Abstract
Atomoxetine was first licensed to treat attention-deficit/hyperactivity disorder (ADHD) in children and adolescents in the US in 2002. The aim of this paper is to comprehensively review subsequent publications addressing the efficacy of atomoxetine in 6- to 18-year-olds with ADHD. We identified 125 eligible papers using a predefined search strategy. Overall, these papers demonstrate that atomoxetine is an effective treatment for the core ADHD symptoms (effect sizes 0.6–1.3, vs. placebo, at 6–18 weeks), and improves functional outcomes and quality of life, in various pediatric populations with ADHD (i.e., males/females, patients with co-morbidities, children/adolescents, and with/without prior exposure to other ADHD medications). Initial responses to atomoxetine may be apparent within 1 week of treatment, but can take longer (median 23 days in a 6-week study; n = 72). Responses often build gradually over time, and may not be robust until after 3 months. A pooled analysis of six randomized placebo-controlled trials (n = 618) indicated that responses at 4 weeks may predict response at 6–9 weeks, although another pooled analysis of open-label data (n = 338) suggests that the probability of a robust response to atomoxetine [≥40 % decrease in ADHD–Rating Scale (ADHD-RS) scores] may continue to increase beyond 6–9 weeks. Atomoxetine may demonstrate similar efficacy to methylphenidate, particularly immediate-release methylphenidate, although randomized controlled trials are generally limited by short durations (3–12 weeks). In conclusion, notwithstanding these positive findings, before initiating treatment with atomoxetine, it is important that the clinician sets appropriate expectations for the patient and their family with regard to the likelihood of a gradual response, which often builds over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Using a predefined search strategy, we identified a total of 125 papers from over a decade of clinical research of atomoxetine. Overall, these papers demonstrate that atomoxetine is an effective treatment for the core attention-deficit/hyperactivity disorder (ADHD) symptoms, and improves functional outcomes and quality of life, in various pediatric populations (i.e., males/females, patients with various co-morbidities, children/adolescents, and with/without prior exposure to other ADHD medications). |
Responses to atomoxetine often build gradually over time, and may not be robust until after 3 months. |
Before initiating treatment with atomoxetine in children/adolescents, it is paramount that clinicians set realistic expectations for the patient and their family with regard to the likelihood of a relatively gradual response that often builds over time. We believe that this approach may increase the chances of attaining successful outcomes with atomoxetine treatment in the long term. |
1 Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder that often has a major negative impact on social functioning [1–4], academic attainment [5, 6], risk of criminal behavior [5, 7], job prospects and finances [6, 8], and has been estimated to affect over 5 % of children/adolescents worldwide [9–12].
Atomoxetine was the first non-stimulant medication to be approved for ADHD, to treat patients of 6 years and older; marketing authorization was first granted in the US in 2002, followed by various other countries, including the UK in 2004. The non-stimulants guanfacine and clonidine are now also licensed to treat ADHD in the US [13]. Before these approvals, psychostimulants such as methylphenidate and amphetamines were the only licensed medications for ADHD. However, while a range of stimulants are available, including several long-acting formulations, about 10–30 % of patients may not respond to or be able to tolerate stimulant treatment and, unlike atomoxetine, their use can be limited by their abuse potential [14–16]. Thus, some patients/families may be reluctant to use stimulant medications. Also, some patients cannot use stimulants due to contraindications.
Between 2001 and 2005, the manufacturer of atomoxetine, Eli Lilly, published the results of six randomized, placebo-controlled core registration studies for atomoxetine in children/adolescents, all performed in North America. In these pediatric patients, the six clinical trials demonstrated to healthcare professionals and licensing authorities that, when given for 6–9 weeks, atomoxetine is an effective treatment for the core symptoms of ADHD, relative to placebo [17–21]. In addition, these registration studies also provided other clinically relevant information, including dosing schedules, functional outcomes, data for subpopulations of patients such as older children/adolescents only, and the time to onset of improvement following atomoxetine treatment. While these registration studies for atomoxetine were extremely informative, providing baseline expectations for treatment response, early experiences of this novel treatment in the clinical setting suggested that subsequent data were needed to enhance understanding of the pharmacodynamics and clinical effects of the medication. Indeed, the registration studies may not have been adequate to set appropriate expectations for atomoxetine treatment. This may be particularly related to their short study durations, which were not sufficient to fully describe the pattern of response over time, and therefore may not have helped clinicians to optimize treatment. Thus, over the decade that followed the launch of atomoxetine, many additional studies were performed by Eli Lilly and independent researchers to further investigate atomoxetine treatment in relation to outcomes that were included in the registration studies but which warranted further investigation. For instance, several prospective studies [randomized controlled trials (RCTs) or open-label] and retrospective analyses have focused on the effect of atomoxetine over durations longer than 9 weeks, the effects of different atomoxetine dosing schedules, and outcomes in various patient subgroups (e.g., single-sex cohorts, patients with ADHD and a particular co-morbidity, or only treatment-naïve patients). However, to the best of our knowledge, this extensive body of atomoxetine efficacy data from RCTs and non-randomized analyses has not yet been comprehensively reviewed to provide a summary of the key clinical implications, although a systematic review of atomoxetine data from 2009 to 2011 has previously been published [22], and efficacy and safety data for atomoxetine monotherapy in 25 RCTs has been meta-analyzed [23].
The objective of this comprehensive review is to help organize the available data from over a decade of clinical research, so that it can be appropriately critiqued and discussed, with the overall aim being to enhance healthcare professionals’ knowledge and understanding of the efficacy of atomoxetine as a treatment for ADHD in children and adolescents. Therefore, in this paper we have summarized the evidence relating to the efficacy of atomoxetine that was initially established in the six registration studies, and what we have subsequently learnt from the ensuing decade of atomoxetine research. We will review safety data in a separate publication.
2 Literature Search Strategy
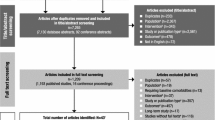
We used a predefined search strategy to identify literature examining the efficacy of atomoxetine for the treatment of ADHD in children and adolescents in clinical trials and real-life studies. The predefined search strategy was comprised of three stages:
-
Stage 1 As shown in Fig. 1, three bibliographic databases were searched.
-
Stage 2 Using the lists of titles and abstracts of publications electronically identified in the database searches, two authors (JKB and NCS) acted as independent reviewers, manually screening the titles and the abstracts of each publication (but not the main body of each paper) against predefined exclusion criteria, and then against predefined inclusion criteria (see Fig. 1). If there was any doubt about whether a paper should be excluded based on the contents of the title and abstract, the inclusion/exclusion decision was finalized by checking the main body of the paper. The two reviewers compiled their own list of eligible publications, and then consolidated their lists by discussing any discrepancies, resolved by means of a consensus. It was planned that a third independent reviewer (DC) would adjudicate if no consensus was reached, although this was in fact not needed.
-
Stage 3 From the consolidated list of papers selected during Stage 2, the two reviewers manually grouped each paper into one or more of ten topics of clinical relevance, based on the efficacy of atomoxetine, which were defined before selecting literature from the searches (see Fig. 1). In addition to needing to be related to efficacy, the rationale for choosing the ten predefined topics was that each topic had been addressed to some extent in one or more of the six registration studies [17–21] and, as such, is predominantly covered by the licenses granted for the clinical use of atomoxetine. This was also why the lower age cut-off point was set at 6 years old. While atomoxetine treatment data are available for children younger than 6 years of age, atomoxetine has been approved to treat ADHD in patients of 6 years and older.
This article was written using the ten predefined topics as subheadings, with all the papers selected during Stage 3 referred to under the appropriate subheading(s). Another 42 documents [1–13, 15, 16, 22–46, 136, 154], not selected via the predefined search strategy, were included in this review to provide relevant background information and discussion of the selected literature. These included the European Summary of Product Characteristics (SPC) [45] and a US Full Prescribing Information document [46] for atomoxetine. In addition, one paper in which patients had ADHD and substance use disorder (SUD) was included because of the importance of SUD and the lack of other atomoxetine studies addressing SUD in young patients, even though the inclusion criteria were not completely met because patients were admitted who were 19 years of age [47].
3 Topics of Clinical Relevance for the Efficacy of Atomoxetine
As shown in Fig. 1, using the three-stage approach, a total of 819 papers were identified in the electronic literature searches (Stage 1). Of these, 127 papers met none of the exclusion criteria and all of the inclusion criteria (Stage 2). Overall, 125 papers were then included in the review, in at least one of the ten predefined topics of clinical relevance, while two papers were excluded due to a lack of relevant clinical data in relation to the ten predefined topics (Stage 3). The number of papers that was identified for each predefined topic during Stage 3, and their citations, are shown in Table 1.
3.1 Mode of Action and Pharmacokinetics
The pathophysiology of ADHD appears to involve dysregulation of noradrenergic and dopaminergic pathways that are associated with modulation of higher cortical functions, including attention and executive functioning [24, 25]. Atomoxetine is a potent and selective inhibitor of the norepinephrine (noradrenaline) transporter. Although the precise mechanism of action is not clear, preclinical studies suggest that atomoxetine exerts its therapeutic effect on ADHD by increasing the concentration of synaptic norepinephrine and dopamine in the prefrontal cortex, but without an increase in dopamine in the nucleus accumbens or striatum [26–30]. The apparent lack of an effect of atomoxetine on dopamine in the mesolimbic system is compatible with the lack of abuse potential of this medication [16]. By contrast, in preclinical studies, medications such as methylphenidate and amphetamine increase dopamine and norepinephrine levels in the prefrontal cortex and striatum [24, 30–34]. The mechanism of action of atomoxetine was not investigated in the six registration studies. Nevertheless, in a clinical study of 36 youths with ADHD [mean (standard deviation; SD) age 11.2 (2.7) years], using functional magnetic resonance imaging, 6–8 weeks of atomoxetine (n = 18) or methylphenidate (n = 18) treatment were associated with improvements in ADHD symptoms “via both common and divergent neurophysiologic actions in frontoparietal regions that have been implicated in the pathophysiology of ADHD” [48].
The two proof-of-concept atomoxetine registration studies reported by Spencer et al. [17] were the first to demonstrate that a medication working on the norepinephrine transporter may be effective in the treatment of ADHD. Since then, this has been taken a stage further, to determine how genetic polymorphisms in the noradrenergic pathway may impact efficacy outcomes of atomoxetine treatment [49, 50]. In a recent study of 111 pediatric patients with ADHD, mutations in the SLC6A2 and ADRA2A genes were associated with altered responses to atomoxetine after 8–12 weeks of treatment [50]. Specifically, patients with the C allele of the rs3785143 mutation in the SLC6A2 gene were more likely to be responders, and those with the T allele were more likely to be non-responders (p = 0.0048)Footnote 1, and the GG haplotype of rs1800544 and rs553668 in the ADRA2A gene was associated with non-remission (p = 0.0219).Footnote 2 The SLC6A2 gene encodes a sodium-dependent norepinephrine transporter, and the ADRA2A gene encodes the α2A-adrenergic receptor.
