Abstract
Down syndrome (DS), often due to trisomy 21, is the most common genetic cause of intellectual disability (ID). In addition, virtually all individuals with DS develop the neuropathology of Alzheimer’s disease (AD) by the age of 40 years and almost 60 % will manifest symptoms of AD dementia by the age of 65 years. Currently, there are no pharmacological treatments available for ID in individuals with DS and only limited symptomatic treatments for AD dementia. Advances in our understanding in both the molecular basis of ID and the pathogenesis of AD have created opportunities to study potential therapeutic targets. Recent studies in animal models of DS continue to provide a rational basis for translating specific compounds into human clinical trials. However, target and compound selection are only initial steps in the drug development pathway. Other necessary considerations include appropriate study designs to assess efficacy in the DS population, as well as operational aspects specifically tailored to assess cognition in this population. We discuss recent progress in the development of compounds for both ID and AD in individuals with DS, as well as concepts for the design and conduct of clinical trials with such compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent advances in understanding of the molecular basis of intellectual disability (ID) and Alzheimer’s disease (AD) in Down syndrome (DS) have created an array of potential therapeutic targets. |
The two main therapeutic strategies that have been examined for DS in recent years include restoration of synaptic plasticity to treat ID and modification of the amyloid cascade for the treatment of AD. |
Longitudinal studies of aging are ongoing to better understand biomarker changes across the lifespan in anticipation of disease-modifying clinical trials. |
1 Introduction
In the USA, approximately 5000 children are born with Down syndrome (DS) (1/792 live births) per year [1] and at least 250,000 people are living with DS [2]. With improvements in medical care, the number of older adults with DS in the USA is increasing dramatically, just as in the general population.
People with DS display a variety of characteristic physical and behavioral features, as well as cognitive abilities that can vary from mild to profound in intellectual disability (ID) [3, 4]. In addition, with increasing age, people with DS are at increased risk of developing dementia. The cognitive phenotype of DS can therefore be characterized by two distinct conditions, one being a lifelong ID that manifests during childhood and the second being the emergence of dementia due to Alzheimer’s disease (AD) in older adulthood. Different therapeutic strategies will be required for each condition due to their differing etiologies. The two main strategies that have been examined in recent years include restoration of synaptic plasticity to treat ID and modification of the amyloid cascade for the treatment of AD.
2 Intellectual Disability in Down Syndrome
DS is associated with intellectual abilities that range from only slightly below average for the general population to profound disability [4, 5]. One key animal model for DS, the Ts65Dn mouse, has been extensively studied to better elucidate the neural mechanisms, including perturbations in neurotransmission thought to underlie the intellectual disabilities seen in individuals with DS [6].
Particularly well-characterized phenotypes in the Ts65Dn mouse are deficits in learning, memory, object recognition, and fear conditioning [7]. The ID in individuals with DS has been proposed to be linked to an imbalance in neurotransmitter function, specifically excess gamma-aminobutyric acid (GABA) activity and therefore enhanced synaptic inhibition. Electrophysiological abnormalities reflect concurrent alterations in GABA-ergic and glutamatergic transmission in the hippocampus [8]. A number of studies have demonstrated diminished synaptic long-term potentiation (LTP) and increased long-term depression (LTD) in the Ts65Dn mouse leading to an enhanced inhibitory tone within neuronal circuits in areas of the brain sub-serving memory, including the hippocampus [9–12].
Administration of chronically low doses of GABAA receptor antagonists to Ts65Dn mice resulted in normalization of learning and memory impairments as well as synaptic plasticity [13]. This and related work [14] set the stage for human studies to improve synaptic function in DS. One of the first such human studies to target the GABA-ergic imbalance in DS was the phase I multi-center, randomized, double-blind, placebo-controlled, multiple-dose study that investigated the safety and tolerability of the GABAA α5 antagonist, RG1662 (NCT01436955). The study started in November of 2011 and was completed in September 2013. Participants (N = 35) aged 18–30 years were treated for approximately 38 days with 16 weeks’ safety follow-up. The primary outcome measure was safety and tolerability, that is, incidence of adverse events. The secondary outcome measures included pharmacokinetics and performance on a brief neurocognitive battery.
