate and confirmed disability progression in patients receiving continuous ocrelizumab or switching from interferon beta-1a to ocrelizumab therapy in the open-label extension period of the phase III trials of ocrelizumab in patients with relapsing multiple sclerosis [abstract no. P1.366]. Neurology. 2018;90(Suppl 15).
Arnold D, Kappos L, Hauser S, et al. Brain MRI activity and atrophy measures in patients receiving continuous ocrelizumab or switching from interferon beta-1a to ocrelizumab therapy in the open-label extension period of the phase III trials of ocrelizumab in patients with relapsing multiple sclerosis [abstract no. S6.002]. Neurology. 2018;90(Suppl 15).
Benedict R, De Seze J, Hauser S, et al. Impact of ocrelizumab on cognition in patients at increased risk of progressive disease [abstract no. P1.420]. Neurology. 2018;90(Suppl 15).
Turner B, Cree B, Lorscheider J, et al. Confirmed disability progression in different subgroups of patients with relapsing multiple sclerosis who received ocrelizumab or interferon beta-1a in the phase III OPERA I and OPERA II studies [abstract no. P1.371]. Neurology. 2018;90(Suppl 15).
Havrdova E, Arnold DL, Bar-Or A, et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin. 2018. https://doi.org/10.1177/2055217318760642.
Wolinsky JS, Montalban X, Hauser SL, et al. Sustained and durable reduction in confirmed disability progression in patients with primary progressive multiple sclerosis receiving ocrelizumab: findings from the phase III ORATORIO study extended control period [abstract no. P1234]. Mult Scler J. 2017;23(Suppl 3):656–7.
Fox EJ, Markowitz C, Applebee A, et al. Effect of ocrelizumab on upper limb function in patients with primary progressive multiple sclerosis in the ORATORIO study [abstract no. P1236]. Mult Scler J. 2017;23(Suppl 3):658–9.
Giovannoni G, De Seze J, Kappos L, et al. An exploratory analysis of the risk of being restricted to wheelchair in patients with primary progressive multiple sclerosis in the ORATORIO trial [abstract no. PR1087]. Eur J Neurol. 2017;24(Suppl 1):494.
Kappos L, Giovannoni G, De Seze J, et al. Impact of ocrelizumab on reducing more severe disability progression in primary progressive multiple sclerosis [abstract no. O1216]. Eur J Neurol. 2017;24(Suppl 1):44.
Montalban X, Wolinsky J, Kappos L, et al. Evaluation of No Evidence of Progression or Active Disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial [abstract no. PR2086]. Eur J Neurol. 2017;24(Suppl 1):576.
De Seze J, Montalban X, McDougall F, et al. Patient-reported outcomes in the phase III double-blind, placebo-controlled ORATORIO study of ocrelizumab in primary progressive multiple sclerosis [abstract no. P171]. Mult Scler J. 2017;23(Suppl 1):84.
Wolinsky J, Montalban X, Arnold DL, et al. Evaluation of no evidence of progression (NEP) in patients with primary progressive multiple sclerosis in the ORATORIO trial [abstract no. P015]. Mult Scler J. 2017;23(Suppl 1):17.
Ocrevus™ (ocrelizumab): US prescribing information. 2017. https://www.fda.gov. Accessed 3 Aug 2018.
Hauser S, Kappos L, Montalban X. Safety of ocrelizumab in multiple sclerosis: updated analysis in patients with relapsing and primary progressive multiple sclerosis [abstract no. S36.001]. Neurology. 2018;90(Suppl 15).
Gajofatto A, Turatti M, Benedetti MD. Primary progressive multiple sclerosis: current therapeutic strategies and future perspectives. Expert Rev Neurother. 2017;17(4):393–406.
National Institute for health and Care Excellence. Ocrelizumab for treating relapsing multiple sclerosis: final appraisal document. 2018. https://www.nice.org.uk. Accessed 3 Aug 2018.
Acknowledgements
During the peer review process, the manufacturer of ocrelizumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Yahiya Y. Syed is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: P. K. Coyle, Department of Neurology, Stony Brook University, Stony Brook, NY, USA; M. S. Freedman, Department of Medicine, Division of Neurology, University of Ottawa; The Ottawa Hospital Research Institute, Multiple Sclerosis Research Unit, The Ottawa Hospital - General Campus; A. Traboulsee, Division of Neurology, University of British Columbia, Vancouver, BC, Canada.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Ocrelizumab: A Review in Multiple Sclerosis. CNS Drugs 32, 883–890 (2018). https://doi.org/10.1007/s40263-018-0568-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0568-7