Abstract
Background
Observational studies have suggested an increased risk of intracranial hemorrhage (ICH) associated with selective serotonin reuptake inhibitors (SSRIs) and other antidepressants primarily inhibiting serotonin reuptake.
Objectives
Our aim was to systematically review the available epidemiologic evidence regarding the risk of ICH associated with SSRIs and antidepressants inhibiting serotonin reuptake.
Methods
MEDLINE/PubMed and EMBASE were searched for all relevant articles in English, French, or German published before April 2017. Observational studies with SSRIs or any antidepressants classified by strength of serotonin reuptake inhibition as primary exposure, a comparison group, and ICH as outcome were eligible.
Results
Among twelve identified studies (six nested case-control, three cohort, two case-control, one case-crossover), seven assessed the risk of ICH associated with SSRIs (some also including other antidepressants primarily inhibiting serotonin reuptake), two the risk of ICH associated with inhibitors of serotonin reuptake according to the degree of reuptake inhibition, and three addressed both objectives. Four of ten studies showed an increased risk of ICH associated with SSRIs, with the two largest studies suggesting a moderate effect. Three of five studies showed an increased risk of ICH associated with strong inhibitors of serotonin reuptake. Limitations including residual confounding, inclusion of prevalent users, potentially inappropriate study designs, and lack of power may have influenced these results, especially in studies showing no association or a highly increased risk.
Conclusion
This systematic review suggests an increased risk of ICH with antidepressants primarily inhibiting serotonin reuptake, such as SSRIs. An increased risk of ICH with strong inhibitors of serotonin reuptake compared with weak inhibitors is also possible but the available evidence is limited. Antidepressants only moderately or weakly inhibiting serotonin reuptake might be preferred in high-risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The evidence to date suggests that the use of antidepressants that primarily inhibit the reuptake of serotonin, such as selective serotonin reuptake inhibitors, may increase the risk of intracranial hemorrhage. |
The use of antidepressants strongly inhibiting serotonin reuptake could also increase the risk of intracranial hemorrhage as compared with weak inhibitors, although the available evidence is limited. |
Antidepressants only moderately or weakly inhibiting serotonin reuptake might be preferred in patients with a high propensity for bleeding. |
1 Introduction
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed antidepressants that show a favorable risk–benefit ratio in comparison with older drugs such as tricyclic antidepressants (TCAs) [1]. However, use of SSRIs has been suggested to increase the risk of bleeding, including gastrointestinal bleeding [2] and intracranial hemorrhage (ICH) [3]. Although a moderate increase in the risk of gastrointestinal bleeding has been replicated in several observational studies and subsequent meta-analyses [2, 4], and can now be found in the summary of product characteristics of SSRIs [5], the findings on ICH have been contradictory [3, 6, 7].
The bleeding risk associated with the use of SSRIs, and any other antidepressant that primarily inhibits the reuptake of serotonin, probably results from their mechanism of action on the serotonin transporter expressed by thrombocytes. In particular, SSRIs can inhibit the reuptake of serotonin into thrombocytes, and the depletion of serotonin is considered responsible for their antiplatelet action [8]. Importantly, the strength of serotonin reuptake inhibition varies among different SSRIs and antidepressants in general [9]. Therefore, the potential bleeding risk of SSRIs and other antidepressants has been linked to the varying strength of this inhibition.
Given the high fatality rate of ICH such as intracerebral and subarachnoid hemorrhage [10, 11] and the increasing use of antidepressants, including SSRIs, observed in the past decades [12], this potential association is of high clinical importance. Previous systematic reviews or meta-analyses have focused mostly on the risk of ICH (or ICH subtypes) associated with SSRIs [3, 7]. However, while several studies on the risk of ICH according to the strength of serotonin reuptake inhibition of antidepressants were recently published [13,14,15,16,17], this topic has not been specifically reviewed yet. Thus, we conducted a systematic review of observational studies to synthesize the available data regarding the risk of ICH associated with SSRIs and with antidepressants in general according to their strength of inhibition of serotonin reuptake.