This was followed by a multi-center, randomized, double-blind, three-arm, parallel-group, 26-week phase II trial of RG1662 that started in May 2014 (NCT02024789). This is an international trial with approximately 180 participants aged 12–30 years; enrollment was completed in early 2016. Primary outcomes are cognition as assessed by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) sub-tests, adaptive behavior as assessed by the Vineland Adaptive Behavior Scales-II (VABS-II) standard scores, and Clinical global impression as assessed by Clinician Rated Global Impression Scale-Improvement (CGI-I). A second study with the same compound is being conducted in approximately 46 children with DS, aged 6–11 years; estimated study completion date is June 2017.
Also targeting the GABA-ergic system is a phase II trial using the GABAA receptor antagonist pentylenetetrazole, BTD-001. This is a randomized, double-blind, placebo-controlled, parallel-group study being conducted in Australia with approximately 90 subjects with DS aged 13–35 years, with a 12-week treatment period and a 4-week follow-up.
Postsynaptic targets of locus coeruleus (LC) axons, such as in the hippocampus, are rich in β1-adrenergic receptors and have been shown to be markedly reduced in both DS and AD [15, 16]. Substantial defects in the noradrenergic system have also been reported in the Ts65Dn mouse, including the hippocampus [17]. A significant improvement in contextual fear conditioning was found after treatment of Ts65Dn mice with droxidopa (l-threo-3,4-dihydroxyphenylserine; l-DOPS), a norepinephrine precursor [18]. In addition, treatment with the β1-adrenergic receptor agonist xamoterol rescued some of the learning deficits in Ts65Dn mice [19]. These results suggest that behavioral deficits observed in the Ts65Dn mouse may be mediated by an imbalance in the noradrenergic system and be potentially reversible. Human trials are being considered.
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), has been shown in animal models to stimulate learning and memory, promote hippocampal neurogenesis and synaptogenesis, and reduce levels of GABA-ergic inhibition [20–23]. Chronic treatment with fluoxetine administered in drinking water to adult Ts65Dn mice normalized GABA release and promoted recovery of spatial memory abilities and hippocampal synaptic plasticity [24]. The defects in dendritic hypotrophy and neuronal connectivity, in addition to neurogenesis, were further studied by Guidi et al. [25]. Here, pregnant Ts65Dn females were treated with fluoxetine from embryonic day 10 until delivery. As compared with untreated Ts65Dn mice, which exhibit a severe reduction in neurogenesis throughout the developing brain, the embryonically treated Ts65Dn mice had neurogenesis nearly fully restored throughout all brain regions. Moreover, treatment during the embryonic stage restored brain volume in the Ts65Dn mice to a volume similar to that of normal controls.
A pilot clinical study is being planned at the University of Texas Southwestern Medical Center; 21 pregnant mothers carrying a fetus with confirmed DS will be randomized 2:1 to fluoxetine:placebo beginning at 13–24 weeks’ gestation. Fluoxetine (or placebo) will be taken orally by the mother and initiated at 20 mg/day and increased weekly as tolerated to 80 mg/day and continued throughout pregnancy. The primary outcomes of the study will assess feasibility and safety. Efficacy will be assessed at 6 months, 1 and 2 years, and will be the composite score of the Bayley Scales of Infant Development–third edition.
The DYRK1A gene, located on the 21st chromosome is thought to play an important role in the pathogenesis of the ID associated with DS by virtue of its involvement in neuronal differentiation [26]. Its overexpression, as studied in transgenic mice (TgDyrk1A) is thought to lead to cognitive impairment and deficient synaptic architecture [27]. Epigallocatechin gallate (EGCG) is a flavonoid and natural antioxidant found in green tea and has been shown to be a potent DYRK1A inhibitor [28, 29]. A phase II clinical trial involving 31 participants with DS aged 14–29 years enrolled in a 6-month, placebo-controlled study of oral EGCG (9 mg/kg/day) found significant effects on episodic memory function after 3 months of treatment as compared with placebo [30]. These participants had moderate ID (IQ ~45) at baseline and were enrolled from a large cohort of outpatients in the “Fundació Catalana Síndrome de Down” program in Barcelona, Spain. The primary outcome measures were pattern recognition memory (PRM), Fuld object memory evaluation, and paired-associate learning (PAL) as well as plasma homocysteine, NAD(P)H: quinone oxireductase activity and DYRK1A gene expression in lymphocytes. Overall, EGCG was well tolerated. Two participants experienced side effects: one in the EGCG group presented increased excitability that required withdrawal of treatment and one in the placebo-treated group required dose reduction due to abdominal pain.
