Abstract
Oral brexpiprazole (Rexulti®) is a partial dopamine D2 agonist, which also has activity at several other receptors. This article reviews the pharmacological properties of brexpiprazole and its clinical efficacy and tolerability in patients with schizophrenia; its use in patients with major depressive disorder is beyond the scope of this review. Brexpiprazole 2–4 mg/day was generally effective in short-term, phase III studies at improving Positive and Negative Symptom Scale scores and other schizophrenia symptoms in patients with acute schizophrenia. Moreover, maintenance treatment with brexpiprazole 1–4 mg/day was associated with a significantly longer time to exacerbation of disease or impending relapse than placebo. The drug was well tolerated in clinical trials, with most serious adverse events in the short term being associated with the underlying disorder. Overall, oral brexpiprazole is a useful treatment option for the treatment of patients with schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dopamine D2 receptor partial agonist, serotonin 5-HT1A receptor partial agonist and potent serotonin 5-HT2A receptor antagonist |
Generally effective at improving schizophrenia symptoms in patients with acute schizophrenia |
Longer time to exacerbation or impending relapse than placebo with maintenance treatment |
Associated with moderate weight gain; no clinically relevant changes in lipid profiles or other metabolic parameters occurred in clinical trials |
1 Introduction
Affecting 1 % of the global population, schizophrenia is characterized by multiple symptom domains: e.g. positive symptoms (hallucinations, delusions, behavioural disturbances and disordered thinking), negative symptoms (affective blunting, emotional withdrawal, apathy), and cognitive deficits (attention problems, impaired memory) [1, 2]. Positive symptoms appear to be associated with an overactive mesolimbic dopamine pathway, and negative symptoms with dopaminergic hypoactivity; serotonin also appears to play a part in schizophrenia [1].
There have historically been two main classes of antipsychotics used to treat schizophrenia: first-generation (typical) and second-generation (atypical) antipsychotics [1]. First-generation antipsychotics (e.g. haloperidol) block dopamine D2 receptors and have generally beneficial effects on positive but not negative or cognitive symptoms; second-generation antipsychotics target either multiple receptors (e.g. quetiapine) or are high-affinity D2 and serotonin 5-HT2A receptor antagonists (e.g. risperidone), and have generally beneficial effects on both positive and negative symptoms [1]. First-generation antipsychotics are often associated with extrapyramidal symptoms, hyperprolactinemia, and cardiovascular adverse events; while these specific tolerability issues are less common with second-generation antipsychotics, there is a potentially increased risk of body-weight gain, diabetes mellitus and hypercholesterolemia with these drugs [1, 2]. These adverse events may lead to a lack of compliance, preventing effective disease management [1].
The heterogeneity of antipsychotic efficacy and tolerability profiles mean that each patient may react differently to each drug; thus, the development of new drugs is expected to be of benefit, as this should lead to a more diverse set of treatment options [3, 4]. The most recent drug development programmes have focused on improving negative symptoms and cognitive dysfunction as well as positive symptoms, as these are associated with more long-term disability [1].
The second-generation antipsychotic brexpiprazole (Rexulti®) is indicated in the USA for use in patients with schizophrenia and as an adjunctive therapy to antidepressants in patients with major depressive disorder (MDD) [5]. This article reviews the pharmacological properties of brexpiprazole and its clinical efficacy and tolerability in patients with schizophrenia. The use of brexpiprazole in patients with MDD is beyond the scope of this review and is discussed elsewhere [6].
2 Pharmacodynamic Properties of Brexpiprazole
While the mechanism of action of brexpiprazole is unknown, it may be mediated through its combined serotonin 5-HT1A receptor partial-agonist, dopamine D2 receptor partial-agonist and potent serotonin 5-HT2A receptor antagonist activity [5]. Brexpiprazole binds with high affinity to human serotonin 5-HT1A (Ki 0.12 nmol/L), 5-HT2A (Ki 0.47 nmol/L), 5-HT2B (Ki 1.9 nmol/L) and 5-HT7 (Ki 3.7 nmol/L), dopamine D2 (Ki 0.30 nmol/L) and D3 (Ki 1.1 nmol/L) receptors, and noradrenergic α1A (Ki 3.8 nmol/L), α1B (Ki 0.17 nmol/L), α1D (Ki 2.6 nmol/L) and α2C (Ki 0.59 nmol/L) receptors [5, 7]. The drug also has moderate affinity for the human histamine H1 receptor (Ki 19 nmol/L) and very low affinity for the human muscarinic M1 receptor (Ki >1000 nmol/L) [5, 7]. Brexpiprazole binds with an approximately threefold higher affinity to the D2 receptor and an approximately 10-fold higher affinity to the 5-HT1A and 5-HT2A receptors than aripiprazole, and with approximately 200- and 65-fold higher affinity to the α1B and α2C receptors, respectively [7].