The rate at which atomoxetine is metabolized by the cytochrome P450 (CYP) 2D6 enzyme pathway may also affect the efficacy of atomoxetine, as shown in a registration study and in a pooled analysis of data from four registration studies by Michelson et al. [18, 51]. About 7 % of Caucasian people may be “poor metabolizers”, as suggested by a German study of 589 individuals who received one of two drugs (dextromethorphan or debrisoquine) that are metabolized by CYP2D6 [35]. In the pooled analysis by Michelson et al. [51], poor metabolizers (n = 30) were more likely than extensive metabolizers (n = 559) to experience greater efficacy when treated with similar doses of atomoxetine. However, as this was accompanied by greater cardiovascular tone and greater prevalence of several adverse events (e.g., tremor, decreased appetite), the European atomoxetine SPC states that “for patients with a known poor metabolizer genotype, a lower starting dose and slower up-titration of the dose may be considered” [45]. In the Michelson et al. [18] registration paper, the authors speculated that poor metabolizers may experience greater efficacy either because of a “longer plasma half-life (more constant and consistent receptor blockade) or higher drug exposures”. Conversely, in a post hoc analysis of another study, involving patients with co-morbid ADHD/oppositional defiant disorder (ODD), it was found that higher atomoxetine plasma concentrations were not indicative of improvements in ODD and ADHD at 12 weeks of treatment, thus leading to the suggestion that “other factors are involved in determining therapeutic response to atomoxetine” [52]. Indeed, it may be the case that neuroadaptation occurs within the brain as a consequence of long-term exposure to atomoxetine, as suggested by several molecular changes in rats that included a reduction in the level of the norepinephrine transporter [43]. Hence, plasma concentrations of atomoxetine may not always correlate with the presentation of symptoms. In pediatric patients, we postulate that the relatively gradual onset of atomoxetine efficacy [53–55], and the gradual relapse of ADHD symptoms that occur upon discontinuation of atomoxetine treatment [56], may be indicative of neuroadaptation. However, it is also possible that the gradual relapse of ADHD symptoms after discontinuing atomoxetine treatment is attributable to patients having gained better skills to cope with their symptoms.
3.2 Dose
Since dosing schedules and dose–response outcomes were first explored in depth in the registration studies by Michelson et al. (a twice-daily dosing, dose-response study; n = 297 [18], and a once-daily dosing study; n = 171 [19]), dose recommendations have not changed for children/adolescents [45, 46]. In the registration studies, once-daily dosing had the same magnitude of response as twice-daily dosing, versus placebo [18, 19, 21, 57]. With regard to once- or twice-daily dosing, and morning versus afternoon/evening dosing, similar advice is issued in the European atomoxetine SPC [45] and US Full Prescribing Information document [46]. The European SPC states that atomoxetine “can be administered as a single daily dose in the morning, with or without food”, although “patients who do not achieve a satisfactory clinical response (tolerability [e.g. nausea or somnolence] or efficacy) … might benefit from taking it as twice daily evenly divided doses in the morning and late afternoon or early evening”. Indeed, as reported in a congress abstract from 2012, in a retrospective review of the referrals of 147 pediatric patients who had failed to respond to once-daily atomoxetine, 75 % appeared to respond to twice-daily dosing [58].Footnote 3 It is also possible that the time of day that once-daily atomoxetine is administered may affect efficacy and tolerability. In a placebo-controlled study (n = 288), while morning and evening dosing of once-daily atomoxetine both resulted in better symptom improvements when measured up to 24 h later, the efficacy of morning dosing was superior to evening dosing, based on greater reductions in ADHD–Rating Scale (ADHD-RS) total score and hyperactivity/impulsivity subscore, and Clinical Global Impressions (CGI)–ADHD–Severity (CGI-ADHD-S) scores. Better tolerability was gained with evening dosing [59].
In addition to the timing of atomoxetine dosing per day, the impact of various other dosing variables were also assessed in the registration studies, including the speed of titration and the level of the maintenance dose. Body weight-based dosing is recommended for children/adolescents up to 70 kg in the European SPC and US Full Prescribing Information for atomoxetine [45, 46]. Based on the registration studies, the European and US labels recommend that atomoxetine should be administered at a starting dose of approximately 0.5 mg/kg/day (for at least 7 days in Europe, and at least 3 days in the USA), and should then be up-titrated to a maintenance dose of approximately 1.2 mg/kg/day, according to clinical response and tolerability [45, 46]. According to the US label, the maximum daily dose of atomoxetine “should not exceed 1.4 mg/kg/day or 100 mg, whichever is less”, while the European SPC states that the safety of atomoxetine doses >1.8 mg/kg/day “have not been systematically evaluated”. In the USA and Europe, the recommendation for children/adolescents above 70 kg is to up-titrate atomoxetine from a daily initiation dose of 40–80 mg, and the daily dose should not exceed 100 mg.
Speed of titration has been explored in recent studies. According to Dittmann et al. [60], slower up-titration (i.e., 0.5 mg/kg/day for 7 days, followed by 0.8 mg/kg/day for 7 days, and then 1.2 mg/kg/day) may be better tolerated than faster up-titration (0.5 mg/kg/day for 7 days, then 1.2 mg/kg/day), although both titration schedules were equally as effective in this 9-week study of 181 children/adolescents with ADHD and co-morbid ODD. Similarly, different switching speeds (2 vs. 10 weeks) from stimulants to atomoxetine (standard target dose 1.2 mg/kg/day) were recently investigated in 6- to 16-year-olds (111 patients); no clinically relevant differences in efficacy measures were seen in this 14-week study [14].
The currently recommended maintenance dose of atomoxetine, 1.2 mg/kg/day, was established through the 8-week dose–response registration study by Michelson et al. [18] (n = 297), in which a dose of 1.2 mg/kg/day was more efficacious than 0.5 mg/kg/day, although 1.2 mg/kg/day appeared to be as effective as 1.8 mg/kg/day. Similar doses had previously been used in a small (n = 10) open-label pilot study [61]. The efficacy of 1.2 mg/kg/day atomoxetine has now been demonstrated for the core ADHD symptoms and functional outcomes in various non-registration studies of children/adolescents of different ages, genders, and ethnicities (e.g., Cardo et al. [14], Dittmann et al. [60], Cho et al. [62], and Takahashi et al. [63]). Similarly, according to Wietecha et al. [64], in patients (n = 178) who previously responded to 8 weeks of atomoxetine (defined as a decrease of ≥1 point on the CGI-ADHD-S), treatment benefit was better maintained over an additional 40-week period with 1.4 mg/kg/day, the maximum recommended maintenance dose of atomoxetine in the USA, than with atomoxetine 0.8 mg/kg/day. Conversely, in patients (n = 229) who had a robust response to atomoxetine (defined as ≥40 % reduction in ADHD-RS total score), Newcorn et al. [65] reported that it may be possible to sustain the response with low doses of atomoxetine (0.5 mg/kg/day) for 8 months; this is lower than the maintenance dose (approximately 1.2 mg/kg/day) recommended in the European and US labels [45, 46]. The discrepant findings of Wietecha et al. [64] and Newcorn et al. [65] may be due to methodological differences between the studies, such as the aforementioned different definitions of response used in each study, and differences in sample characteristics between the studies.
As in the Michelson et al. [18] registration study, in other studies [52, 65–67] the efficacy of atomoxetine has also been investigated using maintenance doses (up to 3.0 mg/kg/day) that exceed the current recommendations in the European SPC and US Full Prescribing Information [45, 46], and which, therefore, are off-label. Overall, better efficacy outcomes do not appear to be gained with higher doses of atomoxetine [52, 65–67], compatible with the findings of Michelson et al. [18], and similar to the statement in the US Full Prescribing Information that “there are no data that support increased effectiveness at higher doses” [46]. For instance, Kratochvil et al. [67] demonstrated that, in patients (n = 247) who were “non-responders” (defined as “<40 % reduction in ADHD-RS total score” or “ADHD-RS score remained ≥1 SD above age and sex norms”) to a standard dose of atomoxetine over a 6- to 8-week period, persisting with this dose for between 4 weeks and 8 months led to improvements in ADHD symptoms, and no statistically significant advantage was gained by changing to a higher dose of atomoxetine.
3.3 Clinical Responses
Different definitions of clinical response may be used in different trials [e.g., response may be defined as a particular percentage decrease in ADHD-RS total scores, such as ≥25 %, or as any statistically significant decrease versus placebo, or as a CGI-Improvement (CGI-I) score of 1 or 2], thus complicating comparisons between trials. Various clinical response-related issues have been considered in the six registration studies and/or subsequent non-registration studies. These issues are discussed in Sects. 3.3.1–3.3.4.
3.3.1 Time to Clinical Response
Clinical responses may be detected at 1 week of atomoxetine treatment, as shown for changes in mean ADHD-RS total scores versus placebo in the registration study of once-daily atomoxetine by Kelsey et al. [21]. Conversely, in two non-registration studies, initial clinical responses (defined as a CGI-I score of 1 or 2 [68], or a >25 % reduction in ADHD-RS total score [69]) appeared to take appreciably longer; a median of 21 days (95 % CI 15–23; n = 134) in one study [68], while the median of 23 days (95 % CI 13–32; n = 72) was significantly (p < 0.001) faster than the 50 days (95 % CI 32—not estimated; n = 33) with placebo in an RCT [69]. However, in other non-registration studies, clinical responses have been detected much earlier than after 23 days. In a 9-week study in 51 girls by Biederman et al. [70], statistically significant differences in efficacy were apparent between atomoxetine and placebo at the first follow-up visit, 1 week after starting treatment, and this was maintained until the end of the study. In a 24-week study, changes in Global Impression of Perceived Difficulties (GIPD) scores (which reflect the degree of impairment, the level of psychosocial functioning and quality of life [QOL]) were greatest in the first 2 weeks of atomoxetine treatment [71].
3.3.2 Trajectories of Response Over Time, and Long-Term Efficacy
Clinically meaningful improvements in ADHD symptoms are often observed after a few weeks of atomoxetine treatment. For instance, in an open-label study of 604 pediatric patients with ADHD, ADHD-RS total scores decreased by 56.7 % after 10 weeks, such that 69 % of patients had “no or minimal symptoms” [72]. There appears to be some heterogeneity in response rates in geographic regions, although atomoxetine is an effective treatment in all locations (Asia, Europe, North America, and Russia) [40], and responses appear to be similar by ethnic group in the USA, i.e., Caucasian, African American, and Latino [73, 74].