2 Methods
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18], and following a prespecified protocol.
2.1 Systematic Search
Potentially eligible studies were identified through a systematic search on the electronic publication databases MEDLINE/PubMed and EMBASE from the earliest available online year of indexing up to and including April 29, 2017. A detailed search algorithm was developed, based on a combination of concepts that addressed the exposure of interest and the outcome of interest: SSRIs or antidepressants classified by strength of serotonin reuptake inhibition AND ICH. The search algorithm applied to Medical Subject Headings and free-text words in MEDLINE/PubMed can be found in eTable 1 (see electronic supplementary material). A similar search strategy was applied to EMBASE. We also scanned the bibliographies of the included articles and relevant reviews for further references. Duplicate publications from the same study were removed using EndNote X7. All publications written in English, German, and French were eligible for screening.
2.2 Eligibility Criteria
The articles eligible for systematic review were studies assessing the risk of ICH or any subtype of ICH (e.g., hemorrhagic stroke) associated with SSRIs or with antidepressants in general classified according to strength of serotonin reuptake inhibition (strong, intermediate, or weak). The strength of inhibition is defined based on the affinity (ki) of the antidepressant for the serotonin transporter [9]. SSRIs are either strong or intermediate inhibitors of serotonin reuptake. Non-SSRI antidepressants can rarely be strong inhibitors (e.g., duloxetine, clomipramine), but are mostly either intermediate or weak inhibitors of serotonin reuptake. Studies eligible for the systematic review were observational studies (case-control, nested case-control, cohort, within-subject designs) containing original data and with a comparison group. Depending on study design, the comparison group could be non-exposed individuals, patients treated with other antidepressants such as TCAs, or the patient him-/herself at different time periods (in the case of within-subject designs). No disease-specific definitions (e.g., depression) for the study population were used. Only studies in which antidepressants were the primary exposure of interest and where antidepressants with various strengths of serotonin reuptake inhibition were analysed separately were included. As such, studies which primarily focused on another medication while reporting risk associated with other drugs, including SSRIs, were excluded.
Studies were excluded if they did not provide any description of characteristics of the study population, or if the frequency of ICH given exposure status could not be calculated. Screening of titles and abstracts for articles potentially eligible for further review was performed independently by two investigators (AD and CR) in accordance with the PRISMA checklist [19]. Differences were resolved by consensus.
2.3 Data Collection
We developed a structured data extraction form, which was first tested on four included studies, to record the following data from each individual study if available: year of publication, country, years of study conduct, study design, source population, sample size, inclusion and exclusion criteria, percentage of males and mean age, type of comparison group (e.g., treated with TCAs, other antidepressants, untreated individuals), number of participants per treatment group, exposure of interest (e.g., new users, prevalent users), definition of exposure, definition of the outcome (e.g., ICH, hemorrhagic stroke, intracerebral hemorrhage), number of events per treatment group, matching variables or covariates adjusted for in the analysis, and adjusted estimates of measure of association between treatment with SSRIs or other inhibitors of serotonin reuptake and the outcome. Furthermore, we used the data extraction form to assess the potential biases and/or conflicts of interest, and to evaluate the overall quality of the study, adapted from the A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI updated version ROBINS 1) [20]. We performed quality assessment considering aspects such as study design, exposure and outcome definition, statistical analysis, and classical biases, as well as methodological issues particular to pharmacoepidemiology such as inclusion of prevalent users or time-related biases [21, 22]. Two independent reviewers (AD and MA) extracted data, with disagreements resolved by consensus or a third reviewer (CR). The quality assessment of the studies was assessed by two independent reviewers (AD and CR), with disagreements also resolved by consensus.