In preclinical studies, brexpiprazole demonstrated partial agonist activity at the human 5-HT1A receptor, with an effect that was similar to [7] or significantly greater than [8] that of aripiprazole. Brexpiprazole was also a human D2 and D3 receptor partial agonist, with (significantly; p < 0.05 [7]) lower intrinsic activity than aripiprazole [7, 8]. The intrinsic activity at the D2 receptor for brexpiprazole is between those for aripiprazole and D2 antagonist antipsychotics; however, it is as yet unclear whether the difference between aripiprazole and D2 antagonists is wide enough to be clinically significant [9]. Moreover, brexpiprazole demonstrated antagonist activity at the human 5-HT2A, 5-HT2B, α1B and α2C receptors [7, 8]; the highest potency was observed at α1B receptors (50 % inhibitory concentration of 0.66 nmol/L), with slightly lower potency at the other receptors [7]. Brexpiprazole was associated with potentiated nerve-growth factor-induced neurite outgrowth in PC12 cells, via the activation of 5-HT1A and 5-HT2A receptors and Ca2+ signaling via inositol 1,4,5-triphosphate receptors; neurite outgrowth may play a part in the therapeutic effect of antipsychotic drugs [10].
In animal models, brexpiprazole demonstrated antipsychotic-like properties at clinically relevant occupancies of the D2 receptor, while showing a low potential to induce extrapyramidal symptoms [11]. Brexpiprazole was also associated with a reduction in impulsive behavior [12] and significant (p < 0.05) improvements in models of cognitive impairment associated with schizophrenia [11, 13, 14]. Aripiprazole was not associated with any significant changes in cognitive impairment in animal models [11].
3 Pharmacokinetic Properties of Brexpiprazole
Maximum plasma brexpiprazole concentration was reached within 4 h of a single oral dose; steady state was reached within 10 to 12 days of treatment initiation [5]. The absolute oral bioavailability was 95 %. Brexpiprazole exposure was not significantly affected by coadministration of a high-fat meal; it can thus be taken with or without food. Intravenous brexpiprazole has a high volume of distribution (1.56 L/kg), indicating extravascular distribution. It is highly (>99 %) protein bound in plasma [5].
In vitro and in vivo studies indicate that brexpiprazole metabolism is mainly mediated by cytochrome P450 (CYP)3A4 and CYP2D6 [5]. Systemically, brexpiprazole and its major metabolite (DM-3411) were the main drug moieties after single and multiple doses; DM-3411 does not appear to contribute to the therapeutic effects of brexpiprazole. At steady state, 23–48 % of brexpiprazole exposure in plasma was represented by DM-3411 [5].
In a radio-labelled oral brexpiprazole study, ≈25 and 46 % of the total radioactivity was recovered in the urine and faeces; <1 and ≈14 % of unchanged drug was found in the urine and feces [5]. After multiple once-daily oral doses of brexpiprazole, its apparent oral clearance was 19.8 mL/h/kg, and the terminal elimination half-lives of brexpiprazole and DM-3411 were 91 and 86 h [5].
As brexpiprazole is metabolized by CYP2D6, patients known to be poor CYP2D6 metabolizers have higher brexpiprazole concentrations than normal CYP2D6 metabolizers [5]. Patients with moderate to severe hepatic impairment (Child-Pugh score ≥7) and patients with impaired renal function (creatinine clearance <60 mL/min) generally had higher brexpiprazole exposure than patients with normal hepatic or renal function. Dosage adjustment is recommended in these patients (Sect. 6) [5].
Brexpiprazole exposure is increased with concomitant use of strong CYP3A4 inhibitors (e.g. itraconazole, clarithromycin, ketoconazole) and strong CYP2D6 inhibitors (e.g. paroxetine, fluoxetine, quinidine), and brexpiprazole dosage adjustment is recommended when these drugs are coadministered alone or when moderate or strong CYP3A4 and CYP2D6 inhibitors are both coadministered (Sect. 6) [5]. Brexpiprazole exposure is decreased with concomitant use of strong CYP3A4 inducers (e.g. rifampin, St. John’s wort); dosage adjustment is recommended (Sect. 6) [5].