3 Alzheimer’s Disease in Down Syndrome
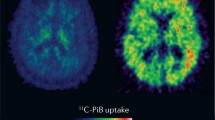
The triplication of chromosome 21 results in a life-long overproduction of brain amyloid [31]. Overproduction of β amyloid invariably leads to the early development of AD-like pathology in DS [32]. Because of this overproduction of Aβ, by the age of 40 years, virtually all people with DS show the same neuropathological changes as seen in AD [33]. Moreover, the cholinergic losses seen in brains of individuals with DS are identical to those observed in AD [34]. However, in contrast to sporadic AD, amyloid plaques and neurofibrillary tangles in DS can start developing as early as in the teen years [35]. AD dementia has been reported to affect at least 60 % of individuals with DS over the age of 65 years [36].
Recent data also indicate AD biomarker changes in DS are similar to those observed in familial and sporadic AD [37]. Results of amyloid positron emission tomography (PET) imaging from individuals with DS are also consistent with those seen in non-DS individuals with AD [38]. Furthermore, as in familial and sporadic AD, presence of the Apo ɛ4 allele is associated with greater accumulation of Aβ protein in the brains of adults with DS [39], and greater risk of an earlier age of onset of dementia [40]. The link between triplication of the APP gene and subsequent overproduction of Aβ leading to AD dementia in DS is further supported by the case of an individual with DS with partial trisomy of chromosome 21 who was disomic for the APP gene and who did not develop dementia or any AD pathology [41].
As individuals with DS represent a population at high risk for AD, the launch of longitudinal studies of AD biomarkers in DS, including the DSBI (Down Syndrome Biomarker Initiative) and the NIAD (Neurodegeneration in Aging Down Syndrome), will be important in understanding the natural history study of AD from the pre-symptomatic through dementia stages and will help inform the design of future clinical trials in this, and perhaps the sporadic AD population.
Elevated levels of myo-inositol are also thought to contribute to the cognitive impairment in DS [42]. ELND005 (scyllo-inositol), an endogenous myo-inositol isomer, is hypothesized to reduce amyloid toxicity and regulate myo-inositol levels to improve cognitive function in patients with DS. ELND005 (scyllo-inositol; cyclohexane-1,2,3,4,5,6-hexol) has also been evaluated as a potential disease-modifying treatment of AD. In preclinical studies, ELND005 has shown amyloid anti-aggregation effects in vitro, protective effects on oligomer-induced neuronal toxicity, and positive effects on learning in animal models of AD [43–45]. ELND005 has shown both amyloid- and myo-inositol-lowering effects in cerebrospinal fluid (CSF) and brain, respectively, in patients with AD [46]. At a dose with acceptable long-term safety, ELND005 showed beneficial trends on cognition in mild AD [46]. A phase II randomized, double-blind, placebo-controlled study of oral ELND005 in 26 individuals with DS (mean age 28 years) without dementia was recently completed [47]. The compound was well-tolerated, and results are expected to be published later this year.
ACI-24 is a new-generation liposomal vaccine that is designed to elicit an antibody response only against aggregated Aβ peptides without concomitant pro-inflammatory T-cell activation [48, 49]. ACI-24 is based on the truncated Aβ1-15 sequence, which is devoid of T-cell epitopes located closer to the peptide’s C-terminus. The peptide sequence is anchored into the surface of liposomes in such a way that peptides adopt an aggregated β-sheet structure, forming a conformational epitope.
Previous active vaccines (e.g., AN-1792) elicited a T-cell response, which led to an increased risk of meningoencephalitis [50]. In the view of potentially adverse inflammatory responses caused by a T-cell response, new-generation vaccines have been designed in which the epitopes for T-cell activation have been removed such that there is only a B-cell-mediated or humoral response, with generation of Aβ-specific antibodies only. In addition to ACI-24, these new-generation active vaccines include ACC-001 [51] and CAD-106 [52]. Phase I trials for these approaches showed positive antibody responses with no signs of adverse inflammation [53, 54].