3 Results
3.1 Included Studies
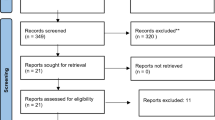
The initial search identified 226 publications, of which 196 were excluded based on review of the title and abstract (Fig. 1). The main reasons for exclusion were irrelevance based on the study question addressed or the type of study or publication (e.g., reviews, case reports, comments, and letters). The remaining 30 articles were reviewed in detail independently by two reviewers and 19 articles were further excluded. During the review process, we identified one article based on the bibliographies of the included articles. Therefore, twelve studies were included in this systematic review [6, 13,14,15,16,17, 23,24,25,26,27,28]. All were observational studies, consisting of six nested case-control studies [6, 14, 15, 23,24,25], three cohort studies [13, 27, 28], two case-control studies [16, 26], and one case-crossover study [17]. Seven studies assessed the risk of ICH associated with the use of SSRIs [6, 23,24,25,26,27,28], two studies assessed the risk associated with antidepressants according to the degree of serotonin reuptake inhibition [13, 14], and three studies addressed both objectives [15,16,17]. Three of the seven studies assessing the risk of ICH associated with the use of SSRIs also considered drugs that, while inhibiting serotonin reuptake to some extent, are not specifically classified as SSRIs (clomipramine [6, 24], trazodone [24], venlafaxine [25], mirtazapine [25], duloxetine [25]). From this point on in the manuscript, we have used the term ‘SSRIs’ in the same way as in the cited studies to cover the classical SSRIs and these related drugs, given that the data from these studies was predominantly that from SSRIs.
3.2 Risk of Intracranial Hemorrhage (ICH) Associated with Selective Serotonin Reuptake Inhibitors (SSRIs)
Among the ten studies that addressed the risk of ICH associated with the use of SSRIs, five were nested case-control studies [6, 15, 23,24,25], two were cohort studies [27, 28], two were case-control studies [16, 26], and one was a case-crossover study [17]. Their characteristics are presented in Table 1. The studies were conducted in North America (n = 4) [15, 23, 26, 28], Europe (n = 4) [6, 16, 24, 25], and Asia (n = 2) [17, 27]. The sizes of the study cohorts ranged from 136,293 to 1,363,990 patients. Four studies considered new users of antidepressants [15, 24, 25, 27], three studies considered the general population [6, 16, 26], one study considered only patients with a diagnosis of depression [23], one study considered women between 50 and 79 years of age [28], and the case-crossover study considered patients with ICH and antidepressant use in the year prior to the event [17]. Overall, 12,797 events of ICH, 1552 events of hemorrhagic stroke, and 659 events of intracerebral hemorrhage were identified (Table 2). Seven studies compared use of SSRIs (six studies assessed current use and one study assessed use at cohort entry) with no use [6, 16, 23,24,25,26, 28], two studies compared current use of SSRIs with current use of serotonin-norepinephrine reuptake inhibitors and TCAs, respectively [15, 27], and the case-crossover study compared the use of SSRIs in the risk period with the use of SSRIs in the control periods among cases [17].
Overall, three studies observed an increased risk of ICH associated with SSRIs [15,16,17] and one study observed an increased risk of hemorrhagic stroke associated with SSRIs [28]. Of those, a nested case-control study using TCAs as an active comparator reported a 17% increased risk of ICH (rate ratio [RR] 1.17; 95% confidence interval [CI] 1.02–1.35) [15]. The three other studies reported much higher increases in the risk including 112% for hemorrhagic stroke (hazard ratio [HR] 2.12; 95% CI 1.10–4.07) [28], 39% for ICH (odds ratio [OR] 1.39; 95% CI 1.13–1.70) [16], and 324% for ICH (OR 4.24; 95% CI 1.95–9.26) [17], comparing SSRIs with no use. In contrast, three studies showed no increased risk of ICH associated with SSRIs (HR 1.18; 95% CI 0.64–2.16 [23]/OR 0.8; 95% CI 0.3–2.3 [24]/HR 1.17; 95% CI 0.91–1.48 [27]), two studies showed no increased risk of hemorrhagic stroke associated with SSRIs (OR 1.11; 95% CI 0.82–1.50 [25]/OR 0.8; 95% CI 0.5–1.2[26]), and one study showed no increased risk of intracerebral hemorrhage associated with SSRIs (OR 1.0; 95% CI 0.6–1.6) [6]. Of those, one study compared SSRIs with serotonin-norepinephrine reuptake inhibitors [27], while the five other studies compared SSRIs with ‘no use’ [6, 23,24,25,26].