Brexpiprazole is not a substrate of efflux transporters [e.g. P-glycoprotein or breast cancer resistant protein (BCRP)] and shows little to no inhibition of CYP enzymes, according to in vitro studies [5]. No brexpiprazole dosage adjustment is required with concomitant use of CYP2B6 inhibitors, gastric pH modifiers, CYP2D6, CYP3A4 or CYP2B6 substrates, or BCRP or P-glycoprotein substrates.
4 Therapeutic Efficacy of Brexpiprazole
4.1 Acute Schizophrenia
While data from a phase II study investigating the efficacy of brexpiprazole in patients with acute schizophrenia are available [15], this section discusses data from phase III trials, with a focus on large (n = 459–674), randomized, double-blind, placebo-controlled, multinational [16, 17] or multicentre [18], quetiapine extended release-referenced [18], phase III trials [BEACON [17], VECTOR [16] (both fully published) and LIGHTHOUSE (data taken from an abstract) [18, 19]]. Data (taken from an abstract) from an exploratory, randomized, open-label, aripiprazole-referenced, phase III trial are briefly discussed [20].
Patients were randomized to 6 weeks of treatment with oral brexpiprazole 0.25–4 mg/day (see Table 1 for dosage allocations and titration details) [16–18], placebo [16–18] or quetiapine extended release 400–800 mg/day [18]. The dosages of 1 mg/day in BEACON and 0.25 mg/day in VECTOR were included for exploratory purposes (the studies were not powered for statistical comparisons between these groups and placebo recipients) [16, 17] and the 0.25 mg/day dosage does not fall within the approved dosage range [5]; as a result, they are not discussed further here (some results from the 1 mg/day dosage are presented in Table 1 for completeness).
Inclusion criteria were: adult patients with schizophrenia [16, 17, 19] and a history of relapse and/or symptom exacerbation when they were not receiving antipsychotics [17, 18], who were experiencing an acute exacerbation of psychotic symptoms [16, 17, 19] and marked deterioration of usual functioning [17, 18] that would benefit from hospitalization [16, 17, 19]. Exclusion criteria included comorbid psychological or neurological disorders (e.g. tardive dyskinesia) [16, 17, 19], patients undergoing their first schizophrenia episode [16, 17], a recent history of substance abuse [16, 17], and the current or recent use of prohibited concomitant medication or treatment [17].
The primary endpoint was the change from baseline at week 6 in Positive and Negative Syndrome Scale (PANSS) total score [16–18] in the modified intent-to-treat population [16, 17], using mixed model for repeated measures (MMRM) analysis [16–18]. Where reported, demographic and baseline characteristics did not significantly differ between treatment groups [16, 17]. The time since first diagnosis was ≈12 to 13 years [16, 17], and the duration of the current episode was a mean of 2.5 [17] and 2.6 [16] weeks. A total of 41–52 % of patients were receiving concomitant lorazepam (the most frequently used concomitant benzodiazepine) [16, 17]. In VECTOR, before the study, 91, 71 and 12 % of patients had received treatment with antipsychotics, anxiolytics/hypnosedatives and antidepressants, respectively [16].
Individual trials showed mixed results with regard to the primary endpoint of PANSS total score (Table 1) [16–18]. Recipients of brexpiprazole 2 or 4 mg/day, analysed as a single brexpiprazole group, had a significantly greater change from baseline in PANSS total score than placebo at 6 weeks (p < 0.01) in both BEACON and VECTOR [16, 17]. Recipients of brexpiprazole 4 mg/day also had a significantly greater change from baseline than placebo in both BEACON and VECTOR [−6.47 (95 % CI −10.6 to −2.35) in BEACON and −7.64 (95 % CI −12.0 to −3.30) in VECTOR] [16, 17], as did recipients of brexpiprazole 2 mg/day in VECTOR (−8.72; 95 % CI −13.1 to −4.37) [16]; however, recipients of brexpiprazole 2 mg/day in BEACON did not significantly differ from placebo recipients (−3.08; 95 % CI −7.23 to +1.07) (Table 1) [17]. As significance was not reached for both the 2 and the 4 mg/day dosages for this endpoint in BEACON, all subsequent statistical analyses were considered exploratory in this trial [17]. In LIGHTHOUSE, recipients of brexpiprazole 2–4 mg/day (flexible dose) and placebo did not significantly differ in terms of change in PANSS total score (Table 1) [18].
