Abstract
Background
Lennox–Gastaut syndrome (LGS) is a severe developmental epileptic encephalopathy, and available interventions fail to control seizures in most patients. Cannabidiol (CBD) is a major chemical of marijuana, which has anti-seizure properties and different mechanisms of action compared with other approved antiepileptic drugs (AEDs).
Objective
The aim was to evaluate the efficacy and safety of CBD as adjunctive treatment for seizures in patients with LGS using meta-analytical techniques.
Methods
Randomized, placebo-controlled, single- or double-blinded trials were identified. Main outcomes included the ≥ 50% reduction in baseline drop and non-drop seizure frequency, and the incidence of treatment withdrawal and adverse events (AEs). Risk ratios (RRs) with 95% confidence intervals (CIs) were estimated through the inverse variance method.
Results
Two trials were included involving 396 participants. Patients presenting ≥ 50% reduction in drop seizure frequency during the treatment were 40.0% with CBD and 19.3% with placebo [RR 2.12 (95% CI 1.48–3.03); p < 0.001]. The rate of non-drop seizure frequency was reduced by 50% or more in 49.4% of patients in the CBD and 30.4% in the placebo arms [RR 1.62 (95% CI 1.09–2.43); p = 0.018]. The RR for CBD withdrawal was 4.93 (95% CI 1.50–16.22; p = 0.009). The RR to develop any AE during CBD treatment was 1.24 (95% CI 1.11–1.38; p < 0.001). AEs significantly associated with CBD were somnolence, decreased appetite, diarrhea and increased serum aminotransferases.
Conclusions
Adjunctive CBD resulted in a greater reduction in seizure frequency and a higher rate of AEs than placebo in patients with LGS presenting seizures uncontrolled by concomitant AEDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cannabidiol (CBD) is a major chemical of marijuana displaying anti-seizure properties without psychoactive effects. |
Adjunctive CBD was effective in controlling drop and non-drop seizures in patients with Lennox–Gastaut syndrome. |
The most common adverse events associated with CBD were somnolence, decreased appetite, diarrhea and increased serum aminotransferases. |
1 Introduction
Epilepsies are one of the most common groups of brain disorders, affecting approximately 70 million people worldwide [1, 2]. Treatment is mainly symptomatic, and although most patients have a favorable prognosis and achieve a long-term remission, almost one-third continue to experience seizures despite adequate treatment [3,4,5].
Lennox–Gastaut syndrome (LGS) is a severe drug-resistant developmental epileptic encephalopathy with various causes [6]. The syndrome is clinically characterized by the occurrence of multiple seizures types, slow spike-wave activity on the electroencephalogram, and moderate to severe cognitive impairment [6]. Seizures usually begin in early childhood and have a peak age of onset between 3 and 5 years. They persist into adulthood in more than 90% of patients, are usually hard to control and need life-long treatment [7]. Drop seizures due to the increase (tonic) or loss (atonic) of motor tone are characteristic of this syndrome and are very upsetting given the potential for body injuries following sudden falls [8].
The antiepileptic drugs (AEDs) licensed for LGS in the USA and Europe include felbamate, lamotrigine, topiramate, rufinamide, clobazam, and clonazepam [9]. The use of valproate is also common on the basis of clinical experience and study data [10]. Non-pharmacological strategies, like vagus nerve stimulation [11], ketogenic diet [12], and corpus callosotomy [13], have further shown to be beneficial in some cases [14, 15]. All currently available interventions, however, fail to control seizures in most patients. LGS presents great challenges to both patients and their caregivers, and there still remains the need to identify new effective therapeutic strategies.
The interest in cannabis-based therapies for epilepsy dates back more than a millennium and has skyrocketed in the recent years [16]. Cannabidiol (CBD) is a major chemical of marijuana that is devoid of adverse psychoactive effects and abuse liability [16]. Compared with approved AEDs, CBD has a distinctive chemical structure and mechanisms of action [17], which have not been fully ascertained. The anti-seizure properties do not relate to the direct effect on cannabinoid receptors, but appeared to be mediated by the agonism or antagonism of multiple 7-transmembrane receptors, ionic channels and neurotransmitter transporters [16, 17]. In the preclinical setting, CBD has shown activity against seizures in both in vitro and in vivo models [18]. The evidence about the anti-seizure potential of CBD has further increased with the results from an open-label expanded access program in children and young adults with drug-resistant epilepsy [19], and placebo-controlled, randomized trials in patients with difficult-to-treat epileptic syndromes, including LGS and Dravet syndrome [20].