With regard to response rates, the IDEA (Integrated Data Exploratory Analysis) study is a notable pooled analysis of six US studies, including five of the six registration RCTs and one other RCT. In the IDEA study, at 6–9 weeks of atomoxetine treatment (n = 618), 47 % of patients were “much improved” (defined as ≥40 % decrease in ADHD-RS scores), only 13 % of patients were “minimally improved” (25 to <40 % decrease), and the remaining 40 % of patients were “non-responders” (<25 % decrease) [53]. The authors of the IDEA study noted that, at endpoint, “subjects who ultimately achieved a much improved response were likely to be at least minimal responders by week 4” [53]. Specifically, the likelihood of being “much improved” at 6–9 weeks was 75 % for patients who were “at least minimally improved” at 4 weeks and 21 % for those who were “non-responders” at week 4 [53]. This paper [53] and results from other pooled analyses of data from several clinical trials suggest that responses to the first few weeks of treatment may help predict responses over time, including changes in individual ADHD-RS items [75] and, in a conference abstract, health-related QOL (HR-QOL) [76].
Conversely, another pooled analysis of data from three Canadian open-label trials by Dickson et al. [54], involving 338 children with ADHD, suggests that the number of children gaining a “robust improvement” (defined as ≥40 % decrease in ADHD-RS scores) may increase substantially beyond the 6-to 9-week endpoint used in the IDEA study. According to Dickson et al. [54], the probabilities of gaining a robust improvement were 47 % at 4 weeks, 76 % at 12 weeks, 85 % at 26 weeks, and 96 % at 52 weeks. In addition, in two other open-label studies that were reported together [67], for patients who were said to be “non-responders” (defined as “<40 % reduction in ADHD-RS total score” at 8 weeks in Study 1, and as patients “whose ADHD-RS score remained ≥1 SD above age and sex norms” at 6 weeks in Study 2) to a standard dose of atomoxetine, 53 % (32/60 patients in Study 1) and 26 % (15/58 patients in Study 2) responded when persisting with this dose for 8 months and 4 weeks, respectively. Based on these data, it seems likely that a significant proportion of those who only have partial responses, and possibly those considered to be non-responders to atomoxetine at 4 weeks, will go on to have robust responses beyond this timepoint, and thus stopping atomoxetine in these patients at 4 weeks will potentially deny a treatment that could eventually benefit them.
However, caution should be exercised when viewing the data from all of these analyses [53, 54, 72, 75, 76], as methodological details could affect their results. In Dickson et al. [54], the open-label study design could have encouraged clinician bias when estimating improvements, and thus response rates may have been overestimated, although this is unlikely to have affected the overall finding of increased probability of response beyond 4 weeks of atomoxetine treatment. Similarly, in the IDEA [53] and in the Dickson et al. [54] studies, response rates may have been overestimated due to discontinuations: 79.9 % of 618 patients completed atomoxetine treatment (6–9 weeks) in the IDEA study [17–19, 21, 44, 53], and 70.4 % of 338 patients completed the three longer clinical trials (with endpoints at 13, 26, and 52 weeks) in Dickson et al. [44, 54], although only 10.7 % of the 338 patients discontinued due to lack of efficacy [54]. In addition, symptoms were only reported at ≥1-month intervals in the Dickson et al. [54] paper; this may have affected the time-to-effect of atomoxetine. Furthermore, in the Dickson et al. paper [54] a relatively slow upward titration schedule was used: 0.5 mg/kg/day for 10 days, followed by 0.8 mg/kg/day for 10 days, 1.2 mg/kg/day for at least 10 days, and up to 1.4 mg/kg day allowed thereafter in two of the three studies in this pooled analysis. The authors acknowledged that slow titration “may have had some impact on early response rates, but it is unlikely that the dosing of atomoxetine during the initial part of the study impacted the major finding of the study, which was that there is slow but progressive improvement over time during atomoxetine treatment long after titration is complete”.
Long-term efficacy of atomoxetine was not addressed in the six registration studies, which were 6–9 weeks in duration. Once clinical responses to atomoxetine treatment have been established, they can be sustained in the long term, as shown in various post-registration studies, with durations ranging between 9 months and 2 years, in children and adolescents [55, 56, 77–79]. For instance, in a pooled analysis of data from 13 atomoxetine studies (including short-term double-blind or short-term open-label phases, and open-label extension phases), Wilens et al. [55] reported that 219 (36.4 %) of 601 adolescents completed at least 2 years of atomoxetine treatment. Only 16.5 % (99 of 601) adolescents discontinued due to lack of efficacy during this 2-year period, and ADHD-RS total scores decreased from a mean (SD) of 34.7 (10.2) to 14.5 (10.7). Notably, the maximum response to atomoxetine did not appear to be reached until 6 months of atomoxetine treatment, and symptoms remained improved up to 2 years without increasing the dosage (mean dose of atomoxetine at endpoint was 1.41 mg/kg/day) [55].
Atomoxetine has also been shown to be effective at preventing relapse of ADHD symptoms in three clinical studies with initial treatment periods of 10–52 weeks [56, 77, 78] and was not influenced by the presence of co-morbid ODD [78]. Buitelaar et al. [56] reported that for children/adolescents (n = 163) who had responded to treatment with atomoxetine for 1 year, who were then randomized either to atomoxetine or placebo for a further 6 months, atomoxetine was superior to placebo at preventing relapse and maintaining the response. For instance, only two (2.5 %) of 81 patients relapsed with atomoxetine versus 10 (12.2 %) of 82 patients with placebo [the relative risk ratio for relapse was 5.6 (95 % CI 1.2–25.6)]Footnote 4, and the mean (SD) number of days to relapse was 160.5 (0.7) with atomoxetine versus 130.8 (3.1) with placebo (p = 0.008). However, as can be seen by these data, relapse rates with placebo were limited, at least partly due to the study design, e.g., all patients had to be clear clinical responders before randomization to treatment with atomoxetine or placebo. The limited relapse rates with placebo suggest that many patients may stay well for several months without atomoxetine treatment, after being treated with atomoxetine for 1 year. We speculate that for some of these patients this may be due to adaptive changes at the brain level that have stabilizing effects, a hypothesis that is supported by observations of molecular changes in rats [43], although it could also be due to numerous other factors, e.g., it may be the case that patients gained better skills to cope with their symptoms. Indeed, for the patients who were assigned to discontinue atomoxetine in the 1-year study, when symptoms returned they were generally less severe than at study entry [56]. Moreover, the findings of Buitelaar et al. [56] are also compatible with atomoxetine treatment guidelines [45, 46]. Specifically, the European SPC states that treatment with atomoxetine “need not be indefinite. Re-evaluation of the need for continued therapy beyond 1 year should be performed, particularly when the patient has reached a stable and satisfactory response” [45], while the US label states that the physician should “periodically reevaluate the long-term usefulness of the drug for the individual patient” [46].
3.3.3 Maintenance of Clinical Responses to Atomoxetine Throughout the Day
Several studies support the maintenance of clinical responses throughout the day gained from atomoxetine administered once daily in the morning. In the Michelson et al. [19] and Kelsey et al. [21] registration studies, after a single administration in the morning, efficacy ratings in parent diaries suggested that the effects of atomoxetine were sustained late in the day [19, 21] and into the morning of the following day [21]. More recently, in a single report of two almost identical open-label studies of a morning dose of atomoxetine, ADHD-related difficulties, assessed in the mornings and evenings using the Weekly Rating of Evening and Morning Behavior–Revised (WREMB-R) scale, were reduced significantly by week 2, and scores then remained stable to study endpoint (week 24) in pediatric patients (n = 421) [80]. This response was accompanied by improvements in ADHD-related difficulties, when assessed in the morning, during school, during homework, and in the evening using the GIPD scale [80]. Compatible findings were also reported for an 8-week trial when ADHD-related symptoms were measured using a computer-based continuous performance test combined with a motion-tracking device [81].
3.3.4 Clinical Responses to Combined Treatment with Atomoxetine and Methylphenidate
Clinical responses to combined treatment with atomoxetine and methylphenidate have been assessed in an open-label study [82]. Patients who were only partial responders to a minimum of 4 weeks of atomoxetine monotherapy were subsequently treated for 3 weeks with a combination of atomoxetine and osmotic release oral system (OROS) methylphenidate. While statistically significant improvements were detected in ADHD symptoms and functioning, according to the ADHD-RS, CGI-S, and Behavior Rating Inventory of Executive Functioning (BRIEF), unfortunately the lack of a control group makes it unclear whether these improvements were a consequence of adding the methylphenidate treatment or whether they would have occurred anyway through continuation of atomoxetine monotherapy [82].
3.4 Comparator Data
All six registration studies were designed to assess the effectiveness of atomoxetine relative to placebo, but not in relation to other comparators. In the two registration studies by Spencer et al. [17], patients were also randomized to a methylphenidate arm. However, these two proof-of-concept studies were not statistically powered for head-to-head comparison of atomoxetine and methylphenidate; the methylphenidate arm was only included as a means to validate study design in the event that atomoxetine failed to separate from placebo.
The need to know how atomoxetine compares with other treatments has been an area of ongoing clinical research. However, these comparative analyses are often limited by various factors. Most notably, comparisons are usually restricted to very short treatment durations. While patients tend to respond quickly to stimulant medications, responses to atomoxetine tend to build over time and may not be maximal until after 3 months of treatment [55], thereby complicating these comparisons. Indeed, faster responses have been detected with mixed amphetamine salts extended release (MAS XR [83, 84]) and lisdexamfetamine [68, 85], both of which are stimulants, and guanfacine extended release (GXR [86]), versus atomoxetine in studies or meta-analyses that were ≤9 weeks in duration. The comparison of MAS XR with atomoxetine is extremely limited because data were only collected up to 3 weeks after starting treatment [83] and these data were only extrapolated to 3 months [84]. Moreover, two meta-analyses, comparing lisdexamfetamine and GXR with atomoxetine, are limited by indirect comparison as well as short durations [85, 86].
When treated over longer timespans, other studies suggest that atomoxetine is at least as effective as alternative ADHD medications, including stimulants. In a retrospective chart review, after approximately 6 months of treatment with the same medication, patients with ADHD who received atomoxetine (n = 85) “improved at least as well” as patients using stimulants (n = 81) [87]. In addition, in a 1-week study using electronic diaries, in which patients had been treated for at least 2 months, behavioral profiles were similar for children treated with atomoxetine (n = 25) versus stimulants (n = 26), with “indications of better functioning in the ATX group during mornings” [88]. Moreover, over a 12-month period, starting with two groups of 199 patients who received atomoxetine versus any other type of ADHD medication, mainly methylphenidate, improvements in QOL and functional impairment were said to be “meaningful and stable” for each group. Differences between the two groups were described as “small and not considered clinically relevant” [89].