However, a meta-analysis of all three large phase III trials found that brexpiprazole 2–4 mg/day (n = 868) was associated with a significantly greater change from baseline in PANSS total score than placebo (n = 517) [−20.1 vs. −14.3; treatment difference −5.8 (95 % CI −8.0 to −3.6); p < 0.001] [21]. Moreover, a pooled analysis of BEACON and VECTOR found that both dosages of brexpiprazole were associated with significantly greater improvements in PANSS total score than placebo [2 mg/day: −5.46 (95 % CI −8.46 to −2.47; p = 0.0004); 4 mg/day: −6.69 (95 % CI −9.67 to −3.70; p < 0.0001)] [22]. The effect size for the primary endpoint in VECTOR was 0.41 and 0.36 for brexpiprazole 2 and 4 mg/day, respectively, versus placebo [16]; effect sizes were not reported for BEACON.
In general, brexpiprazole was associated with a significant improvement in Clinical Global Impressions-Severity scale (CGI-S) score (Table 1) [16–18]. In BEACON and VECTOR, change from baseline in CGI-S total score was significantly greater in brexpiprazole 4 mg/day than placebo recipients in both trials [mean treatment difference −0.38 (95 % CI −0.62 to −0.15; p = 0.0015) in BEACON and −0.38 (95 % CI −0.61 to −0.15; p = 0.002) in VECTOR] [16, 17] and brexpiprazole 2 mg/day than placebo recipients in VECTOR (−0.33; 95 % CI −0.56 to −0.10; p = 0.006) [16]; no significant difference was observed between brexpiprazole 2 mg/day and placebo recipients in BEACON (−0.19; 95 % CI −0.42 to +0.05) [17]. In LIGHTHOUSE, recipients of brexpiprazole 2–4 mg/day had a greater change in CGI-S score than placebo recipients (Table 1) [18].
The changes from baseline in PANSS positive and negative subscale scores were significantly greater with brexpiprazole 2 mg/day (in VECTOR) [16] and 4 mg/day (in both BEACON and VECTOR) than with placebo [16, 17], but brexpiprazole 2 mg/day recipients did not significantly differ from placebo recipients in these endpoints in BEACON (Table 1) [17]. While CGI-I scores were significantly lower with both brexpiprazole dosages than with placebo in both BEACON and VECTOR [16, 17], and response rates were significantly higher with both dosages in VECTOR [16], response rate was only significantly greater with brexpiprazole 4 mg/day than placebo in BEACON (Table 1) [17]. When response rate was defined as a ≥30 % reduction from baseline in PANSS total score or a CGI-I score of 1 or 2, the number needed to treat for improvement was 15 [17] and 6 [16] for brexpiprazole 2 mg/day and 6 [17] and 7 [16] for brexpiprazole 4 mg/day. A pooled analysis of brexpiprazole 2 and 4 mg/day across BEACON and VECTOR yielded response rates of 45.5 versus 31.0 % for brexpiprazole versus placebo, with a number need to treat (NNT) of 7 [23]. Other definitions of response elicited NNTs of 14–22 [17] and 6–7 [16] for brexpiprazole 2 mg/day and 6–8 [16, 17] for brexpiprazole 4 mg/day.
In BEACON, Personal and Social Performance (PSP) scale scores were improved to a significantly greater extent than with placebo (+8.52) with brexpiprazole 4 mg (+13.11 [p = 0.0005]) but not with 2 mg (+10.52), while in VECTOR, PSP scale scores were improved to a significantly greater extent than with placebo (+10.26) with brexpiprazole 2 mg (+13.15 [p = 0.03]) but not with 4 mg (+12.72); baseline scores were 43.7, 44.7 and 43.7 [17] and 45.4, 45.3 and 45.1 [16] for brexpiprazole 2 and 4 mg/day and placebo recipients, respectively. Neither dosage of brexpiprazole was associated with a significant difference from placebo in the number of discontinuations due to lack of efficacy in BEACON [17]; however, recipients of brexpiprazole 4 but not 2 mg/day were significantly (p = 0.02) less likely than placebo recipients to discontinue treatment as a result of lack of efficacy in VECTOR [16].
Brexpiprazole 2 mg/day in VECTOR and 4 mg/day in both BEACON and VECTOR were associated with a significantly (p < 0.05) greater improvement than placebo in PANSS excited component score and PANSS Marder scores for the negative, disorganized thought and uncontrolled hostility/excitement factors [16, 17], and both dosages in VECTOR but neither in BEACON were associated with significantly greater improvement than placebo in PANSS Marder positive factor scores [16, 17]. Both brexpiprazole dosages were associated with a significantly (p < 0.05) greater improvement than placebo in PANSS Marder anxiety/depression factor scores in BEACON [17] but neither were significantly different from placebo in VECTOR [16].