A phase I/II trial of ACI-24 in Europe is ongoing and aims to address safety, immunogenicity, and efficacy. The study is enrolling up to 198 patients aged ≥40 with mild to moderate AD dementia who have a positive amyloid PET scan and are receiving stable cholinesterase-inhibitor therapy. This double-blind, randomized, placebo-controlled study compares three doses with placebo delivered subcutaneously. Participants are being treated for 1 year and followed-up for 2 more. Primary outcomes include safety and tolerability measures as well as serum titers of anti-Aβ42 immunoglobulin-G (IgG) antibodies and 1-year change from baseline in cognitive performance on the Neuropsychological Test Battery. As well as clinical endpoints, secondary outcomes include amyloid burden as assessed by PET and biomarker measures such as magnetic resonance imaging (MRI) volumetrics and tau, phospho-tau, and Aβ levels in CSF.
A phase Ib placebo-controlled, multicenter study with ACI-24 for the treatment of AD in individuals with DS was launched in 2016. The study will enroll 24 adults with DS aged 35–45 years and treat them with ACI-24 for 1 year with an additional year of follow-up. Primary endpoints include measures of safety, tolerability, and immunogenicity. Secondary endpoints of this clinical trial are effects on biomarkers of AD pathology, including amyloid burden as measured by amyloid PET, as well as cognitive and clinical function.
4 Designing Clinical Trials for Down Syndrome
The conduct of clinical trials in the DS population raises methodological challenges in studying cognitive functioning. Given the variability in baseline IDs and functioning, assessments in this population require a unique set of considerations. Specifically, demands on the memory, attention, and language ability of individuals with DS must be taken into account for successful and accurate cognitive assessment and interpretation of results. Moreover, thoughtfully designed cognitive testing sessions using validated instruments with consistent examiners who can develop a familiarity with the study participant and vice versa will be critical.
A number of validated instruments currently exist for assessing cognition and functioning in individuals with DS across many ages. These include the Cambridge Neuropsychological Testing Automated Battery (CANTAB), the Arizona Cognitive Test Battery (ACTB), and the VABS-II. The CANTAB is a computerized assessment with touch-screen technology designed for individuals aged 4–90 years that is normed with alternate versions. Its memory tasks, such as the PAL, spatial recognition, and spatial span subtests, may be particularly well-suited for individuals with DS [55, 56]. The ACTB was developed specifically to assess the cognitive phenotype in DS and includes tests of general cognitive ability and prefrontal, hippocampal, and cerebellar function [57]. In a study of 74 participants with DS and 50 mental age-matched controls, the ACTB provided consistent results across contexts, including home versus clinic, cross-site, and among individuals with a wide range of socio-economic backgrounds and differences in ethnicity, and correlated well with benchmark clinical assessments. The VABS-II has also been used in a number of DS clinical trials [58]. It covers birth to age 90 years, is primarily informant based and takes approximately 60 minutes to administer and score.
An additional consideration that may aid in reducing variance associated with significant individual differences in DS is the double-crossover clinical trial design. The essential feature distinguishing a crossover trial from a conventional parallel-group trial is that each participant serves as his/her own control since they receive study drug and placebo for equal intervals during the course of the study [59]. The crossover design may thus help avoid problems of comparability with regard to confounding variables.
Finally, the delayed-start study design, also called the randomized-start design, may be particularly useful in assessing disease-modifying effects of potential treatments for the pre-symptomatic stage of AD. In delayed-start studies, patients are randomly assigned to start active treatment at the beginning of the study or are placed in a “delayed-start” group that receives a placebo for a period of time before receiving the active treatment. Comparison is then made between the two groups at a later pre-defined point in time [60]. If the active treatment is limited to reducing symptoms, both early-start and delayed-start participants experience the same benefit. If the active treatment is disease-modifying, both groups will benefit, but the delayed-start group will remain temporally separated. This design has been applied successfully in clinical trials in AD [61] and other neurodegenerative diseases that develop slowly in their early stages [62].