Another possible limitation, which may bias the interpretation of comparator data, is that patients may or may not have previously received treatment with stimulants, and those who had had an inadequate response to stimulant treatment were often excluded from the comparator studies; however, this was not necessarily the case for patients who received atomoxetine [90]. Furthermore, comparator studies may not be statistically powered to reliably detect differences between the various ADHD treatments. To improve statistical power, data from the two atomoxetine registration studies by Spencer et al. [17] have been incorporated into cumulative datasets, in two large meta-analyses of atomoxetine vs. methylphenidate, by Hazell et al. [90] (n = 1,368) and Hanwella et al. [91] (n = 2,762). These meta-analyses indicate that atomoxetine may be comparable to methylphenidate [90, 91], particularly the immediate-release (IR) formulation [91], as a treatment for the core symptoms of ADHD. Hazell et al. [90] included seven RCTs (five double-blind and two open-label, 6–10 weeks’ duration [17, 92–96]) in their meta-analysis, while Hanwella et al. [91] included the same seven studies [17, 92–96] plus two additional open-label RCTs (3 and 12 weeks’ duration [97–99]). In Hazell et al. [90], atomoxetine was found to be non-inferior to methylphenidate, primarily based on 6-week response rates (≥40 % reduction in ADHD-RS total score)Footnote 5, i.e., 53.6 % (95 % CI 48.6–58.4) of patients responded with atomoxetine versus 54.4 % (95 % CI 47.6–61.1) with methylphenidate, while Hanwella et al. [91] did not find a significant difference between atomoxetine and methylphenidate (p = 0.29, effect size difference 0.09, 95 % CI −0.08 to 0.26). Notably, the IR and OROS types of methylphenidate were used in both meta-analyses; in subgroup analyses by Hanwella et al. [91], the effect size [0.32 (95 % CI 0.12–0.53); p < 0.002] favored OROS methylphenidate, while atomoxetine was similar to IR methylphenidate [–0.04 (95 % CI –0.19 to 0.12); p = 0.64]. Also, in a very small cross-sectional study, marginally higher self-esteem appeared to be gained after 6 months using methylphenidate (n = 18) than using atomoxetine (n = 23), based on mean (SD) Self-Esteem Multidimensional Test total scores of 96.39 (12.3) vs. 85.48 (9.9) (p = 0.048) [100]. Accordingly, overall the evidence indicates that the short-term efficacy of atomoxetine is comparable with methylphenidate, particularly the IR formulation.
Of course, in these comparative analyses, it is standard that average responses are compared between whole treatment populations; however, individual ADHD patients do respond differently to different ADHD medications. In addition, choice of medication may be related to the individual patient’s requirements. For example, if a rapid onset of effect is required then stimulants may be more appropriate than atomoxetine, whereas atomoxetine may be appropriate if the treatment effect is required throughout the day, or if abuse of stimulants is suspected, due to the lack of abuse potential associated with atomoxetine [16]. Furthermore, children with ADHD may experience less sleep disturbance with atomoxetine than with methylphenidate [93].
3.5 Functional Outcomes/Quality of Life
HR-QOL is a multidimensional concept, based on self-reported functional outcomes, such as physical, social, and psychological aspects of health. While HR-QOL is largely distinct from ADHD symptoms and objective functional outcomes, HR-QOL closely depends on the patient’s subjectively perceived impact of the disorder and treatment on the level of physical, social, and psychological health.
In the 8-week registration study (n = 297) by Michelson et al. [18], using the Childhood Health Questionnaire (CHQ; a well-validated QOL instrument with subscales for mental health, self-esteem, general behavior, and emotional distress), atomoxetine was superior to placebo at improving social and family functioning. Since this study was published, an extensive number of studies (at least 35 papers) have demonstrated that various functional outcomes and QOL improve in children/adolescents with ADHD following treatment with atomoxetine, using various outcomes measures, both in the short-term (approximately 8–12 weeks) and long-term (6–24 months) [14, 57, 71, 72, 76, 77, 87, 89, 94, 100–125].
With regard to the short-term, in an 8-week non-registration study of 249 children, using the CHQ, atomoxetine-induced improvements in ADHD symptoms were related to improvements in psychosocial and family functioning, and were also related to reductions in the burden of illness on the child and parent [108]. Similarly, in a large pooled analysis of data from 794 patients from five clinical trials, 8–12 weeks in duration, QOL [based on the Child Health and Illness Profile, Child Edition (CHIP-CE)] was shown to correlate with improvements in the core ADHD symptoms [107]. Also, in 564 atomoxetine-treated patients, using the Life Participation Scale for ADHD–Child Version, improvements in ADHD symptoms correlated with improvements in social and behavioral functioning [114].
In a 24-week study, using the GIPD instrument, which reflects overall impairment, psychosocial functioning, and QOL, 6- to 11-year-old ADHD patients’ QOL appeared to improve over time using atomoxetine (n = 262) [116]. In another 24-week study in 159 adolescents, GIPD scores, self-esteem, and ADHD symptoms improved over time [71, 105]. Two other sizable, long-term studies are also of note. In a global multicenter study of 416 children and adolescents who responded to an initial 12-week period of treatment with atomoxetine, 9 months after being randomized to continued atomoxetine treatment or placebo, these atomoxetine-treated patients had better psychosocial functioning [77]. In the other study, involving 912 children and adolescents over a 24-month period, improvements in QOL, measured using the CHQ, that were gained via atomoxetine treatment were generally stable over time [111].
Improvements in functional outcomes and QOL have also been demonstrated in patients with ADHD and co-morbid conditions such as ODD following 8–9 weeks of treatment with atomoxetine [101, 102, 115, 117, 118].
Accordingly, a large body of clinical evidence suggests that atomoxetine has an impact on QOL as well as on the core ADHD symptoms. However, it must be acknowledged that ADHD symptom measures do not always correlate well with QOL, e.g., as shown by Wehmeier et al. [124], presumably because patients have other aspects of their lives, in addition to their ADHD, that affect QOL.
3.6 Response to Atomoxetine After Previous Exposure to Other Treatments, or in Treatment-Naïve Patients
The six registration studies for atomoxetine all included patients with prior exposure to ADHD treatments, but did not study the effectiveness of atomoxetine in these patients as a subgroup. Given that atomoxetine and psychostimulant medications have somewhat different mechanisms of action [24, 26–30], it is perhaps not surprising that several papers (at least eight) now indicate that pediatric ADHD patients who fail to respond adequately to stimulants, or fail to tolerate stimulants, including methylphenidate and amphetamine, may benefit from switching to atomoxetine treatment [14, 58, 68, 96, 126–129]. For instance, in a recent study of 134 patients who had previously responded inadequately to methylphenidate, 63.6 % responded to atomoxetine treatment 9 weeks after switching to atomoxetine [68]. Conversely, in a study by Niederkirchner et al. [130], subsequent treatment with OROS methylphenidate in 42 patients who did not tolerate or had an insufficient response to atomoxetine was associated with improved ADHD symptoms and HR-QOL.
In addition to lack of response with medications, atomoxetine may be a viable alternative following inadequate responses to non-pharmacological treatments. In a study of 156 patients with co-morbid ADHD/ODD, only two patients responded to parental support (defined as “parents received weekly series of advice on the management of the behavioral problems of their children from qualified psychologists or child neuropsychiatrists, based on standardized procedures”), whereas statistically significant improvements were seen in ADHD and ODD symptoms following treatment with atomoxetine [102]. However, treatment of ODD is an off-label use of atomoxetine.
Evidence from the registration studies for the effectiveness of atomoxetine as a first-line medication for ADHD is limited to the two RCTs by Spencer et al. [17], in which the effects of atomoxetine treatment were assessed in 56 stimulant-naïve school-age children, albeit combined with data from 73 atomoxetine-treated children with prior stimulant exposure. In these two double-blind 12-week trials, atomoxetine significantly reduced ADHD symptoms [shown using three rating scales: ADHD-RS total scores, CGI-ADHD-S and Conners’ Parent Rating Scale (CPRS)-ADHD Index] relative to placebo [17].
Several other papers (at least seven) now indicate that atomoxetine is an effective first-line medication for ADHD, based on reductions in core ADHD symptoms and improved functional outcomes and QOL, determined in cohorts of children/adolescents who were all treatment naïve [106, 120, 121, 131–134]. For instance, in a randomized, double-blind, placebo-controlled trial in treatment-naïve children/adolescents with ADHD (n = 151), symptoms measured using ADHD-RS improved significantly, with a large effect size of 0.8, after 12 weeks of atomoxetine treatment [131]. At the end of the study, 50 % of atomoxetine-treated patients, versus 14 % with placebo, experienced ≥40 % reductions in the total ADHD-RS-IV-Parent Version: Investigator-Administered and Scored (ADHDRS-IV-Parent:Inv) score, and only 29 %, versus 46 % with placebo, were severely ill (by CGI-ADHD-S). Similarly, during an open-label 12-week trial of atomoxetine in 30 drug-naïve boys with ADHD in Taiwan, significant improvements were observed in visual memory, attention, and school functioning [120]. In addition, improvements in core ADHD symptoms and academic grades have also been seen over 24 weeks of atomoxetine treatment, although no correlations were detected in mean improvements in these outcomes [132].
In an RCT by Svanborg et al. [134], 10 weeks of atomoxetine treatment, when combined with psychoeducation, was found to be particularly effective in stimulant-naïve pediatric patients with ADHD (n = 49), relative to placebo (n = 50), with an effect size of 1.3 on the investigator-rated ADHD-RS. The authors noted that this effect size was “considerably higher than the previously published average effect size across 11 investigator-rated atomoxetine trials (effect size of 0.7) and LOCF [last observation carried forward] effect sizes of 0.6-0.8 in an overview of 6 other atomoxetine trials”. We hypothesize that effect sizes may be particularly large in treatment-naïve patients [131, 134] because these patients may be more willing to wait for the onset of action, relative to patients who have previously experienced rapid stimulant-like effects. However, in the IDEA study, there appeared to be no effect of treatment naivety versus previous stimulant use on treatment outcomes when assessing potential predictors of response status at 6–9 weeks relative to at 4 weeks using logistic regression (n = 378) [53]. In the RCT by Svanborg et al. [134], the large effect size of 1.3 was postulated to be “a result of including stimulant-naive patients only”, although the authors also suggested that it may be related to “a positive interaction between atomoxetine treatment and psychoeducation, possibly by increased compliance”. In addition, in another paper by Svanborg et al. [121], the 10 weeks of combined atomoxetine treatment with psychoeducation also positively affected various everyday coping abilities of the treatment-naïve patients and their families, whereas the patients’ self-image and their parents’ image of the climate in the family were not significantly improved.
3.7 Adolescent-Specific Data
Atomoxetine may be appealing for use in adolescents because of the convenience of once-daily administration, the long duration of action per day, and the lack of abuse potential. Indeed, adolescents with ADHD have an increased risk of SUD [16, 36, 37]. Moreover, at this important stage in school life, ADHD symptoms are also linked to academic underachievement [3, 38] as well as criminal behavior [2, 7] and impaired social and family functioning [1–3].
Two of the six registration studies included data from adolescents [18, 19], although data were only analyzed in adolescents and older children (those above the median age of 10.8 years) as a subgroup in the Michelson et al. [18] study. These older pediatric patients responded well to atomoxetine and, unlike in younger children, showed a statistically significant response to atomoxetine 0.5 mg/kg/day, which was to a similar degree as with the 1.2 and 1.8 mg/kg/day doses. However, the authors concluded that “although it is possible that these results indicate that adolescents have a different dose-response pattern compared with younger children based on some other factor, we believe that it is more likely an artifact of the smaller number of adolescents and older children, resulting in less precise measurement”.