In an exploratory, open-label, phase III trial, patients with an acute relapse of schizophrenia who would benefit from hospitalization or continued hospitalization were randomized to 6 weeks’ treatment with brexpiprazole 1–4 mg/day (target dosage 3 mg/day; n = 64) or aripiprazole 10–20 mg/day (target dosage 15 mg/day; n = 33) [20]. At week 6, both treatment groups had experienced a significant decrease from baseline in mean PANSS total score (−22.9 and −19.4; both p < 0.0001 vs. baseline) [baseline scores 94.1 and 93.3, respectively). The study was not powered for a between-treatment comparison [20].
4.2 Maintenance Treatment of Schizophrenia
A randomized, double-blind, placebo-controlled, phase III study investigated the efficacy of 52 weeks’ maintenance treatment with brexpiprazole 1–4 mg/day versus placebo in patients with schizophrenia [24]. The study consisted of three phases: in phase A, adult patients with a ≥3-year history of schizophrenia and a history of relapse and/or symptom exacerbation in the absence of antipsychotic treatment, and who were experiencing a current acute exacerbation (PANSS total score >80), were cross-titrated from their current antipsychotic treatment to brexpiprazole and/or underwent a washout period for contraindicated medication (if required) over 1–4 weeks before moving to phase B, where they underwent single-blind, oral stabilization on brexpiprazole for 12–36 weeks (n = 464). Patients who, for 12 consecutive weeks, were outpatients, had a PANSS total score of ≤70, lacked specific psychotic symptoms on the PANSS (scored ≤4 on each of: conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content), had a CGI-S total score ≤4, and showed no suicidal behavior or violent/aggressive behavior resulting in injury or property damage, and who had a stable dosage of brexpiprazole for the last 4 weeks, continued to phase C, where they were randomized to 52 weeks’ treatment with brexpiprazole (n = 97) or placebo (n = 105) [24].
The primary endpoint was the time to exacerbation of psychotic symptoms or impending relapse, defined as any of: a CGI-S score ≥5 plus increased score to >4 on PANSS conceptual disorganization, suspiciousness, hallucinatory behavior, or unusual thought content (with an absolute increase of ≥2 on that item or ≥4 on the combined four items); hospitalization as a result of worsened psychotic symptoms; suicidal behaviour; or violent or aggressive behaviour resulting in injury or damage [24]. No significant differences in patient characteristics between treatment groups were evident at baseline. The time since first diagnosis was ≈12 to 14 years.
The results presented here are from a pre-specified interim analysis after 50 % of impending-relapse events; as efficacy was demonstrated to a satisfactory significance level at this interim analysis, the study was terminated early [24].
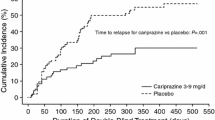
Maintenance treatment with brexpiprazole 1–4 mg/day was associated with a significantly longer time to exacerbation or impending relapse than placebo (primary endpoint) [hazard ratio 0.292; p < 0.0001] [24]. At 52 weeks, 13.5 % of brexpiprazole and 38.5 % of placebo recipients met criteria for exacerbation or impending relapse (p < 0.0001) [24]. A post-hoc analysis calculated an NNT of 4 for this endpoint [23].
Brexpiprazole was also associated with a significantly lower PANSS total score than placebo across all timepoints (treatment difference −4.42; 95 % CI −7.01 to −1.82; p = 0.0011) and a significantly greater improvement from baseline to week 52 in PSP total score (treatment difference +4.8; 95 % CI 1.3–8.2; p = 0.0071) and in Global Assessment of Functioning scale score (+6.6; 95 % CI 3.3–9.8; p = 0.0001) [24].
5 Tolerability of Brexpiprazole
Oral brexpiprazole 1–4 mg/day was well tolerated in patients with schizophrenia in randomized, phase III clinical trials [5, 16–18, 20, 24]. In BEACON, 57, 59 and 63 % of brexpiprazole 1, 2 and 4 mg/day versus 55 % of placebo recipients had at least one adverse event, 3, 2 and 2 versus 5 % had at least one serious adverse event (mostly indicative of underlying schizophrenia), and 9, 6 and 7 versus 12 % discontinued treatment as a result of (mostly psychiatric) adverse events [17]. Corresponding rates in VECTOR were 57 and 57 % of brexpiprazole 2 and 4 mg/day versus 62 % of placebo recipients, 2 and 1 versus 4 % (mostly psychiatric), and 8 and 9 versus 17 %, respectively, and no deaths occurred during the study [16]. The most common adverse events with brexpiprazole 1, 2 or 4 mg/day in acute schizophrenia, from a pooled analysis of BEACON and VECTOR, are presented in Fig. 1 [5]. In BEACON and VECTOR, suicidality had a low incidence, which was similar across all treatment groups [16, 17]. The incidence of adverse events in LIGHTHOUSE was 54 % of brexpiprazole 2–4 g/day and 55 % of placebo recipients [18].