The aim of this systematic review and meta-analysis was to assess the efficacy and safety of CBD for the treatment of seizures in patients with LGS.
2 Methods
2.1 Search Strategy
The report of this systematic review and meta-analysis was made according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]. We systematically searched (May week 3, 2018) MEDLINE (accessed by PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL) and the US National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov) (search strategies are outlined in the electronic supplementary material). There were no date limitations or language restrictions. The reference lists of retrieved studies were reviewed to identify additional reports of relevant trials. The protocol was not registered previously.
2.2 Eligibility Criteria
Studies were selected when they met the following entry criteria: randomized, double- or single-blinded, placebo-controlled, parallel group studies with active and control groups receiving CBD and matched placebo, respectively, in addition to conventional AED treatment. Participants had to meet the following criteria: any gender, any ethnicity, pediatric and/or adult age, and diagnosis of LGS [22].
2.3 Outcome Measures
The primary efficacy outcomes were the proportions of patients who achieved ≥ 50 and 100% reduction in pre-randomization baseline monthly frequency of drop seizures during the treatment and maintenance periods. A drop seizure was defined as an attack or spell (atonic, tonic, or tonic–clonic) involving the entire body, trunk, or head that led or could have led to a fall, injury, slumping in a chair, or hitting the patient’s head on a surface. Secondary efficacy endpoints were the proportions of patients with a ≥ 50% reduction from baseline in non-drop and all seizures frequencies.
The safety outcomes were the proportions of patients: withdrawing from the treatment for any reason; withdrawing from the treatment for adverse events (AEs); experiencing any AE; experiencing any of the AEs found to be commonly related to CBD on the basis of previous evidence [19], i.e., somnolence, decreased appetite, diarrhea, fatigue, increase of serum aminotransferases concentrations by threefold or greater than the upper limit of the normal range; experiencing the five most frequent AEs if different from those listed above; and experiencing any serious adverse event (SAE). We also reviewed the variations from baseline to the end of treatment in measures of global functioning, including patient or caregiver global impression of change, sleep disruption, daytime sleepiness, quality of life and behavioral adaptation, as assessed by validated scales.
2.4 Study Selection, Data Extraction and Assessment of the Risk of Bias
Two review authors (S.L. and C.C.) independently assessed trials for inclusion and extracted the following information from included studies: main study author and age of publication, methods of randomization, allocation concealment and blinding, duration of baseline and treatment periods, dose(s) of CBD tested, number and demographics of participants, and number of participants experiencing each outcome per randomized group. Any disagreement was resolved by discussion with a third review author (F.B.). The risk of bias of the identified studies was assessed in accordance with the recommendations of the Cochrane Collaboration [23].
2.5 Statistical Analysis
Heterogeneity among the trials was assessed by the Chi squared test and the I2 statistics for heterogeneity [23,24,25]. Provided no significant heterogeneity was present (p > 0.05), results were synthesized using a fixed-effect model; if the probability value was ≤ 0.05, heterogeneity determined the choice of a fixed-effect or random-effects model for I2 < 40% or ≥ 40%, respectively [26,27,28,29,30,31]. We presented heterogeneity statistics for all analyses unless only one trial contributed data and heterogeneity was not applicable. Dichotomous outcomes were analyzed by the inverse variance method and risk estimates synthesized by the risk ratio (RR); differences in means of continuous outcomes were pooled with the generic inverse variance model. The intent-to-treat (ITT) population data were used for the analyses. Results were presented according to CBD daily dose, where sufficient data were available. Reported probability values were two-sided, with significance set at < 0.05. Data analysis was performed using STATA/IC 13.1 statistical package (StataCorp LP, College Station, TX, USA).
3 Results
3.1 Results of the Search
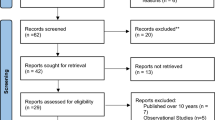
One hundred and twenty-eight records were identified by database and trial registers searching. Three randomized controlled trials were retrieved for detailed assessment, of which one was withdrawn by the sponsor before participants were enrolled (ClinicalTrials.gov number, NCT02318537). Accordingly, two studies [32, 33] were considered in the review, and both were included in the meta-analysis (Fig. 1).