5 Conclusions
Over the past decade, we have seen steady progress being made toward understanding the basis of ID and AD in individuals with DS. Animal models are providing deeper understanding of the cellular and molecular basis of ID in DS. With this knowledge comes an array of potential therapeutic targets. As new compounds enter clinical trials targeting these various mechanisms, study design and conduct will need to be further refined. This is also a significant time for AD research in general, and many of the ongoing secondary-prevention studies will provide results as to whether the amyloid hypothesis, postulating that Aβ accumulation is one of the initial events in AD pathology, is correct. Ongoing longitudinal studies of aging will help us better understand AD biomarker changes and power disease-modifying therapy trials in people with DS.
References
De Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am J Med Genet A. 2015;167A(4):756–67.
Presson AP, Partyka G, Jensen KM, Devine OJ, Rasmussen SA, McCabe LL, McCabe ER. Current estimate of Down Syndrome population prevalence in the United States. J Pediatr. 2013;163(4):1163–8.
Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010;9:623–33.
Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: cognitive and behavioral functioning across the lifespan. Am J Med Genet Part C. 2015;169C:135–49.
Carr J. Six weeks to 45 years: a longitudinal study of a population with Down syndrome. J Appl Res Intellect Disabil. 2012;25(5):414–22.
Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–84.
Belichenko NP, Belichenko PV, Kleschevnikov AM, Salehi A, Reeves RH, Mobley WC. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci. 2009;29:5938–48.
Hanson JE, Blank M, Valenzuela RA, Garner CC, Madison DV. The functional nature of synaptic circuitry is altered in area CA3 of the hippocampus in a mouse model of Down’s syndrome. J Physiol. 2007;579:53–67.
Siarey RJ, Carlson EJ, Epstein CJ, Balbo A, Rapoport SI, Galdzicki Z. Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology. 1999;38(12):1917–20.
Kleschevnikov AM, Belichenko PV, Villar AJ, Epstein CJ, Malenka RC, Mobley WC. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24(37):8153–60.
Costa AC, Grybko MJ. Deficits in hippocampal CA1 LTP induced by TBS but not HFS in the Ts65Dn mouse: a model of Down syndrome. Neurosci Lett. 2005;382(3):317–22.
Siarey RJ, Kline-Burgess A, Cho M, Balbo A, Best TK, Harashima C, Klann E, Galdzicki Z. Altered signaling pathways underlying abnormal hippocampal synaptic plasticity in the Ts65Dn mouse model of Down syndrome. J Neurochem. 2006;98(4):1266–77 (Erratum in: J Neurochem. 2006;99(4):1320).
Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10(4):411–3.
Kleschevnikov AM, Belichenko PV, Gall J, George L, Nosheny R, Maloney MT, Salehi A, Mobley WC. Increased efficiency of the GABAA and GABAB receptor-mediated neurotransmission in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis. 2012;45(2):683–91.
Mann DM, Yates PO, Marcyniuk B, Ravindra CR. Pathological evidence for neurotransmitter deficits in Down’s syndrome of middle age. J Ment Defic Res. 1985;29(Pt 2):125–35.
German DC, Manaye KF, White CL 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32(5):667–76.
Dierssen M, Vallina IF, Baamonde C, García-Calatayud S, Lumbreras MA, Flórez J. Alterations of central noradrenergic transmission in Ts65Dn mouse, a model for Down syndrome. Brain Res. 1997;749(2):238–44.
Salehi A, Faizi M, Colas D, Valletta J, Laguna J, Takimoto-Kimura R, Kleschevnikov A, Wagner SL, Aisen P, Shamloo M, Mobley WC. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci Transl Med. 2009;1(7):7ra17.
Faizi M, Bader PL, Tun C, Encarnacion A, Kleschevnikov A, Belichenko P, Saw N, Priestley M, Tsien RW, Mobley WC, Shamloo M. Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: activation of β1-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol Dis. 2011;43(2):397–413.
Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21(5):1299–303.
Li WL, Cai HH, Wang B, Chen L, Zhou QG, Luo CX, Liu N, Ding XS, Zhu DY. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J Neurosci Res. 2009;87(1):112–22.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–10.
Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–8.
Begenisic T, Baroncelli L, Sansevero G, Milanese M, Bonifacino T, Bonanno G, Cioni G, Maffei L, Sale A. Fluoxetine in adulthood normalizes GABA release and rescues hippocampal synaptic plasticity and spatial memory in a mouse model of Down syndrome. Neurobiol Dis. 2014;63:12–9.