Importantly, the study with the highest point estimate used a case-crossover design [17], which was developed for transient exposures and not for drugs often taken on a regular basis such as SSRIs [29]. Moreover, three of the six studies showing no increased risk were underpowered to assess moderate effects, as seen by the wide 95% confidence intervals [6, 23, 24]. Other methodological shortcomings were the inclusion of prevalent users [6, 16, 23], potential immortal time bias [25], potential selection bias [26], and potential exposure misclassification [28].
3.3 Risk of ICH Associated with the Degree of Serotonin Reuptake Inhibition
Among the five studies that addressed the risk of ICH associated with the strength of inhibition of serotonin reuptake, two were nested case-control studies [14, 15], one was a cohort study [13], one was a case-control study [16], and one was a case-crossover study [17]. Their characteristics are presented in Table 3. The studies were conducted in North America (n = 3) [13,14,15], Europe (n = 1) [16], and Asia (n = 1) [17]. The sizes of the study cohorts ranged from 36,389 to 1,363,990 patients. One study considered only patients with a diagnosis of depression [14], one study considered new users of antidepressants [15], one study considered patients with a diagnosis of depression initiating antidepressants [13], one study considered the general population [16], and the case-crossover study considered patients with ICH and antidepressant use in the year prior to the event [17]. Overall, 10,737 events of ICH and 281 events of hemorrhagic stroke were identified (Table 4). Two studies compared use of strong inhibitors of serotonin reuptake (one study assessed current use and one study assessed ever use) with no use [14, 16], two studies compared current use of strong inhibitors of serotonin reuptake with current use of weak inhibitors of serotonin reuptake [13, 15], and the case-crossover study compared the use of strong inhibitors of serotonin reuptake in the risk period with the use of strong inhibitors of serotonin reuptake in the control periods among cases [17]. The affinity thresholds used to classify strength of serotonin reuptake inhibition in four of the five studies were < 1 nmol/L for strong, 1–10 nmol/L for intermediate, and > 10 nmol/L for weak [13,14,15, 17]. In contrast, the study by Verdel et al. used < 10 nmol/L for strong, 10–1000 nmol/L for intermediate, and > 1000 nmol/L for weak [16].
Overall, three studies observed an increased risk of ICH associated with strong inhibitors of serotonin reuptake [15,16,17]. Of those, a nested case-control study using weak inhibitors of serotonin reuptake as an active comparator reported a 25% increased risk (RR 1.25; 95% CI 1.01–1.54) [15]. The two other studies reported higher increases in the risk when comparing strong inhibitors of serotonin reuptake with no use including 45% (OR 1.45; 95% CI 1.19–1.76) [16] and 255% (OR 3.55; 95% CI 1.68–7.49) [17], respectively. Importantly, in these studies weak or intermediate inhibitors of serotonin reuptake were also associated with increased risks. In contrast, two studies showed no increased risk of ICH associated with strong inhibitors of serotonin reuptake compared either with weak inhibitors of serotonin reuptake (RR 1.13; 95% CI 0.90–1.44) [13] or no use (OR 0.82; 95% CI 0.44–1.55) [14].
Again, a study using the potentially inadequate case-crossover design provided the highest point estimates [17]. Moreover, one of the studies showing no increased risk was underpowered to assess moderate effects, as seen by the wide 95% confidence intervals [14]. Another methodological shortcoming was the inclusion of prevalent users [14, 16]. Finally, the fact that the study by Verdel et al. [16] used different affinity thresholds to classify strength of serotonin reuptake inhibition limits the comparability with other studies.
4 Discussion
In this systematic review, we identified ten observational studies assessing the risk of ICH or ICH subtypes associated with the use of SSRIs. Of those, the two largest studies with no major limitations found an increase in the risk of ICH compared with either TCAs [15] or no use [16], with the magnitude of the effect reported being moderate at best. Several of the remaining studies either suffered from methodological shortcomings [17, 26, 28], or were underpowered to assess such effects [6, 23, 24], which could explain some inconsistencies between studies.