3.2 Characteristics and Risk of Bias of Included Studies
Both included studies were multicenter, randomized, double-blind, placebo-controlled, parallel group trials. The studies included 396 participants according to the ITT: 235 for CBD and 161 for placebo groups, respectively. In both trials, the active treatment was a plant-derived pharmaceutical formulation of purified CBD oral solution (100 mg/mL), which was administered as add-on therapy to the preexisting antiepileptic regimen. In the GWPCARE3 study, patients randomized to the active arm received CBD at a daily dose of either 10 or 20 mg/kg of body weight [32], while in the GWPCARE4, they were all assigned the 20 mg/kg dose [33]. Details of the studies and participants are given in Tables 1 and 2, respectively. Both trials used adequate methods of sequence generation and allocation concealment. We rated both trials as having low risk of performance and detection bias since blinding was ensured by matching placebo, and neither the investigators nor the patients knew the identity of the study treatment being administered. The risks of attrition and selective reporting bias were judged low since patients lost to follow-up and withdrawals were documented, and there was no suspicion of selective outcome reporting. Both trials were sponsored by the CBD manufacturer.
3.3 Reduction in Drop, Non-drop and All Seizures Frequency
The percentages of patients who had at least 50% reduction in drop seizure frequency during the entire treatment period were 40.0% with CBD and 19.3% with placebo, respectively. There were no patients free from drop seizures in either the CBD group or placebo group. The overall RR for 50% response across the trials was 2.12 [95% confidence interval (CI) 1.48–3.03; p < 0.001] (Fig. 2a).
During the entire maintenance phase, the rates of patients who had a ≥ 50 and 100% reduction in drop seizure frequency were 45.3 and 5.5% in the CBD group and 23.5 and 0.6% in the placebo arm. The RRs for 50 and 100% responders were 1.93 (95% CI 1.23–3.02; p = 0.004) and 5.69 (95% CI 1.06–30.38; p = 0.042) (Chi squared = 0.29, df = 1, p = 0.587; I2 = 0.0%), respectively. In the GWPCARE3 trial, data on the maintenance period were available only for freedom from seizures [32].
The overall rates of ≥ 50% reduction of non-drop seizures were 49.4 and 30.4% in the CBD and placebo groups, respectively [RR 1.62 (95% CI 1.09–2.43); p = 0.018] (Fig. 2b). The rates of ≥ 50% reduction of all seizures were also higher among patients randomized to the active drug rather than placebo [37.2% vs 21.2%; RR 1.76 (95% CI 1.07–2.88); p = 0.025] (Fig. 2c).
3.4 Reduction in Drop Seizure Frequency by Dose
During the treatment period, the estimated RRs for ≥ 50% drop seizure frequency reduction were 2.46 (95% CI 1.31–4.61; p = 0.005) for CBD at the dose of 10 mg/kg/day and 2.14 (95% CI 1.49–3.08; p < 0.001) (Chi squared = 0.92, df = 1, p = 0.337; I2 = 0.0%) for the 20 mg CBD group in comparison to placebo.
During the maintenance phase, the RRs to achieve freedom from drop seizures were 3.12 (95% CI 0.33–29.35; p = 0.319) in the 10 mg/kg CBD group and 6.57 (95% CI 1.19–36.31; p = 0.031) (Chi squared = 0.18, df = 1, p = 0.670; I2 = 0.0%) in the 20 mg/kg CBD group. The rates of freedom from drop seizures were 6.2% with CBD at the daily dose of 20 mg/kg and 0.6% with placebo.
3.5 Treatment Withdrawal
Across the trials, treatment was discontinued in 25 (10.6%) and three cases (1.9%) in the CBD and placebo groups, respectively; the overall RR for withdrawal for any reason was 4.93 (95% CI 1.50–16.22; p = 0.009) (Fig. 3a). Drug discontinuation due to AEs occurred in 19 (8.1%) and two patients (1.2%) in the active and control arms, respectively (RR 6.62, 95% CI 1.56–28.15; p = 0.010) (Fig. 3b). In both trials, the most common AEs leading to drug discontinuation comprised increased alanine and aspartate aminotransferases concentrations.