We identified two large meta-analyses in which adolescent-specific data were compared with data from younger children. The meta-analysis by Wilens et al. [135] was based on data from 176 adolescents (12–17 years old) and 851 children (6–11 years old) from the six registration studies, while the other meta-analysis, by Wehmeier et al. [124], was based on five studies that were published more recently, lasted 8–12 weeks, and involved 183 adolescents (12–17 years old) and 611 children (6–11 years old). In both meta-analyses, adolescents had statistically significantly lower baseline ADHD-RS scores than children, due to higher hyperactive/impulsive subscores in children, although there were no significant differences in inattentive subscores. Also at baseline, adolescents in the study by Wehmeier et al. [124] had greater impairment in some subdomains of the CHIP-CE (i.e., Family Involvement, Satisfaction with Self, and Academic Performance), and adolescents subsequently experienced greater efficacy with atomoxetine, versus children, in both the Risk Avoidance domain and the Threats to Achievement subdomain of CHIP-CE. In Wilens et al. [135], there were no statistically significant differences in the overall effects of atomoxetine on ADHD symptoms (based on ADHD-RS, CPRS, and CGI-S), response rates, or time-to-response between the two age groups.
In an adolescent-only, single-site RCT, the efficacy of atomoxetine treatment, combined with motivational interviewing/cognitive behavioral therapy (MI/CBT) [n = 35; mean (SD) 16.06 (1.35) years old], was not statistically significantly different to placebo plus MI/CBT [n = 35; mean (SD) 16.11 (1.78) years old] as a treatment for ADHD or co-morbid non-tobacco SUD over a 12-week study period. The authors postulated that these findings may be related to the use of MI/CBT [47]. Also in this RCT, statistically significant pre–post decreases in ADHD symptoms [indicated by self-report Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) [136] ADHD checklist scores] were reported for atomoxetine plus MI/CBT [mean decrease 18.19 (95 % CI 13.41–22.97)], as well as for placebo plus MI/CBT [mean decrease 19.02 (95 % CI 13.97–24.07)] [47]. However, several other papers (at least six) attest to the effectiveness of atomoxetine solely in adolescent patient populations [55, 64, 71, 105, 125, 137], including a long-term meta-analysis involving 601 adolescents, 219 of whom were treated with atomoxetine for at least 2 years [55], two papers describing a 40-week study (n = 178) [64, 125], and two papers describing a 24-week study (n = 159) [71, 105]. In the 40-week study, for adolescents who previously responded to 8 weeks of atomoxetine, treatment benefit was better maintained with 1.4 mg/kg/day than with 0.8 mg/kg/day, based on the ADHD-RS. Also in this 40-week study, improvements were seen in adaptive functioning, age-appropriate developmental functioning [64], self-reported high-risk behaviors, and overall HR-QOL [125]. QOL, self-esteem, and ADHD symptoms also improved in the 24-week study [71, 105].
3.8 Gender
Given that a greater proportion of boys than girls are diagnosed with ADHD (estimated ratios range between 2:1 and 9:1) [39], it is not surprising that most studies of atomoxetine have been conducted in patient populations predominantly comprised of boys. In the six registration studies, male patients accounted for 71–81 % of the study populations; however, data were not reported for single-sex cohorts in these studies. There is a scarcity of studies comparing the efficacy of atomoxetine between the genders, although core ADHD symptoms and QOL were shown to improve to a similar extent for both genders (658 boys, 136 girls) following 8–12 weeks of atomoxetine treatment in a pooled analysis of five studies by Wehmeier et al. [123]. Similar improvement for the sexes (338 boys, 83 girls) was also shown for QOL following 8 and 24 weeks of atomoxetine treatment in a pooled analysis of two studies [122]. However, in contrast with the aforementioned publication by Wehmeier et al. [123], in a meta-analysis of nine randomized placebo-controlled trials (atomoxetine:placebo n = 1,150:678), male gender (p = 0.02) was associated with smaller reductions in ADHD symptoms [104].
In addition, there are a few small studies of atomoxetine solely in single-sex cohorts [70, 120, 138, 139]. In school-age girls, an RCT indicated that 9 weeks of atomoxetine treatment (n = 30) was superior to placebo (n = 21) at managing ADHD symptoms (according to ADHD-RS, CPRS, CGI-S) [70]. Also in girls, with the combined subtype of ADHD, 18 days of treatment with MAS XR (n = 26) was significantly more effective than atomoxetine (n = 31) in terms of classroom behavior, attention, and academic productivity [139]. However, given that atomoxetine often takes several weeks for responses to build, 18 days of treatment is unlikely to be a sufficient timescale over which to compare these medications.
3.9 Co-Morbid Conditions
In the six registration studies, the patients had often been diagnosed with co-morbid conditions as well as ADHD. However, in the registration studies, the efficacy of atomoxetine was not analyzed in subsets of co-morbid patients. The most commonly reported co-morbid condition in the registrations studies, ODD, affected 19–48 % of patients [17–21]. Other co-morbid conditions included depression, generalized anxiety disorder, conduct disorder (CD), and learning disorder [17–21].
Today, a range of studies have been published suggesting that atomoxetine is an effective medication for ADHD in children and adolescents with various types of co-morbidity. In some cases, it is possible that atomoxetine may positively influence the symptoms of the co-morbid condition. However, as stated in the European SPC and in the US Full Prescribing Information document [45, 46], atomoxetine is only indicated for the treatment of ADHD; it has not been approved as a treatment for any other condition, including co-morbidities of ADHD.
3.9.1 Atomoxetine Treatment in Real-World Attention-Deficit/Hyperactivity Disorder (ADHD) Populations
Patients with ADHD frequently suffer from concomitant psychiatric conditions. In a retrospective chart review of a “real-world ADHD population”, comprised of 166 patients who often had co-morbidities, mainly ODD/CD (25 %), mood disorders (19 %), and anxiety disorders (18 %), the effectiveness and tolerability of atomoxetine (n = 85) was comparable with that of stimulants (n = 81) [87]. Moreover, in another naturalistic study, in which 627 youngsters with ADHD were treated with atomoxetine, “ADHD severity was improved similarly in patients across comorbid conditions (e.g. anxiety, depression, learning disorders)” [129].
3.9.2 Atomoxetine Treatment in Patients with ADHD in Relation to Specific Co-Morbid Conditions
In addition to “real-world ADHD populations”, atomoxetine studies often focus on a single co-morbid condition in patients with ADHD. We have already discussed treatment of ADHD with atomoxetine in adolescents with co-morbid SUD in the Sect 3.7. Other co-morbidities include those discussed in the following sections.
3.9.2.1 Tics and Tourette’s Syndrome
Atomoxetine, when studied for 16–18 weeks, appears to be an effective treatment for ADHD and tics in pediatric patients with co-morbid Tourette’s syndrome or chronic motor tic disorder [140–142]. For instance, in 148 children and adolescents, randomized to up to 18 weeks of atomoxetine or placebo, improvements were observed both in the severity of ADHD (effect size = 0.6) and tics (effect size = 0.3) [142]. This study was the source for all atomoxetine-treatment data in a meta-analysis, alongside eight other placebo-controlled studies of five other medications, in a total of 477 patients with ADHD and co-morbid tics [143]. Significant improvements were gained in ADHD and tic severity following treatment with atomoxetine, methylphenidate, α2 agonists (clonidine and guanfacine), or desipramine. However, no improvement in ADHD was observed with deprenylFootnote 6, ADHD symptoms were not investigated during treatment with dexamphetamine, and tic severity worsened following supratherapeutic doses of dexamphetamine [143].
3.9.2.2 Depression, Bipolar Disorder, and Anxiety
Atomoxetine appears to be an effective treatment for ADHD in children and/or adolescents with co-morbid depression, bipolar disorder, or anxiety [129, 137, 144–146]. In 142 adolescents with co-morbid major depressive disorder (MDD), improvements in ADHD symptoms were greater after 9 weeks of atomoxetine treatment relative to placebo [mean (SD) ADHDRS-IV-Parent:Inv total score −13.3 (10.0) vs. −5.1 (9.9), respectively; p < 0.001), without improvement in MDD [137]. Similarly, in 12 youngsters with co-morbid ADHD and bipolar disorder, 8 weeks of atomoxetine appeared to be effective as a treatment for ADHD symptoms when the patients were receiving mood stabilizers or antipsychotics [144]. In another study, atomoxetine was also an efficacious treatment for ADHD over a 12-week period in pediatric patients with co-morbid anxiety, and was accompanied by improvements in anxiety [mean (SD) Pediatric Anxiety Rating Scale total score −5.5 (4.8), n = 55, vs. placebo −3.2 (5.0), n = 58; p = 0.011] [145].
3.9.2.3 Oppositional Defiant Disorder
ODD is often found in patients with ADHD, and is probably the most extensively studied ADHD co-morbidity. Numerous studies (usually with patient populations in the hundreds, often placebo-controlled, and often with 6–12 weeks of treatment) indicate that ADHD symptoms improve following treatment with atomoxetine in patients who have co-morbid ODD [52, 60, 78, 101, 102, 117, 118, 147–149]. When studied, these improvements are also accompanied by improvements in QOL [117, 118]. However, it is currently uncertain whether or not the efficacy of atomoxetine as a treatment for ADHD is affected by co-morbid ODD [104, 150].
There is evidence that atomoxetine may be associated with improvements in ODD symptoms in patients with ADHD [52, 60, 78, 101, 102, 115, 118, 151]. However, other studies indicate that although ADHD symptoms improved, no statistically significant improvements in ODD are gained in patients with co-morbid ADHD following atomoxetine treatment [147, 148]. The reasons for these discrepancies may be related to differences in study design and treatment schedules [118, 151], e.g., improvements in ODD may be gained using twice-daily versus once-daily atomoxetine [151].
In terms of alternative ADHD treatments, in a meta-analysis of seven trials atomoxetine (n = 823) appeared to have similar efficacy to methylphenidate (n = 568) as a treatment for the core symptoms of ADHD, and response rates to these medications did not appear to be affected by the presence of ODD [152]. In an indirect comparison, GXR (n = 143) appeared to be more efficacious than atomoxetine (n = 98) as a treatment of ODD in patients with ADHD [153], although this meta-analysis was based on results of a trial in which ODD symptoms did not improve with atomoxetine treatment [148], rather than several more recent papers suggesting that ODD symptoms may improve following atomoxetine treatment [52, 60, 102, 115, 151].
3.9.2.4 Pervasive Developmental Disorders: Autism Spectrum Disorder and Asperger’s Syndrome
In the text revised version of DSM-IV, autism spectrum disorder (ASD) was excluded as a co-morbidity of ADHD. However, since the release of DSM-5 [154] in May 2013, this is no longer the case. Indeed, in numerous clinical samples, ADHD is co-morbid with ASD in 20–50 % of cases [41, 42].