We also identified five observational studies assessing the risk of ICH or ICH subtypes associated with the strength of inhibition of serotonin reuptake. The two largest studies found that current use of strong inhibitors was associated with an increased risk of ICH compared with either weak inhibitors of serotonin reuptake [15] or no use [16]. However, the second study also found an increased risk for intermediate or weak inhibitors of serotonin reuptake compared with no use, without any clear trend between strength of serotonin reuptake inhibition and the risk of ICH [16]. Potential explanations include the use of different thresholds for the classification based on strength of serotonin reuptake used in this study, and the inclusion of prevalent users. Among the remaining studies, one suffered from methodological limitations [17], while another one was underpowered to assess the risk of such a rare event [14]. Thus, strong inhibitors could be associated with an increased risk of ICH, but data regarding this potential association are limited.
Residual confounding is a potential limitation of all included studies due to their observational nature. While the use of an active comparator has been suggested in order to decrease the impact of this bias and especially indication bias [30], only three studies compared SSRIs with other classes of antidepressants [15, 27], or compared strong with weak inhibitors of serotonin reuptake [13, 15]. Most of the studies compared SSRIs or strong inhibitors of serotonin reuptake with no use, which can introduce upward bias because the condition being treated (e.g., depression) may be a risk factor for bleeding [15, 31]. Moreover, one study aimed to control for confounding by using a within-subject design, the so-called case-crossover design [17]. While within-subject designs can effectively deal with time-fixed confounders, they were developed to assess the risks of transient exposures such as vaccines or antibiotics. Thus, they are potentially inadequate to study the effects of drugs taken regularly, as is usually the case with antidepressants. Recently, it was shown that when implementing within-subject designs, persistent drug use can lead to an upward bias in the effect estimates [32]. Congruently, the aforementioned case-crossover study found increased risks not only for SSRIs or strong inhibitors of serotonin reuptake, but also for other exposures such as TCAs, monoamine oxidase inhibitors, and intermediate or weak inhibitors of serotonin reuptake [17]. Moreover, SSRIs were also associated with an increased risk of ischemic stroke, which is difficult to explain based on our current understanding of SSRI pharmacology. Finally, another reason why residual confounding may be augmented in some of the studies is the absence of important variables in the analysis such as oral anticoagulants [13, 23, 28] or cardiovascular disease [13].
Four studies did not restrict their study cohorts to new users of SSRIs [6, 14, 16, 23]. The inclusion of prevalent users in observational studies has been criticized for two main reasons [33]. First, prevalent users are ‘survivors’ of the early period of pharmacotherapy, leading to an under-ascertainment of early adverse events. Second, adjusting for covariates at cohort entry may become complicated, since some of these covariates could be affected by the drug itself and thus be in the causal pathway; such overadjustment has been shown to increase bias [33, 34]. Another possible bias limiting the validity of one of the studies is immortal time bias, which can occur when drug exposure is defined after cohort entry and usually leads to decreased effect estimates [21]. In that particular study, at least two prescriptions for SSRIs were required for patients to be considered exposed, thus making the time between the first and the second SSRI prescription ‘immortal’ [25]. Finally, one of the studies using a classic case-control design may suffer from selection bias [26]. In this study, only 27% of the initially identified cases of ICH were included, and interviewed patients were younger than non-interviewed patients. However, the impact of these potential biases on the effect estimates is unclear and possibly minor, and several studies were also underpowered to detect moderately increased risks. The lack of power was often further aggravated in studies subcategorizing their primary outcome [15, 24, 26]. Thus, in our review, point estimates for subtypes of ICH were considered only if assessed as primary outcome.