The RRs for treatment withdrawal were 1.13 (95% CI 0.16–7.83; p = 0.898) for CBD at the dosage of 10 mg/kg/day and 6.41 (95% CI 1.93–21.32; p = 0.002) (Chi squared = 0.88, df = 1, p = 0.348; I2 = 0.0%) for CBD at the dosage of 20 mg/kg/day, in comparison to placebo. The RRs for drug withdrawal due to AEs were 1.13 (95% CI 0.07–17.78; p = 0.928) for CBD at the lower dose and 8.24 (95% CI 1.93–35.22; p = 0.004) (Chi squared = 0.26, df = 1, p = 0.610; I2 = 0.0%) for CBD at the higher dose.
In the GWPCARE3 trial [32], six patients in the 10-mg CBD group temporarily received a dose that was above the target and were therefore included in the 20-mg CBD group for the safety analysis.
3.6 Adverse Events
AEs were reported by 207 (88.1%) and 114 patients (70.8%) treated with CBD and placebo, respectively (RR 1.24, 95% CI 1.11–1.38; p < 0.001) (Fig. 3c). The incidence rates of the selected AEs in the CBD- versus placebo-treated participants were as follows: somnolence 22.1% versus 7.5%, decreased appetite 18.3% versus 5.0%, diarrhea 14.9% versus 8.1%, pyrexia 11.5% versus 11.8%, upper respiratory tract infection 10.2% versus 10.6%, vomiting 9.8% versus 14.3%, and increased alanine or aspartate aminotransferases more than three times the upper normal limit 14.5% versus 0.6%. The AEs significantly associated with CBD in the overall analysis were somnolence, decreased appetite, diarrhea and increased transaminases levels (Table 3). The analysis per daily dose is summarized in Table 4. Serious AEs were reported by 46 (19.6%) and 11 patients (6.8%) treated with CBD and placebo, respectively (RR 2.70, 95% CI 1.44–5.04; p = 0.002) (Chi squared = 2.10, df = 1, p = 0.147; I2 = 52.3%). The RR to have SAEs was 2.11 (95% CI 0.89–4.97; p = 0.089) in the lower dose group and 2.66 (95% CI 1.37–5.16; p = 0.004) (Chi squared = 2.36, df = 1, p = 0.124; I2 = 57.7%) in the higher CBD dose group when compared with placebo.
3.7 Global Functioning Measures
An improvement from baseline in overall condition (slightly improved, much improved, or very much improved) according to the Patient or Caregiver Global Impression of Change (PCGIC) at the last visit was reported in 140 out of 232 patients (60.3%) in the CBD group and in 62 out of 160 patients (38.8%) in the placebo arm [RR 1.51 (95% CI 1.21–1.89); p < 0.001] (Chi squared = 0.78, df = 1, p = 0.378; I2 = 0.0%). The RRs for improvement regarding the PCGIC were 1.49 (95% CI 1.10–2.03; p = 0.010) for CBD at the dosage of 10 mg/kg/day and 1.48 (95% CI 1.17–1.87; p = 0.001) (Chi squared = 1.27, df = 1, p = 0.260; I2 = 21.3%) for CBD at the dosage of 20 mg/kg/day in comparison to placebo.
In both trials, sleep disruption was assessed by the Sleep Disruption Numerical Rating Scale [range 0 (slept extremely well) to 10 (unable to sleep at all)], and the Epworth Sleepiness Scale (range 0–24, with higher scores indicating greater daytime sleepiness) was used to evaluate daytime sleepiness. Quality of life and behavioral adaption were scored through the Quality of Life in Childhood Epilepsy questionnaire (range 0–100, with higher scores indicating better function) and the Vineland Adaptive Behavior Scales, second edition (Vineland-II; range 20–160, with higher scores indicating better behavioral adaptation). The mean variations from baseline to the end of treatment in any of these measures did not significantly differ between the CBD and placebo groups (Table 5).
4 Discussion
CBD was more effective than placebo in reducing the frequency of drop seizures when added to existing AEDs at the daily dose of either 10 or 20 mg/kg in children and adults affected by LGS. The 40% of patients who received the active treatment had a reduction of at least 50% in the baseline drop seizure frequency in comparison to 19% in the placebo group. None of the patients were free from drop seizures throughout the whole 14-week treatment period, while 5.5% of those undergoing treatment with CBD were free during the 12-week maintenance period, as compared with 0.6% of patients in the placebo group. Although there were insufficient available data to perform a dose–response regression analysis, the results suggested a greater likelihood to achieve freedom from drop seizures with CBD treatment at the daily dose of 20 mg/kg than with 10 mg/kg.