At least six studies indicate that atomoxetine may be an effective treatment for ADHD symptoms in patients with co-morbid pervasive developmental disorders [138, 155–160], although five of these studies are small (≤16 patients), with treatment durations up to 10 weeks [138, 157–160]. In the largest study (n = 97), ADHD symptoms “moderately improved” after 8 weeks of atomoxetine versus placebo [155], with further improvements at 28 weeks [156]. However, atomoxetine may be less effective in children with severe autistic disorder, as suggested in a very small study (12 patients with symptoms of ADHD) [157].
3.9.2.5 Intelligence Quotient (IQ)
When children’s and adolescents’ clinical records were retrospectively examined, atomoxetine appeared to be less effective in those with an intelligence quotient (IQ) <85 (n = 29) than with an IQ ≥85 (n = 26). This difference did not appear to be related to the severity of ADHD symptoms, nor to the presence of other co-morbidities [161].
3.9.2.6 Mental Retardation
In a 16-week, open-label study of 48 children with mental retardation, statistically significant reductions in ADHD symptoms were observed following treatment with atomoxetine [162].
3.9.2.7 Dyslexia
In studies by Shaywitz et al. [163] (comprised of a 16-week randomized, double-blind, placebo-controlled phase, followed by a 16-week open-label extension phase) and Sumner et al. [164] (a 16-week open-label, uncontrolled study), the reading scores of patients with co-morbid ADHD/dyslexia (n = 124 and 36, respectively) improved following treatment with atomoxetine. Moreover, following atomoxetine treatment, reading scores also improved in patients who only had dyslexia [163], and ADHD symptoms improved in patients with co-morbid dyslexia [164].
3.9.2.8 Epilepsy
Dumitru and Salan [119] reported the effects of morning administration of atomoxetine, given after 3 months of treatment with anticonvulsants, assessed for a year. School performance improved in 24 of 30 children.
3.10 Teacher Rating of ADHD
Naturally, given the importance of school in children’s lives, it is important to determine the effects of atomoxetine on ADHD symptoms and behavior in the school setting. Teacher ratings of ADHD symptoms were analyzed in the two registration studies by Michelson et al. [19] and Weiss et al. [20] and, while they were planned to be analyzed in other registration studies, this was not possible due to poor rates of completion and return. Teacher rating was the primary method for evaluating core ADHD symptoms in Weiss et al. [20], with the other five registration studies primarily relying on parental rating. In the registration studies, improvements in core ADHD symptoms with atomoxetine treatment were similar when assessed by parents or teachers, with effect sizes of about 0.7 detected by both groups of assessors, relative to placebo [19, 20]. However, Weiss et al. [20] did conclude that “further research is needed to determine the responsiveness to treatment of academic performance, socialization, child perceptions of outcome, and other outcomes beyond the core symptoms of ADHD”.
We identified several non-registration studies in which the impact of atomoxetine treatment on the core symptoms of ADHD has been investigated using teacher-rating scales, often in studies involving hundreds of children/adolescents [63, 102, 104, 109, 138, 149, 151, 155, 165–168]. According to teacher ratings, the core symptoms of ADHD are effectively treated by atomoxetine, with results that are often similar to parent and/or investigator measures.
In terms of the impact of atomoxetine on school-specific outcomes, in an 8-week open-label trial of 56 children with ADHD, treated with atomoxetine alone or with behavior therapy, atomoxetine treatment was associated with a reduction in rule violations in the classroom [115]. Similarly, in a double-blind RCT of atomoxetine (n = 101) versus placebo (n = 52), behavior improved significantly, according to teacher and parent ratings [103], although, despite an effect size of 0.31, no statistically significant effects (p = 0.143) were detected by teachers in relation to atomoxetine treatment on academic productivity/completion of work [103]. These researchers suggested several possible reasons for this lack of statistical significance, including the possibility that the short duration (7 weeks) of the study “was not sufficient to actually detect changes in children’s academic skills”, and continued that “this observation is consistent with much of the stimulant drug literature that has failed to demonstrate the efficacy of stimulant drug therapy on academic achievement”. Indeed, improvements in school performance have been detected in a longer trial (1 year) of atomoxetine treatment, albeit in a small sample (30 children) that had co-morbid ADHD and epilepsy [119].
4 Conclusion
Atomoxetine has been demonstrated to be an effective treatment for the core ADHD symptoms, and improves functional outcomes and QOL, in a broad range of clinical studies and meta-analyses. Atomoxetine often becomes particularly effective 10–12 weeks after initiating treatment. In clinical studies that lasted between 6 and 18 weeks, effect sizes for atomoxetine treatment of the core ADHD symptoms, with/without psychoeducation, ranged between 0.6 and 1.3, versus placebo. The effectiveness of atomoxetine has been shown in various pediatric patient populations (i.e., males/females, patients with various co-morbidities, children/adolescents, and with/without prior exposure to other ADHD medications). Many ADHD patients may stay well for several months after discontinuing atomoxetine treatment, and atomoxetine has been shown to be more effective than placebo at preventing relapse of ADHD symptoms after initial atomoxetine treatment periods of 10–52 weeks.
Based on the current literature, it is difficult to draw strong conclusions in relation to atomoxetine versus other medications, e.g., because the studies were not necessarily powered well enough, used indirect comparison, and/or the durations of the studies were often not long enough due to the relatively gradual onset of the efficacy of atomoxetine. Indeed, responses to atomoxetine tend to be relatively gradual [53–55]. Some researchers, such as the authors of the IDEA study, suggest that responses to the first few weeks of atomoxetine treatment may be used to predict responses over time [53, 75, 76]. In addition, the IDEA study suggested that clinical responses at 6–9 weeks may be bimodal, with pediatric patients either being “responders” [47 % of patients were “much improved” (defined as ≥40 % decrease in ADHD-RS scores); 13 % were “minimally improved” (25 to <40 % decrease)] or “non-responders” [40 % of patients (<25 % decrease in ADHD-RS scores)] [53]. However, in an open-label study [54] that was longer than the IDEA study [53], a sizable proportion of patients who only have partial responses to atomoxetine at 4 weeks go on to have robust responses beyond this timepoint. We are currently unaware of clear predictors of response for ADHD treatments.
Before initiating treatment with atomoxetine in children/adolescents, it is paramount that clinicians set the right expectations for the patient and their family. Thus, the patient/family should be informed of the likelihood of a gradual response to atomoxetine, which often builds over time. Setting the right expectations may increase the chances of attaining successful outcomes with atomoxetine treatment in the long term.
Notes
Response was defined as “at least a 25 % decrease from baseline on the ADHD-RS-IV [ADHD–Rating Scale–IV] total score”.
Remission was defined as “each ADHD-RS-IV item score ≤1 at the end of the treatment”.
However, these results should be viewed with caution, particularly as the congress abstract does not state a definition for “treatment failure”, and we are not aware of these results having been published in a full-length peer-reviewed study paper or of being replicated.
The primary outcome measure was comparison of mean time to relapse, defined as an increase in ADHD-RS total score to 90 % of the score at study entry and an increase in CGI–Severity [CGI-S] score of ≥2 points above the CGI-S score at the end of the initial 10 weeks of treatment. A secondary definition of relapse (≥50 % worsening in ADHD-RS severity score above the last pre-randomization visit) resulted in similar relapse rates [atomoxetine, 6/81 (7.4 %); placebo, 16/82 (19.5 %); p = 0.037; relative risk ratio, 3.0 (95 % CI 1.2–7.6)]. A greater number of patients who received atomoxetine completed the 6-month randomized phase [65/79 (82.3 %)] than did those who received placebo [54/81 (66.7 %); p = 0.030].
The definition of non-inferiority was “if the lower limit of the 2-sided 95 % CI for the difference in proportion of responders (ATX [atomoxetine] minus MPH [methylphenidate]) is greater than −15%, ATX will be considered non-inferior to MPH”.
In the meta-analysis paper [143], and the source study in this paper, it is unclear whether ‘deprenyl’ referred to L-deprenyl (also known as selegiline), D-deprenyl, or a racemic mixture.
References
Young ME, Galvan T, Reidy BL, et al. Family functioning deficits in bipolar disorder and ADHD in youth. J Affect Disord. 2013;150(3):1096–102.
Langley K, Fowler T, Ford T, et al. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2010;196(3):235–40.
Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29(4):546–57.
Babinski DE, Pelham WE Jr, Molina BS, et al. Late adolescent and young adult outcomes of girls diagnosed with ADHD in childhood: an exploratory investigation. J Atten Disord. 2011;15(3):204–14.
Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1424–31.
Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. Med Gen Med. 2006;8(3):12.
Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. Long-term criminal outcome of children with attention deficit hyperactivity disorder. Crim Behav Ment Health. 2013;23(2):86–98.
Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51(10):990–1002.
Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–13.
Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–9.
Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8.
Polanczyk GV, Willcutt EG, Salum GA, et al. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–42.
Kornfield R, Watson S, Higashi AS, et al. Effects of FDA advisories on the pharmacologic treatment of ADHD, 2004–2008. Psychiatr Serv. 2013;64(4):339–46.
Cardo E, Porsdal V, Quail D, et al. Fast vs. slow switching from stimulants to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(4):252–61.
Banaschewski T, Roessner V, Dittmann RW, et al. Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):102–16.
Upadhyaya HP, Desaiah D, Schuh KJ, et al. A review of the abuse potential assessment of atomoxetine: a nonstimulant medication for attention-deficit/hyperactivity disorder. Psychopharmacology. 2013;226(2):189–200.
Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63(12):1140–7.
Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics. 2001;108(5):E83.
Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159(11):1896–901.
Weiss M, Tannock R, Kratochvil C, et al. A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44(7):647–55.
Kelsey DK, Sumner CR, Casat CD, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics. 2004;114(1):e1–8.
Bushe CJ, Savill NC. Systematic review of atomoxetine data in childhood and adolescent attention-deficit hyperactivity disorder 2009–2011: focus on clinical efficacy and safety. J Psychopharmacol. 2014;28(3):204–11.
Schwartz S, Correll CU. Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry. 2014;53(2):174–87.
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145–57.
Agster KL, Bates AT, Cain RE, et al. The role of cortical norepinephrine in the development of executive function. Neuropsychopharmacology. 2011;36:S83.
Gehlert DR, Schober DA, Hemrick-Luecke SK, et al. Novel halogenated analogs of tomoxetine that are potent and selective inhibitors of norepinephrine uptake in brain. Neurochem Int. 1995;26(1):47–52.
Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340(2–3):249–58.
Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther. 1982;222(1):61–5.
Bolden-Watson C, Richelson E. Blockade by newly developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993;52(12):1023–9.
Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699–711.
Volz TJ, Farnsworth SJ, Rowley SD, et al. Methylphenidate-induced increases in vesicular dopamine sequestration and dopamine release in the striatum: the role of muscarinic and dopamine D2 receptors. J Pharmacol Exp Ther. 2008;327(1):161–7.
Calipari ES, Ferris MJ, Salahpour A, et al. Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun. 2013;4:2720.