Guidi S, Stagni F, Bianchi P, Ciani E, Giacomini A, De Franceschi M, Moldrich R, Kurniawan N, Mardon K, Giuliani A, Calzà L, Bartesaghi R. Prenatal pharmacotherapy rescues brain development in a Down’s syndrome mouse model. Brain. 2014;137(Pt 2):380–401.
Becker W, Soppa U, Tejedor FJ. DYRK1A: a potential drug target for multiple Down syndrome neuropathologies. CNS Neurol Disord Drug Targets. 2014;13(1):26–33.
Martinez de Lagran M, Benavides-Piccione R, Ballesteros-Yañez I, Calvo M, Morales M, Fillat C, Defelipe J, Ramakers GJ, Dierssen M. Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. Cereb Cortex. 2012;22(12):2867–77.
Guedj F, Sébrié C, Rivals I, Ledru A, Paly E, Bizot JC, Smith D, Rubin E, Gillet B, Arbones M, Delabar JM. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One. 2009;4(2):e4606.
Souchet B, Guedj F, Penke-Verdier Z, Daubigney F, Duchon A, Herault Y, Bizot JC, Janel N, Créau N, Delatour B, Delabar JM. Pharmacological correction of excitation/inhibition imbalance in Down syndrome mouse models. Front Behav Neurosci. 2015;20(9):267.
De la Torre R, De Sola S, Pons M, Duchon A, de Lagran MM, Farré M, Fitó M, Benejam B, Langohr K, Rodriguez J, Pujadas M, Bizot JC, Cuenca A, Janel N, Catuara S, Covas MI, Blehaut H, Herault Y, Delabar JM, Dierssen M. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014;58(2):278–88.
Schupf N, Sergievsky GH. Genetic and host factors for dementia in Down’s syndrome. Br J Psychiatry. 2002;180:405–10.
Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17(3):278–82.
Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122(3):1131–5.
Yates CM, Simpson J, Maloney AF, Gordon A, Reid AH. Alzheimer-like cholinergic deficiency in Down syndrome. Lancet. 1980;2(8201):979.
Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3(1):16–32.
Janicki MP, Dalton AJ. Prevalence of dementia and impact on intellectual disability services. Ment Retard. 2000;38(3):276–88.
Rafii MS, Wishnek H, Brewer JB, Donohue MC, Ness S, Mobley WC, Aisen PS, Rissman RA. The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer’s disease biomarkers in down syndrome. Front Behav Neurosci. 2015;14(9):239.
Handen BL, Cohen AD, Channamalappa U, Bulova P, Cannon SA, Cohen WI, Mathis CA, Price JC, Klunk WE. Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimers Dement. 2012;8(6):496–501.
Hyman BT, West HL, Rebeck GW, Lai F, Mann DM. Neuropathological changes in Down’s syndrome hippocampal formation. Effect of age and apolipoprotein E genotype. Arch Neurol. 1995;52(4):373–8.
Schupf N, Kapell D, Lee JH, Zigman W, Canto B, Tycko B, Mayeux R. Onset of dementia is associated with apolipoprotein E epsilon4 in Down’s syndrome. Ann Neurol. 1996;40(5):799–801.
Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC, Butler AC. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann Neurol. 1998;43(3):380–3.
Beacher F, Simmons A, Daly E, Prasher V, Adams C, Margallo-Lana ML, Morris R, Lovestone S, Murphy K, Murphy DG. Hippocampal myo-inositol and cognitive ability in adults with Down syndrome: an in vivo proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62(12):1360–5.
Shonk T, Ross BD. Role of increased cerebral myo-inositol in the dementia of Down syndrome. Magn Reson Med. 1995;33(6):858–61.
Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J Mol Med. 2007;85:603–11.
McLaurin J, Kierstead ME, Brown ME, et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12:801–8.
Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, Gilman S, Arnold D, Abushakra S, Hernandez C, Crans G, Liang E, Quinn G, Bairu M, Pastrak A, Cedarbaum JM, ELND005-AD201 Investigators. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77(13):1253–62.
Rafii MS, Skotko B, Lott I, Kesslak P, Abushakra S. Phase 2a study of ELND005 in adults with Down syndrome. Clinical Trials on Alzheimer’s Disease 2014; Philadelphia, PA; 22–22 November 2014.