The significant results seen in the control of non-drop seizures also suggest that CBD can have a broad-spectrum anti-seizure profile. The higher perception of improvement held by patients and caregivers at their last clinical visit in the CBD group versus that in the placebo group, alongside the high rate of enrollment into the open-label extension phase of the blinded trials (ClinicalTrials.gov number, NCT02224573), further reinforced the overall positive effect of CBD treatment. It is noteworthy that statistically and clinically meaningful improvements in seizure frequency were observed in highly treatment-resistant patients who, at baseline, were taking an average of more than three concomitant antiepileptic treatments, had previously tried a median of six AEDs, and had a high frequency of drop and non-drop seizures. Notably, the achievement of freedom seizure by few patients despite their very refractory status could have a genetic basis and deserves further investigation.
Across the phase III trials, there were more treatment withdrawals and AEs in the CBD group, particularly at the 20 mg/kg daily dosage, than in the placebo arm. The overall proportion of patients who withdrew CBD was similar than those associated with the use of other AEDs [34, 35]. Most patients reported mild to moderate AEs, which generally resolved on treatment and were consistent with the tolerability profile previously reported during the open-label use of the compound in severe refractory epilepsy [19]. Somnolence was the most frequent AE associated with CBD treatment, and it was more likely to occur in patients who were concomitantly taking clobazam. Notably, CBD can inhibit the catalytic activity of the cytochrome P450 2C19 and increase by 500% the concentrations of N-desmethylclobazam, the biologically active metabolite of clobazam [36, 37]. Accordingly, it would be prudent to strictly observe patients on concomitant clobazam and adjust doses as necessary to manage AEs.
The efficacy of CBD might be influenced by the pharmacokinetic interaction with clobazam. In this regard, add-on CBD treatment resulted in clinically meaningful drop-seizure reductions versus add-on placebo, regardless of concomitant use of clobazam in a post hoc analysis of the GWPCARE3 and GWPCARE4 trials. Although the re-assessment of non-randomized subgroups could limit the interpretability and generalizability of the conclusions, these findings provided useful insights into the independent effect of CBD in reducing seizures [38].
The increase in serum alanine or aspartate aminotransferases concentrations was reported by near to 15% of the patients randomized to CBD, which was the most common reason for its discontinuation. None of the elevations, however, suggested lasting liver damage, and no patient met the criteria for severe drug-induced liver injury as concomitant increases in bilirubin concentration were not observed [39]. Noteworthy, more than two-thirds of the cases occurred in patients on AED regimens that included valproate. Since CBD has shown to have no effects on the systemic levels of valproate [36], this interaction is thought to be mostly pharmacodynamic rather than pharmacokinetic. Elevations of aminotransferases tended to appear early during the treatment and reversed either spontaneously or after the reduction in concomitant valproate use, tapering or cessation of CBD, or entry into the open-label extension trial. All these issues should be considered when adding CBD to a preexisting drug regimen; slow up-titration and close monitoring of serum transaminases and signs suggestive of hepatic toxicity, mostly during the initial phases of the treatment and in patients concomitantly taking valproate, are recommended.
This systematic review with meta-analysis represents a comprehensive quantitative synthesis of the currently available randomized controlled clinical trials on the use of CBD in patients with LGS. Compared with the studies that previously addressed the same question [40, 41], our meta-analysis provides an updated and more detailed assessment of efficacy, safety, global functioning and quality-of-life endpoints according to drug daily dosages. Different limits should be taken into account when interpreting the findings. One main pitfall relates to the limited literature available. Indeed, only two trials met the inclusion criteria, and both were sponsored by a pharmaceutical company. In this respect, further evidence about the therapeutic potential of CBD has been shown in the interim analysis of the Expanded Access Program data, as 94 out of 607 enrolled patients were diagnosed with LGS. Notably, CBD was associated with a reduction of approximately 50% in the median monthly frequency of total seizures after 12 weeks of treatment, and response rates were consistent at each visit window through 96 weeks. Overall, CBD was generally well tolerated and treatment-emergent AEs were consistent with those reported in the randomized controlled trials, with the most common being somnolence and diarrhea [42]. The ethnic diversity of trial populations was low and likely to reflect the demographics of the study sites, with more than 90% of patients being Caucasian; furthermore, the evidence for the 10 mg/kg dose was derived from one trial and a small sample size. Due to the short, double-blind treatment period of the trials, this meta-analysis does not allow us to draw definitive conclusions about the long-term efficacy and safety of CBD, including the estimation of rare AEs, the occurrence of phenomena such as habituation and tolerance, and the effects on growth, brain development and learning in subpopulations potentially at risk as young children and offspring of pregnant women. All these questions could be assessed in the ongoing open-label extension of the trials and using real-world data, once available. Likewise, this meta-analysis cannot provide information on the effectiveness of CBD in comparison with other AEDs licensed for LGS. Finally, the use of individual-participant level data rather than aggregate results could clarify the independent effects of CBD and the relationship between the improvement in seizure control and the interaction with concomitant drugs, particularly with clobazam [16].