Hutson PH, Pennick M, Secker R. Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: a novel d-amphetamine pro-drug. Neuropharmacology. 2014;87C:41–50.
Fan X, Hess EJ. D2-like dopamine receptors mediate the response to amphetamine in a mouse model of ADHD. Neurobiol Dis. 2007;26(1):201–11.
Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60(2):284–95.
Molina BS, Pelham WE Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112(3):497–507.
Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799–809.
Galéra C, Melchior M, Chastang JF, et al. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: the GAZEL Youth study. Psychol Med. 2009;39(11):1895–906.
Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33(2):357–73.
Tanaka Y, Rohde LA, Jin L, et al. A meta-analysis of the consistency of atomoxetine treatment effects in pediatric patients with attention-deficit/hyperactivity disorder from 15 clinical trials across four geographic regions. J Child Adolesc Psychopharmacol. 2013;23(4):262–70.
Rommelse NN, Geurts HM, Franke B, et al. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 2011;35(6):1363–96.
Rommelse NN, Franke B, Geurts HM, et al. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19(3):281–95.
Udvardi PT, Föhr KJ, Henes C, et al. Atomoxetine affects transcription/translation of the NMDA receptor and the norepinephrine transporter in the rat brain—an in vivo study. Drug Des Devel Ther. 2013;7:1433–46.
STRATTERA®. Clinical study results. Eli Lilly and Co. http://www.lillytrials.com/results/Strattera.pdf. Accessed 5 Dec 2014.
STRATTERA®. Summary of product characteristics. Eli Lilly and Co. http://www.medicines.org.uk/emc/medicine/14482. Accessed 27 Nov 2014.
STRATTERA®. Full prescribing information. Eli Lilly and Co. http://www.pi.lilly.com/us/strattera-pi.pdf. Accessed 27 Nov 2014.
Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573–82.
Schulz KP, Fan J, Bédard AC, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69(9):952–61.
Ramoz N, Boni C, Downing AM, et al. A haplotype of the norepinephrine transporter (Net) gene Slc6a2 is associated with clinical response to atomoxetine in attention-deficit hyperactivity disorder (ADHD). Neuropsychopharmacology. 2009;34(9):2135–42.
Yang L, Qian Q, Liu L, et al. Adrenergic neurotransmitter system transporter and receptor genes associated with atomoxetine response in attention-deficit hyperactivity disorder children. J Neural Transm. 2013;120(7):1127–33.
Michelson D, Read HA, Ruff DD, et al. CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46(2):242–51.
Hazell P, Becker K, Nikkanen EA, et al. Relationship between atomoxetine plasma concentration, treatment response and tolerability in attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Atten Defic Hyperact Disord. 2009;1(2):201–10.
Newcorn JH, Sutton VK, Weiss MD, Sumner CR. Clinical responses to atomoxetine in attention-deficit/hyperactivity disorder: the Integrated Data Exploratory Analysis (IDEA) study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):511–8.
Dickson RA, Maki E, Gibbins C, et al. Time courses of improvement and symptom remission in children treated with atomoxetine for attention-deficit/hyperactivity disorder: analysis of Canadian open-label studies. Child Adolesc Psychiatry Ment Health. 2011;5(14).
Wilens TE, Newcorn JH, Kratochvil CJ, et al. Long-term atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Pediatr. 2006;149(1):112–9.
Buitelaar JK, Michelson D, Danckaerts M, et al. A randomized, double-blind study of continuation treatment for attention-deficit/hyperactivity disorder after 1 year. Biol Psychiatry. 2007;61(5):694–9.
Perwien AR, Faries DE, Kratochvil CJ, et al. Improvement in health-related quality of life in children with ADHD: an analysis of placebo controlled studies of atomoxetine. J Dev Behav Pediatr. 2004;25(4):264–71.
Urion DK. Atomoxetine “treatment failures” usually respond to dosing changes. Ann Neurol. 2012;72:S186–7.
Block SL, Kelsey D, Coury D, et al. Once-daily atomoxetine for treating pediatric attention-deficit/hyperactivity disorder: comparison of morning and evening dosing. Clin Pediatr. 2009;48(7):723–33.
Dittmann RW, Schacht A, Helsberg K, et al. Atomoxetine versus placebo in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a double-blind, randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol. 2011;21(2):97–110.
Kratochvil CJ, Bohac D, Harrington M, et al. An open-label trial of tomoxetine in pediatric attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11(2):167–70.
Cho S, Lee SI, Yoo H, et al. A randomized, open-label assessment of response to various doses of atomoxetine in Korean pediatric outpatients with attention-deficit/hyperactivity disorder. Psychiatry Investig. 2011;8(2):141–8.
Takahashi M, Takita Y, Yamazaki K, et al. A randomized, double-blind, placebo-controlled study of atomoxetine in Japanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(4):341–50.
Wietecha LA, Williams DW, Herbert M, et al. Atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(6):719–30.
Newcorn JH, Michelson D, Kratochvil CJ, et al. Low-dose atomoxetine for maintenance treatment of attention-deficit/hyperactivity disorder. Pediatrics. 2006;118(6):e1701–6.
Spencer T, Biederman J, Heiligenstein J, et al. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11(3):251–65.
Kratochvil CJ, Michelson D, Newcorn JH, et al. High-dose atomoxetine treatment of ADHD in youths with limited response to standard doses. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1128–37.
Dittmann RW, Cardo E, Nagy P, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs. 2013;27(12):1081–92.
Martenyi F, Zavadenko NN, Jarkova NB, et al. Atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: a 6-week, randomized, placebo-controlled, double-blind trial in Russia. Eur Child Adolesc Psychiatry. 2010;19(1):57–66.
Biederman J, Heiligenstein JH, Faries DE, et al. Efficacy of atomoxetine versus placebo in school-age girls with attention-deficit/hyperactivity disorder. Pediatrics. 2002;110(6):e75.
Dittmann RW, Wehmeier PM, Schacht A, et al. Atomoxetine treatment and ADHD-related difficulties as assessed by adolescent patients, their parents and physicians. Child Adolesc Psychiatry Ment Health. 2009;3(1):21.
Buitelaar JK, Danckaerts M, Gillberg C, et al. A prospective, multicenter, open-label assessment of atomoxetine in non-North American children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2004;13(4):249–57.
Tamayo JM, Pumariega A, Rothe EM, et al. Latino versus Caucasian response to atomoxetine in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2008;18(1):44–53.
Durell TM, Pumariega AJ, Rothe EM, et al. Effects of open-label atomoxetine on African-American and Caucasian pediatric outpatients with attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2009;21(1):26–37.
Block SL, Williams D, Donnelly CL, et al. Post hoc analysis: early changes in ADHD-RS items predict longer term response to atomoxetine in pediatric patients. Clin Pediatr. 2010;49(8):768–76.
Montoya A, Quail D, Anand E, et al. Prognostic factors of improvement in health related quality of life in children and adolescents with attention deficit/hyperactivity disorder, after atomoxetine treatment. Eur Psychiatry. 2012;27(Suppl 1):1.
Michelson D, Buitelaar JK, Danckaerts M, et al. Relapse prevention in pediatric patients with ADHD treated with atomoxetine: a randomized, double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2004;43(7):896–904.
Hazell P, Zhang S, Wolańczyk T, et al. Comorbid oppositional defiant disorder and the risk of relapse during 9 months of atomoxetine treatment for attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2006;15(2):105–10.
Kratochvil CJ, Wilens TE, Greenhill LL, et al. Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(8):919–27.
Wehmeier PM, Dittmann RW, Schacht A, et al. Morning and evening behavior in children and adolescents treated with atomoxetine once daily for attention-deficit/hyperactivity disorder (ADHD): findings from two 24-week, open-label studies. Child Adolesc Psychiatry Ment Health. 2009;3(1):5.
Wehmeier PM, Schacht A, Ulberstad F, et al. Does atomoxetine improve executive function, inhibitory control, and hyperactivity? Results from a placebo-controlled trial using quantitative measurement technology. J Clin Psychopharmacol. 2012;32(5):653–60.
Wilens TE, Hammerness P, Utzinger L, et al. An open study of adjunct OROS-methylphenidate in children and adolescents who are atomoxetine partial responders: I. Effectiveness. J Child Adolesc Psychopharmacol. 2009;19(5):485–92.
Wigal SB, McGough JJ, McCracken JT, et al. A laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with attention deficit/hyperactivity disorder. J Atten Disord. 2005;9(1):275–89.
Faraone SV, Wigal SB, Hodgkins P. Forecasting three-month outcomes in a laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with ADHD. J Atten Disord. 2007;11(1):74–82.
Roskell NS, Setyawan J, Zimovetz EA, Hodgkins P. Systematic review and network meta-analysis of treatments used for attention deficit hyperactivity disorder (ADHD). Value Health. 2012;15(7):A334.
Sikirica V, Findling RL, Signorovitch J, et al. Comparative efficacy of guanfacine extended release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in children and adolescents: applying matching-adjusted indirect comparison methodology. CNS Drugs. 2013;27(11):943–53.
Bastiaens L. Effectiveness and tolerability of atomoxetine in a real-world ADHD population: nonrandomized comparison with stimulants. Psychiatry. 2007;4(12):44–8.
Whalen CK, Henker B, Ishikawa SS, et al. Atomoxetine versus stimulants in the community treatment of children with ADHD: an electronic diary study. J Atten Disord. 2010;13(4):391–400.
Fuentes J, Danckaerts M, Cardo E, et al. Long-term quality-of-life and functioning comparison of atomoxetine versus other standard treatment in pediatric attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2013;33(6):766–74.
Hazell PL, Kohn MR, Dickson R, et al. Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis. J Atten Disord. 2011;15(8):674–83.
Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176.
Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry. 2002;41(7):776–84.
Sangal RB, Owens J, Allen AJ, et al. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep. 2006;29(12):1573–85.
Prasad S, Harpin V, Poole L, et al. A multi-centre, randomised, open-label study of atomoxetine compared with standard current therapy in UK children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Curr Med Res Opin. 2007;23(2):379–94.
Wang Y, Zheng Y, Du Y, et al. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust NZ J Psychiatry. 2007;41(3):222–30.
Newcorn JH, Kratochvil CJ, Allen AJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165(6):721–30.
Kemner JE, Starr HL, Ciccone PE, et al. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Adv Ther. 2005;22(5):498–512.
Yildiz O, Sismanlar SG, Memik NC, et al. Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry Hum Dev. 2011;42(3):257–69.
Starr HL, Kemner J. Multicenter, randomized, open-label study of OROS methylphenidate versus atomoxetine: treatment outcomes in African-American children with ADHD. J Natl Med Assoc. 2005;97(10):11S–6S.
Mazzone L, Postorino V, Reale L, et al. Self-esteem evaluation in children and adolescents suffering from ADHD. Clin Pract Epidemiol Ment Health. 2013;9:96–102.
Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology. 2007;190(1):31–41.