Adverse events occurring in ≥2 % of brexpiprazole 1–4 mg/day recipients with a greater incidence than in placebo recipients in patients with acute schizophrenia in a 6-week, pooled analysis of BEACON and VECTOR [5]. CPK creatine phosphokinase
In the 52-week maintenance study, 43 % of brexpiprazole and 56 % of placebo recipients experienced at least one adverse event, and 5 and 12 % discontinued as a result of adverse events [24]. The most common (incidence ≥5 % in either group) adverse events were headache (6 vs. 10 %), insomnia (5 vs. 8 %), nasopharyngitis (3 vs. 7 %), schizophrenia (3 vs. 7 %) and psychotic disorder (1 vs. 6 %) [24].
In a pooled analysis of two open-label, noncomparative, 52-week extension studies (n = 813), 59 % of brexpiprazole 1-6 mg/day recipients experienced at least one adverse event and 16 % discontinued treatment as a result of adverse events [25]. Most adverse events were of mild or moderate severity, and the incidence of activating (akathisia, insomnia, anxiety, restlessness) and sedating (sedation, somnolence) adverse events was low. The most common adverse events (incidence ≥5 % in either group) were schizophrenia (12 %), insomnia (9 %), increased body weight (7 %), headache (6 %) agitation (5 %) and akathisia (5 %) [25].
Akathisia onset mainly occurred in the first 3 weeks of treatment, and no patients discontinued treatment as a result of akathisia [16, 17]. All instances of akathisia were of mild or moderate severity [26]. Excluding akathisia, the incidence of extra-pyramidal symptom-related adverse events was 5 % of brexpiprazole and 4 % of placebo recipients [5]. The mean changes from baseline in score on the Simpson Angus Rating Scale for extrapyramidal symptoms, the Barnes Akathisia Scale for akathisia, and the Abnormal Involuntary Movement Scale for dyskinesia were similar between brexpiprazole and placebo recipients [5].
Atypical antipsychotic use has been associated with metabolic changes (including hyperglycaemia, diabetes mellitus, dyslipidemia and body-weight gain); while moderate weight gain was observed with brexpiprazole treatment, no clinically relevant changes in lipid profiles or other metabolic parameters occurred in the short-term studies [5]. In a pooled analysis of BEACON and VECTOR, similar proportions of patients in brexpiprazole and placebo groups experienced shifts in fasting glucose levels from normal/borderline to high [5]. Fasting total, low-density and high-density cholesterol changed to similar degrees, regardless of treatment group [5]. Increases in body weight of +1.0, +1.2 and +1.2 versus +0.2 kg were observed in brexpiprazole 1, 2 and 4 mg/day versus placebo recipients, respectively, and potentially clinically relevant (≥7 %) increases occurred in 10, 11 and 10 versus 4 % of patients [5].
Of patients in the long-term, open-label studies with normal or borderline baseline fasting glucose levels, 8 and 17 % experienced a shift to high levels, respectively, with brexpiprazole treatment [5]. Of patients with normal triglyceride levels at baseline, 13 % experienced shifts to high and 0.4 % to very high levels with 52-weeks’ treatment. Long-term, 0.6 % of brexpiprazole recipients discontinued treatment as a result of weight gain; mean increase in body weight was 1.3 kg at week 26 and 2.0 kg at week 52, and potentially clinically relevant increases in body weight were observed in 20 %. A total of 10 % of patients experienced a ≥7 % decrease in body weight [5].
Increases to abnormal prolactin levels occurred in similar, low proportions of brexpiprazole and placebo recipients in BEACON and VECTOR [16, 17].
The US prescribing information (PI) contains a boxed warning about an increased risk of suicidal thoughts and behaviours in patients aged ≤24 years receiving antidepressants (the efficacy and safety of brexpiprazole has not been established in paediatric patients) [5]. It also states that antipsychotic drug use has been associated with neuroleptic malignant syndrome, tardive dyskinesia, leukopenia, neutropenia, agranulocytosis, body-temperature dysregulation, dysphagia and dystonia. Brexpiprazole, like other antipsychotic drugs, may cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure [5].