5 Conclusion
CBD as add-on therapy to an existing antiepileptic regimen significantly reduced the frequency of drop, non-drop, and total seizures in highly treatment-resistant patients with LGS. Adjunctive CBD was associated with more AEs than placebo, and most events were mild or moderate.
The last months have signed a tipping point for the use of cannabis-based treatments in the field of epilepsy. After the positive results obtained in the treatment of Dravet syndrome [43], there is also evidence that adjunctive CBD can represent an effective treatment option in children and adults with LGS. Future studies should be directed to further define the therapeutic potential of CBD by evaluating its effectiveness in patients with drug-resistant epilepsies other than those currently investigated.
References
Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–37.
Cagnetti C, Lattanzi S, Foschi N, Provinciali L, Silvestrini M. Seizure course during pregnancy in catamenial epilepsy. Neurology. 2014;83:339–44.
Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the national general practice study of epilepsy. Lancet. 1995;346:140–4.
Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Lacosamide monotherapy for partial onset seizures. Seizure. 2015;27:71–4.
Lattanzi S, Cagnetti C, Foschi N, Lorusso A, Provinciali L, Silvestrini M. Eslicarbazepine acetate as adjunctive treatment in partial-onset epilepsy. Acta Neurol Scand. 2018;137:29–32.
Arzimanoglou A, French J, Blume WT, Cross JH, Ernst JP, Feucht M, Genton P, Guerrini R, Kluger G, Pellock JM, Perucca E, Wheless JW. Lennox–Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8:82–93.
Kim HJ, Kim HD, Lee JS, Heo K, Kim DS, Kang HC. Long-term prognosis of patients with Lennox–Gastaut syndrome in recent decades. Epilepsy Res. 2015;110:10–9.
Camfield PR. Definition and natural history of Lennox–Gastaut syndrome. Epilepsia. 2011;52(Suppl 5):3–9.
Doring JH, Lampert A, Hoffmann GF, Ries M. Thirty years of orphan drug legislation and the development of drugs to treat rare seizure conditions: a cross sectional analysis. PLoS One. 2016;11:e0161660.
Montouris GD, Wheless JW, Glauser TA. The efficacy and tolerability of pharmacologic treatment options for Lennox–Gastaut syndrome. Epilepsia. 2014;55(suppl 4):10–20.
Cersosimo RO, Bartuluchi M, Fortini S, Soraru A, Pomata H, Caraballo RH. Vagus nerve stimulation: effectiveness and tolerability in 64 paediatric patients with refractory epilepsies. Epileptic Disord. 2011;13:382–8.
Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005;46:272–729.
Douglass LM, Salpekar J. Surgical options for patients with Lennox–Gastaut syndrome. Epilepsia. 2014;55(suppl 4):21–8.
Kossoff EH, Shields WD. Nonpharmacologic care for patients with Lennox–Gastaut syndrome: ketogenic diets and vagus nerve stimulation. Epilepsia. 2014;55(suppl 4):29–33.
Lattanzi S, Cagnetti C, Matricardi S, Silvestrini M. Palliative non-resective surgery for drug-resistant epilepsy. Brain Dev. 2018;40:512–3.
Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res. 2017;7:61–76.
Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730.
Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, Stephens GJ. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332:569–77.
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F, Wong M, Tilton N, Bruno P, Bluvstein J, Hedlund J, Kamens R, Maclean J, Nangia S, Singhal NS, Wilson CA, Patel A, Cilio MR. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–8.
Ružić Zečević D, Folić M, Tantoush Z, Radovanović M, Babić G, Janković SM. Investigational cannabinoids in seizure disorders, what have we learned thus far? Expert Opin Investig Drugs. 2018 Jun 6. https://doi.org/10.1080/13543784.2018.1482275. [Epub ahead of print].