Given the various study designs applied, the different populations considered, and the different comparator groups used, as well as the several biases potentially limiting the validity of the findings of a few of the included studies, we do not deem a meta-analysis to be feasible. Importantly, previously published meta-analyses of observational studies on the risk of ICH associated with SSRIs also considered studies where oral anticoagulants or antiplatelet drugs and not SSRIs were the primary exposure of interest, making the interpretation of the results even more challenging [3, 7].
ICH is a rare event in the general population, with the added incidence rate of its two major components (i.e., intracerebral hemorrhage and subarachnoid hemorrhage) being approximately 30 per 100,000 persons per year [35]. Thus, even a moderately increased risk of ICH conferred by exposure to SSRIs or antidepressants strongly inhibiting serotonin reuptake would translate to very few additional events. Indeed, in one study the absolute rate difference was shown to be 6.7 per 100,000 persons per year for the comparison between SSRIs and TCAs, and 9.5 per 100,000 persons per year for the comparison between strong and weak inhibitors of serotonin reuptake [15]. Importantly, previous RCTs failed to determine whether SSRIs are associated with an increased risk of ICH [36]. The rarity of the event, the small sample size, and the incomplete reporting of adverse events in those RCTs probably account for this failure [36].
While ICH is rare in the general population, an increase in the relative risk associated with the use of SSRIs can become clinically relevant in high-risk groups, such as patients treated with oral anticoagulants or antiplatelet agents, patients with arterial hypertension or bleeding disorders, or patients with a history of bleeding or stroke [37]. However, SSRIs have a more favorable overall safety profile compared with other antidepressants such as TCAs and a comparable effectiveness. Thus, according to current guidelines, they remain the drug of choice in various psychiatric conditions such as moderate or severe depression [1] or anxiety disorders [38]. In this context, the clinically relevant question relates to which antidepressant medication to use as opposed to the question of whether to prescribe an antidepressant. Although only a few studies have assessed the risk of ICH according to the degree of serotonin reuptake inhibition, the potentially increased risk with strong inhibitors suggests that intermediate or weak inhibitors may be favored in patients at high risk of ICH.
5 Conclusions
In this systematic review, we found evidence suggesting that antidepressants primarily inhibiting serotonin reuptake, such as SSRIs, and strong inhibitors of serotonin reuptake may increase the risk of ICH. However, data are limited, particularly regarding the risk associated with strong inhibitors of serotonin reuptake, and a firm conclusion cannot be drawn because of heterogeneity between studies in terms of methods, comparator group, and outcome definition. Based on the individual studies, their methodological shortcomings, and their power (or lack thereof) to reliably quantify this rare event, the increased risk associated with antidepressants primarily inhibiting serotonin reuptake, such as SSRIs, might be moderate. Assumptions regarding the magnitude of the effect associated with antidepressants strongly inhibiting serotonin reuptake are challenging given the scarcity of available evidence. Since antidepressants primarily inhibiting the reuptake of serotonin are widely used for a number of conditions due to their overall positive safety profile, agents of this class only moderately or weakly inhibiting serotonin reuptake might be preferred over agents strongly inhibiting serotonin reuptake in patients with a high propensity for bleeding.
References
NICE. Depression in adults: recognition and management. 2016. https://www.nice.org.uk/guidance/cg902016.
Jiang HY, Chen HZ, Hu XJ, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13(42–50):e3.
Hackam DG, Mrkobrada M. Selective serotonin reuptake inhibitors and brain hemorrhage: a meta-analysis. Neurology. 2012;79:1862–5.
Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D, Leontiadis GI. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811–9.
Summary of Product Characteristics—Citalopram. 2017. https://www.medicines.org.uk/emc/medicine/238612017.
Bak S, Tsiropoulos I, Kjaersgaard JO, et al. Selective serotonin reuptake inhibitors and the risk of stroke: a population-based case-control study. Stroke. 2002;33:1465–73.
Shin D, Oh YH, Eom CS, Park SM. Use of selective serotonin reuptake inhibitors and risk of stroke: a systematic review and meta-analysis. J Neurol. 2014;261:686–95.
Halperin D, Reber G. Influence of antidepressants on hemostasis. Dialogues Clin Neurosci. 2007;9:47–59.
Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–58.
Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394–9.
Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–7.
Mojtabai R. Increase in antidepressant medication in the US adult population between 1990 and 2003. Psychother Psychosom. 2008;77:83–92.
Castro VM, Gallagher PJ, Clements CC, et al. Incident user cohort study of risk for gastrointestinal bleed and stroke in individuals with major depressive disorder treated with antidepressants. BMJ Open. 2012;2:e000544.
Chen Y, Guo JJ, Patel NC. Hemorrhagic stroke associated with antidepressant use in patients with depression: does degree of serotonin reuptake inhibition matter? Pharmacoepidemiol Drug Saf. 2009;18:196–202.
Renoux C, Vahey S, Dell’Aniello S, Boivin JF. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. 2017;74:173–80.
Verdel BM, Souverein PC, Meenks SD, Heerdink ER, Leufkens HG, Egberts TC. Use of serotonergic drugs and the risk of bleeding. Clin Pharmacol Ther. 2011;89:89–96.
Wu CS, Wang SC, Cheng YC, Gau SS. Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry. 2011;168:511–21.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Sterne J, Higgins J, Reeves B, On behalf of the development group for ACROBAT-NRSI. A Cochrane risk of bias assessment tool: for non-randomized studies of interventions (ACROBAT-NRSI), Version 1.0. 0, 24 September 2014. 2015.
Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–9.
Suissa S, Dell’aniello S, Vahey S, Renoux C. Time-window bias in case-control studies: statins and lung cancer. Epidemiology (Cambridge, Mass). 2011;22:228–31.
Chen Y, Guo JJ, Li H, Wulsin L, Patel NC. Risk of cerebrovascular events associated with antidepressant use in patients with depression: a population-based, nested case-control study. Ann Pharmacother. 2008;42:177–84.
de Abajo FJ, Jick H, Derby L, Jick S, Schmitz S. Intracranial haemorrhage and use of selective serotonin reuptake inhibitors. Br J Clin Pharmacol. 2000;50:43–7.
Douglas I, Smeeth L, Irvine D. The use of antidepressants and the risk of haemorrhagic stroke: a nested case control study. Br J Clin Pharmacol. 2011;71:116–20.
Kharofa J, Sekar P, Haverbusch M, et al. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke. 2007;38:3049–51.
Lee YC, Lin CH, Lin MS, Lu Y, Chang CH, Lin JW. Comparison of the effects of serotonin-norepinephrine reuptake inhibitors versus selective serotonin reuptake inhibitors on cerebrovascular events. J Clin Psychiatry. 2016;77:e1–7.
Smoller JW, Allison M, Cochrane BB, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med. 2009;169:2128–39.
Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53.
Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2:221–8.
Daskalopoulou M, George J, Walters K, et al. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PLoS One. 2016;11:e0153838.
Hallas J, Pottegard A, Wang S, Schneeweiss S, Gagne JJ. Persistent user bias in case-crossover studies in pharmacoepidemiology. Am J Epidemiol. 2016;184:761–9.
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20.
Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology (Cambridge, Mass). 2009;20:488–95.
Caceres JA, Goldstein JN. Intracranial Hemorrhage. Emerg Med Clin N Am. 2012;30:771–94.
Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry Revue canadienne de psychiatrie. 2006;51:923–9.
Meretoja A, Strbian D, Putaala J, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012;43:2592–7.
NICE. Obsessive-compulsive disorder and body dysmorphic disorder: treatment. 2005. https://www.nice.org.uk/guidance/cg312005.
Acknowledgements
A.D. is a recipient of a Research Fellowship from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no funding for this study.
Conflict of interest
A.D., M.A., and C.R. do not have any conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Douros, A., Ades, M. & Renoux, C. Risk of Intracranial Hemorrhage Associated with the Use of Antidepressants Inhibiting Serotonin Reuptake: A Systematic Review. CNS Drugs 32, 321–334 (2018). https://doi.org/10.1007/s40263-018-0507-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0507-7