6 Dosage and Administration of Brexpiprazole
Oral brexpiprazole is indicated in the USA for the treatment of schizophrenia [5]. The US PI recommends initiating treatment at a dosage of 1 mg/day on days 1–4, titrating through 2 mg/day (on days 5–7) up to a maximum dosage of 4 mg/day, based on clinical response and tolerability, with a recommended dosage range of 2–4 mg/day. Brexpiprazole can be taken with or without food [5].
Patients with moderate to severe hepatic impairment or moderate to end-stage renal impairment should receive a maximum brexpiprazole dosage of 3 mg/day (Sect. 3) [5]. The dosage should be halved in patients who are known CYP2D6 poor metabolizers or receiving concomitant strong CYP2D6 or CYP3A4 inhibitors, quartered in patients who are known CYP2D6 poor metabolizers and receiving concomitant strong/moderate CYP3A4 inhibitors or in patients receiving concomitant strong/moderate CYP2D6 inhibitors and strong/moderate CYP3A4 inhibitors, and doubled in patients receiving concomitant strong CYP3A4 inducers (Sect. 3) [5]. Local prescribing information should be consulted for detailed information, including contraindications, precautions, drug interactions and use in special patient populations.
7 Current Status of Brexpiprazole in Schizophrenia
Oral brexpiprazole was approved in 2015 in the USA for the treatment of schizophrenia. Brexpiprazole 2–4 mg/day was generally effective at improving PANSS total, positive and negative scores, as well as CGI-S scores and rates of response, in patients with acute schizophrenia in short-term, phase III studies (Sect. 4.1). The effect sizes reported in VECTOR are both within the range of effect sizes observed with other antipsychotic drugs in a meta-analysis of 212 studies involving 15 drugs [27]. Maintenance treatment with brexpiprazole 1–4 mg/day was associated with a significantly longer time to exacerbation of disease or impending relapse than placebo (Sect. 4.2). The drug was well tolerated in clinical trials, with most serious adverse events in the short term being associated with the underlying disorder (Sect. 5). Brexpiprazole is associated with moderate weight gain.
As yet, no direct comparisons between brexpiprazole and other antipsychotics in patients with schizophrenia have been undertaken, although some results from studies with other antipsychotic treatments as active references are available (Sect. 4.1). Trials powered for head-to-head comparisons between brexpiprazole and other antipsychotics, particularly aripiprazole, would be of great interest in the future.
Additionally, clinical trials (including head-to-head comparisons with other antipsychotics) that specifically investigate the effect of brexpiprazole on cognitive function in patients with schizophrenia would be of interest, particularly as brexpiprazole, but not aripiprazole, appeared to improve cognitive dysfunction in animal models. Cognitive-function outcomes were not reported for the phase III clinical trials. Trials including patients with common comorbid conditions would also be of interest. For example, trials including patients with a recent history of substance abuse, as patients with schizophrenia had a higher prevalence of cigarette (72 vs. 29 % smoked daily for >1 month), marijuana (43 vs. 18 % smoked marijuana >21 times in a year), heavy alcohol (28 vs. 8 % often drank >4 alcoholic drinks in a day) and recreational drug use (35 vs. 12 % had used drugs >10 times) than the general population in a large cohort study [28].
In conclusion, oral brexpiprazole demonstrated clinical efficacy and was well tolerated in well-designed phase III trials in patients with schizophrenia, and is a useful treatment option in this indication.
Data selection sources:
Relevant medical literature (including published and unpublished data) on brexpiprazole was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 26 February 2016], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Brexpiprazole, Lu-AF41156, OPC-34712, Rexulti, schizophrenia, schizophrenias, schizophrenic.
Study selection: Studies in patients with schizophrenia who received brexpiprazole. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Zajdel P, Partyka A, Marciniec K, et al. Quinoline- and isoquinoline-sulfonamide analogs of aripiprazole: novel antipsychotic agents? Future Med Chem. 2014;6(1):57–75.
National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: the NICE guideline on treatment and management (cg178). 2014. http://www.nice.org.uk/. Accessed 2 Mar 2016.
Citrome L. Brexpiprazole: a new dopamine D2 receptor partial agonist for the treatment of schizophrenia and major depressive disorder. Drugs Today. 2015;51(7):397–414.
Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879–911.
Otsuka Pharmaceutical Co Ltd. Prescribing Information for Rexulti® (brexpiprazole tablets). 2015. http://www.accessdata.fda.gov. Accessed 2 Mar 2016.
McKeage K. Adjunctive brexpiprazole: a review in major depressive disorder. CNS Drugs. 2016. doi:10.1007/s40263-016-0320-0.
Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589–604.