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Niedermeyer E. The Lennox–Gastaut syndrome and its frontiers. Clin Electroencephalogr. 1986;17:117–26.
Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Higgins JPT, Green S, editors. The Cochrane Collaboration, 2011. Available at http://handbook-5-1.cochrane.org/. Accessed October 2017.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Brivaracetam add-on for refractory focal epilepsy: a systematic review and meta-analysis. Neurology. 2016;86:1344–52.
Lattanzi S, Brigo F, Grillo E, Cagnetti C, Verrotti A, Zaccara G, Silvestrini M. Adjunctive eslicarbazepine acetate in pediatric patients with focal epilepsy: a systematic review and meta-analysis. CNS Drugs. 2018;32:189–96.
Lattanzi S, Grillo E, Brigo F, Silvestrini M. Efficacy and safety of perampanel in Parkinson’s disease. A systematic review with meta-analysis. J Neurol. 2018;265:733–40.
Lattanzi S, Cagnetti C, Danni M, Provinciali L, Silvestrini M. Oral and intravenous steroids for multiple sclerosis relapse: a systematic review and meta-analysis. J Neurol. 2017;264:1697–704.
Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis. 2017;43:207–13.
Lattanzi S, Brigo F, Cagnetti C, Di Napoli M, Silvestrini M. Patent foramen ovale and cryptogenic stroke or transient ischemic attack: to close or not to close? A systematic review and meta-analysis. Cerebrovasc Dis. 2018;45:193–203.
Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, VanLandingham KE. Zuberi SM; GWPCARE3 Study Group. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378:1888–97.
Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C. Sommerville K; GWPCARE4 Study Group. Cannabidiol in patients with seizures associated with Lennox–Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–96.
Ng YT, Conry JA, Drummond R, Stolle J. Weinberg MA; OV-1012 Study Investigators. Randomized, phase III study results of clobazam in Lennox–Gastaut syndrome. Neurology. 2011;77:1473–81.
Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox–Gastaut syndrome. Neurology. 2008;70:1950–8.
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, Greenwood S, Morrison G, Sommerville K, GWPCARE1 Part A Study Group. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–11.
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51.
Thiele EA, Devinsky O, Checketts D, Knappertz V. Cannabidiol (CBD) treatment responders analysis in patients with Lennox–Gastaut syndrome (LGS) on and off clobazam (CLB). The American Epilepsy Society Annual Meeting; Washington, DC; December 1–5, 2017. Abst. 1.436. Available at: https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/381224 (Accessed on July, 2018)].
US Department of Health and Human Services. Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. 2009. https://www.fda.gov/downloads/Guidances/UCM174090.pdf. Accessed Jun 11, 2018.
Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, Herkes GK, Farrell M, Degenhardt L. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry. 2018 Mar 6. https://doi.org/10.1136/jnnp-2017-317168. [Epub ahead of print].
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
Szaflarski JP, Bebin EM, Comi AM, Patel AD, Joshi C, Checketts D, Beal JC, Laux LC, De Boer LM, Wong MH, Lopez M, Devinsky O, Lyons PD, Zentil PP, Wechsler R; CBD EAP study group. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: expanded access program results. Epilepsia. 2018 Jul 12. https://doi.org/10.1111/epi.14477. [Epub ahead of print].
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S, Cannabidiol in Dravet Syndrome Study Group. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to conduct this study.
Conflict of interest
Simona Lattanzi, Claudia Cagnetti and Mauro Silvestrini have no conflicts of interest directly relevant to the content of this study. Francesco Brigo acted as a consultant for Eisai. Eugen Trinka received speaker’s honoraria from UCB, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, Sunovion Pharmaceuticals Inc., LivaNova and Novartis; consultancy funds from UCB, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, GW Pharmaceuticals, Sunovion Pharmaceuticals Inc., and Novartis; and directorship funds from Neuroconsult GmbH. E. Trinka’s Institution received grants from Biogen, Red Bull, Merck, UCB, European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, and Bundesministerium für Wissenschaft und Forschung.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lattanzi, S., Brigo, F., Cagnetti, C. et al. Efficacy and Safety of Adjunctive Cannabidiol in Patients with Lennox–Gastaut Syndrome: A Systematic Review and Meta-Analysis. CNS Drugs 32, 905–916 (2018). https://doi.org/10.1007/s40263-018-0558-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0558-9