Muhs A, Hickman DT, Pihlgren M, Chuard N, Giriens V, Meerschman C, van der Auwera I, Van Leuven F, Sugawara M, Weingertner MC, Bechinger B, Greferath R, Kolonko N, Nagel-Steger L, Riesner D, Brady RO, Pfeifer A, Nicolau C. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci. 2007;104(23):9810–5.
Hickman DT, López-Deber MP, Ndao DM, Silva AB, Nand D, Pihlgren M, Giriens V, Madani R, St-Pierre A, Karastaneva H, Nagel-Steger L, Willbold D, Riesner D, Nicolau C, Baldus M, Pfeifer A, Muhs A. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem. 2011;286(16):13966–76.
Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM, AN1792(QS-21)-201 Study Team. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–62.
Ryan JM, Grundman M. Anti-amyloid-beta immunotherapy in Alzheimer’s disease: ACC-001 clinical trials are ongoing. J Alzheimers Dis. 2009;17(2):243.
Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, Maguire RP, Blennow K, Lundmark J, Staufenbiel M, Orgogozo JM, Graf A. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11(7):597–604.
Pasquier F, Sadowsky C, Holstein A, Leterme Gle P, Peng Y, Jackson N, Fox NC, Ketter N, Liu E, Ryan JM, ACC-001 (QS-21) Study Team. Two phase 2 multiple ascending-dose studies of vanutide cridificar (ACC-001) and QS-21 adjuvant in mild-to-moderate Alzheimer’s disease. J Alzheimers Dis. 2016;51(4):1131–43.
Farlow MR, Andreasen N, Riviere ME, Vostiar I, Vitaliti A, Sovago J, Caputo A, Winblad B, Graf A. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res Ther. 2015;7(1):23.
Boada R, Hutaff-Lee C, Schrader A, Weitzenkamp D, Benke T, Goldson E, Costa A. Antagonism of NMDA receptors as a potential treatment for Down syndrome: a pilot randomized controlled trial. Transl Psychiatry. 2012;17(2):e141.
Liogier d’Ardhuy X, Edgin JO, Bouis C, de Sola S, Goeldner C, Kishnani P, Nöldeke J, Rice S, Sacco S, Squassante L, Spiridigliozzi G, Visootsak J, Heller J, Khwaja O. Assessment of cognitive scales to examine memory, executive function and language in individuals with Down syndrome: implications of a 6-month observational study. Front Behav Neurosci. 2015;9:300.
Edgin JO, Mason GM, Allman MJ, Capone GT, DeLeon I, Maslen C, Reeves RH, Sherman SL, Nadel L. Development and validation of the Arizona Cognitive Test Battery for Down syndrome. J Neurodev Disord. 2010;2(3):149–64.
Sparrow S, Cicchetti DV, Balla DA. Vineland II: vineland adaptive behavior scales. Circle Pines: American Guidance Service; 2005.
Jones B, Kenward MG. Design and analysis of cross-over trials. 2nd ed. Boca Raton: Chapman & Hall/CRC; 2003.
D’Agostino RB Sr. The delayed-start study design. N Engl J Med. 2009;361(13):1304–6.
Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361:1268–78.
Liu-Seifert H, Siemers E, Holdridge KC, Anderson SW, Lipkovich I, Carlson C, Sethuraman G, Hoog S, Hayduk R, Doody R, Aisen PS. Delayed-start analysis: mild Alzheimer’s disease patients in solanezumab trials, 3.5 years. Alzheimers Dement Transl Res Clin Inter. 2015;1(2):111–121.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no specific funding used to assist with the preparation of this review.
Conflict of interest
MSR is funded by an NIH R01 as principal investigator for a multicenter clinical trial of ACI-24 in Down syndrome (AG047922-01). He has also received research grants from Elan Corporation (Dublin, Ireland), Hoffmann-La Roche (Basel, Switzerland) and Janssen Pharmaceuticals (Titusville, New Jersey). He was a site investigator for the ELND005 study as well as the RG1662 phase I and phase II studies.
Rights and permissions
About this article
Cite this article
Rafii, M.S. Improving Memory and Cognition in Individuals with Down Syndrome. CNS Drugs 30, 567–573 (2016). https://doi.org/10.1007/s40263-016-0353-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-016-0353-4