Oosterhof CA, El Mansari M, Blier P. Acute effects of brexpiprazole on serotonin, dopamine, and norepinephrine systems: an in vivo electrophysiologic characterization. J Pharmacol Exp Ther. 2014;351(3):585–95.
Goff DC. Brexpiprazole: a new antipsychotic following in the footsteps of aripiprazole. Am J Psychiatry. 2015;172(9):820–1.
Ishima T, Futamura T, Ohgi Y, et al. Potentiation of neurite outgrowth by brexpiprazole, a novel serotonin-dopamine activity modulator: a role for serotonin 5-HT1A and 5-HT2A receptors. Eur Neuropsychopharmacol. 2015;25(4):505–11.
Maeda K, Lerdrup L, Sugino H, et al. Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):605–14.
Lerdrup L, Arnt J, Maeda K, et al. Brexpiprazole reduces impulsive behaviour of rats in a delayed discounting test and 5-choice-serial-reaction-time-test [abstract no. 733]. Biol Psychiatry. 2015;77(9 Suppl 1):273S–4S.
Yoshimi N, Fujita Y, Ohgi Y, et al. Effects of brexpiprazole, a novel serotonin-dopamine activity modulator, on phencyclidine-induced cognitive deficits in mice: a role for serotonin 5-HT1A receptors. Pharmacol Biochem Behav. 2014;124(Suppl C):245–9.
Yoshimi N, Futamura T, Hashimoto K. Improvement of dizocilpine-induced social recognition deficits in mice by brexpiprazole, a novel serotonin-dopamine activity modulator. Eur Neuropsychopharmacol. 2015;25(3):356–64.
Otsuka Pharmaceutical Co Ltd. Study to evaluate the efficacy, safety, and tolerability of oral OPC-34712 and aripiprazole for treatment of acute schizophrenia [NCT00905307]. 2015. http://www.clinicaltrials.gov. Accessed 2 Mar 2016.
Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870–80.
Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1–3):127–35.
Marder SR, Hakala MJ, Gislum M, et al. An interventional, multicenter, randomized, double-blind, placebo-controlled, active reference, flexible dose study of brexpiprazole in adults with acute schizophrenia [abstract]. In: 24th European Congress of Psychiatry; 2016.
H. Lundbeck A/S. Brexpiprazole in patients with acute schizophrenia [NCT01810380]. 2015. http://www.clinicaltrials.gov. Accessed 22 Feb 2016.
Citrome L, Ota A, Nagamizu K, et al. The effect of brexpiprazole (OPC-34712) versus aripiprazole in adult patients with acute schizophrenia: an exploratory study [abstract no. 560]. In: 70th Society of Biological Psychiatry Annual Meeting; 2015.
Weiss C, Zhang P, Hakala MJ, et al. Efficacy and safety of brexpiprazole in schizophrenia: meta-analysis of three double-blind, randomized, placebo-controlled phase 3 studies [abstract]. In: 24th European Congress of Psychiatry; 2016.
Skuban A, Correll CU, Kane JM, et al. Efficacy of brexpiprazole (OPC-34712) in acute schizophrenia: results of two pooled pivotal studies [abstract no. 2089994]. In: 15th International Congress on Schizophrenia Research; 2015.
Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978–97.
Hobart M, Ouyang J, Forbes A, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study [abstract no. 70]. In: American Society of Clinical Psychopharmacology annual meeting; 2015.
Correll CU, Skuban A, Ouyang J, et al. Long-term safety of brexpiprazole (OPC-34712) in schizophrenia: results from two 52-week open-label studies [abstract no. 2089877]. In: 15th International Congress on Schizophrenia Research; 2015.
Eramo A, Skuban A, Ouyang J, et al. Incidence, onset, duration and severity of akathisia with brexpiprazole (OPC-34712) in acute schizophrenia: a pooled analysis of two pivotal studies [abstract no. 78]. In: The American Society of Clinical Psychopharmacology Annual Meeting; 2015.
Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62.
Hartz SM, Pato CN, Medeiros H, et al. Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry. 2014;71(3):248–54.
Acknowledgments
During the peer review process, the manufacturer of brexpiprazole was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Karly P. Garnock-Jones is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: M. Shumway, Department of Psychiatry, University of California, San Francisco/San Francisco General Hospital, San Francisco, CA, USA; J. Volavka, Department of Psychiatry, New York University School of Medicine, Big Sky, MT, USA.
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P. Brexpiprazole: A Review in Schizophrenia. CNS Drugs 30, 335–342 (2016). https://doi.org/10.1007/s40263-016-0325-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-016-0325-8