Dell’Agnello G, Maschietto D, Bravaccio C, et al. Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled Italian study. Eur Neuropsychopharmacol. 2009;19(11):822–34.
Brown RT, Perwien A, Faries DE, et al. Atomoxetine in the management of children with ADHD: effects on quality of life and school functioning. Clin Pediatr. 2006;45(9):819–27.
Cheng JY, Chen RY, Ko JSN, et al. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents—meta-analysis and meta-regression analysis. Psychopharmacology. 2007;194(2):197–209.
Dittmann RW, Wehmeier PM, Schacht A, et al. Self-esteem in adolescent patients with attention-deficit/hyperactivity disorder during open-label atomoxetine treatment: psychometric evaluation of the Rosenberg Self-Esteem Scale and clinical findings. Atten Defic Hyperact Disord. 2009;1(2):187–200.
Escobar R, Montoya A, Polavieja P, et al. Evaluation of patients’ and parents’ quality of life in a randomized placebo-controlled atomoxetine study in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):253–63.
Escobar R, Schacht A, Wehmeier PM, Wagner T. Quality of life and attention-deficit/hyperactivity disorder core symptoms: a pooled analysis of 5 non-US atomoxetine clinical trials. J Clin Psychopharmacol. 2010;30(2):145–51.
Donnelly C, Faries D, Swensen A, et al. The effect of atomoxetine on the social and family functioning of children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Eur Neuropsychopharmacol. 2002;12(Suppl 3):S437.
Maziade M, Rouleau N, Lee B, et al. Atomoxetine and neuropsychological function in children with attention-deficit/hyperactivity disorder: results of a pilot study. J Child Adolesc Psychopharmacol. 2009;19(6):709–18.
Van Der Kolk A, Bouwmans C, Schawo S, et al. The quality of life of children with ADHD and their parents: results of EQ-5D and Kidscreen-10. Value Health. 2011;14(7):A294–5.
Perwien AR, Kratochvil CJ, Faries DE, et al. Atomoxetine treatment in children and adolescents with attention-deficit hyperactivity disorder: what are the long-term health-related quality-of-life outcomes? J Child Adolesc Psychopharmacol. 2006;16(6):713–24.
Matza LS, Rentz AM, Secnik K, et al. The link between health-related quality of life and clinical symptoms among children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2004;25(3):166–74.
Bastiaens L. Improvement in global psychopathology increases quality of life during treatment of ADHD with atomoxetine or stimulants. Psychiatr Q. 2011;82(4):303–8.
Buitelaar JK, Wilens TE, Zhang S, et al. Comparison of symptomatic versus functional changes in children and adolescents with ADHD during randomized, double-blind treatment with psychostimulants, atomoxetine, or placebo. J Child Psychol Psychiatry. 2009;50(3):335–42.
Waxmonsky JG, Waschbusch DA, Pelham WE, et al. Effects of atomoxetine with and without behavior therapy on the school and home functioning of children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2010;71(11):1535–51.
Wehmeier PM, Dittmann RW, Schacht A, et al. Effectiveness of atomoxetine and quality of life in children with attention-deficit/hyperactivity disorder as perceived by patients, parents, and physicians in an open-label study. J Child Adolesc Psychopharmacol. 2007;17(6):813–30.
Wehmeier PM, Schacht A, Dittmann RW, et al. Effect of atomoxetine on quality of life and family burden: results from a randomized, placebo-controlled, double-blind study in children and adolescents with ADHD and comorbid oppositional defiant or conduct disorder. Qual Life Res. 2011;20(5):691–702.
Newcorn JH, Spencer TJ, Biederman J, et al. Atomoxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(3):240–8.
Dumitru I, Salan A. Treating children with epilepsy and comorbid attention-deficit/hyperactivity disorder (ADHD). Eur J Neurol. 2012;19:208.
Shang CY, Gau SS. Improving visual memory, attention, and school function with atomoxetine in boys with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(5):353–63.
Svanborg P, Thernlund G, Gustafsson PA, et al. Atomoxetine improves patient and family coping in attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in Swedish children and adolescents. Eur Child Adolesc Psychiatry. 2009;18(12):725–35.
Wehmeier PM, Schacht A, Dittmann RW, Banaschewski T. Minor differences in ADHD-related difficulties between boys and girls treated with atomoxetine for attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2010;2(2):73–85.
Wehmeier PM, Schacht A, Escobar R, et al. Health-related quality of life in ADHD: a pooled analysis of gender differences in five atomoxetine trials. Atten Defic Hyperact Disord. 2012;4(1):25–35.
Wehmeier PM, Schacht A, Escobar R, et al. Differences between children and adolescents in treatment response to atomoxetine and the correlation between health-related quality of life and attention deficit/hyperactivity disorder core symptoms: meta-analysis of five atomoxetine trials. Child Adolesc Psychiatry Ment Health. 2010;4:30.
Saylor K, Williams DW, Schuh KJ, et al. Effects of atomoxetine on self-reported high-risk behaviors and health-related quality of life in adolescents with ADHD. Curr Med Res Opin. 2010;26(9):2087–95.
Quintana H, Cherlin EA, Duesenberg DA, et al. Transition from methylphenidate or amphetamine to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder—a preliminary tolerability and efficacy study. Clin Ther. 2007;29(6):1168–77.
Heiligenstein JH, Dunn DW, Busner J, et al. Efficacy of tomoxetine versus placebo in school-age children with ADHD who failed psychostimulant treatment. 154th Annual Meeting of the American Psychiatric Association. 2001 5–10 May; New Orleans, LA.
Hammerness P, Doyle R, Kotarski M, et al. Atomoxetine in children with attention-deficit hyperactivity disorder with prior stimulant therapy: a prospective open-label study. Eur Child Adolesc Psychiatry. 2009;18(8):493–8.
Bakken RJ, Paczkowski M, Kramer HP, et al. Effects of atomoxetine on attention-deficit/hyperactivity disorder in clinical pediatric treatment settings: a naturalistic study. Curr Med Res Opin. 2008;24(2):449–60.
Niederkirchner K, Slawik L, Wermelskirchen D, et al. Transitioning to OROS® methylphenidate from atomoxetine is effective in children and adolescents with ADHD. Expert Rev Neurother. 2011;11(4):499–508.
Montoya A, Hervas A, Cardo E, et al. Evaluation of atomoxetine for first-line treatment of newly diagnosed, treatment-naïve children and adolescents with attention deficit/hyperactivity disorder. Curr Med Res Opin. 2009;25(11):2745–54.
Mendez L, Singh P, Harrison G, et al. Academic outcomes in Asian children aged 8–11 years with attention-deficit/hyperactivity disorder treated with atomoxetine hydrochloride. Int J Psychiatry Clin Pract. 2011;15(2):145–56.
Wehmeier PM, Dittmann RW, Banaschewski T, Schacht A. Does stimulant pretreatment modify atomoxetine effects on core symptoms of ADHD in children assessed by quantitative measurement technology? J Atten Disord. 2014;18(2):105–16.
Svanborg P, Thernlund G, Gustafsson PA, et al. Efficacy and safety of atomoxetine as add-on to psychoeducation in the treatment of attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in stimulant-naïve Swedish children and adolescents. Eur Child Adolesc Psychiatry. 2009;18(4):240–9.
Wilens TE, Kratochvil C, Newcorn JH, Gao H. Do children and adolescents with ADHD respond differently to atomoxetine? J Am Acad Child Adolesc Psychiatry. 2006;45(2):149–57.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994.
Bangs ME, Emslie GJ, Spencer TJ, et al. Efficacy and safety of atomoxetine in adolescents with attention-deficit/hyperactivity disorder and major depression. J Child Adolesc Psychopharmacol. 2007;17(4):407–20.
Zeiner P, Gjevik E, Weidle B. Response to atomoxetine in boys with high-functioning autism spectrum disorders and attention deficit/hyperactivity disorder. Acta Paediatr. 2011;100(9):1258–61.
Biederman J, Wigal SB, Spencer TJ, et al. A post hoc subgroup analysis of an 18-day randomized controlled trial comparing the tolerability and efficacy of mixed amphetamine salts extended release and atomoxetine in school-age girls with attention-deficit/hyperactivity disorder. Clin Ther. 2006;28(2):280–93.
Spencer TJ, Sallee FR, Gilbert DL, et al. Atomoxetine treatment of ADHD in children with comorbid Tourette syndrome. J Atten Disord. 2008;11(4):470–81.
Gilbert DL, Zhang J, Lipps TD, et al. Atomoxetine treatment of ADHD in Tourette syndrome: reduction in motor cortex inhibition correlates with clinical improvement. Clin Neurophysiol. 2007;118(8):1835–41.
Allen AJ, Kurlan RM, Gilbert DL, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology. 2005;65(12):1941–9.
Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884–93.
Chang K, Nayar D, Howe M, Rana M. Atomoxetine as an adjunct therapy in the treatment of co-morbid attention-deficit/hyperactivity disorder in children and adolescents with bipolar I or II disorder. J Child Adolesc Psychopharmacol. 2009;19(5):547–51.
Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1119–27.
Kratochvil CJ, Newcorn JH, Arnold LE, et al. Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms. J Am Acad Child Adolesc Psychiatry. 2005;44(9):915–24.
Bangs ME, Hazell P, Danckaerts M, et al. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314–20.
Kaplan S, Heiligenstein J, West S, et al. Efficacy and safety of atomoxetine in childhood attention-deficit/hyperactivity disorder with comorbid oppositional defiant disorder. J Atten Disord. 2004;8(2):45–52.
Ercan ES, Akyol Ardic U, Kabukcu Basay B, et al. Atomoxetine response in the inattentive and combined subtypes of attention deficit hyperactivity disorder: a retrospective chart review. Atten Defic Hyperact Disord. 2013;5(4):377–85.
Wehmeier PM, Kipp L, Banaschewski T, et al. Does comorbid disruptive behavior modify the effects of atomoxetine on ADHD symptoms as measured by a continuous performance test and a motion tracking device? J Atten Disord. doi:10.1177/1087054712456739 (Epub 2012 Aug 28).
Waxmonsky JG, Waschbusch DA, Akinnusi O, Pelham WE. A comparison of atomoxetine administered as once versus twice daily dosing on the school and home functioning of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(1):21–32.
van Wyk GW, Hazell PL, Kohn MR, et al. How oppositionality, inattention, and hyperactivity affect response to atomoxetine versus methylphenidate: a pooled meta-analysis. J Atten Disord. 2012;16(4):314–24.
Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):130–7.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733–41.
Harfterkamp M, Buitelaar JK, Minderaa RB, et al. Long-term treatment with atomoxetine for attention-deficit/hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder: an open-label extension study. J Child Adolesc Psychopharmacol. 2013;23(3):194–9.
Charnsil C. Efficacy of atomoxetine in children with severe autistic disorders and symptoms of ADHD: an open-label study. J Atten Disord. 2011;15(8):684–9.
Posey DJ, Wiegand RE, Wilkerson J, et al. Open-label atomoxetine for attention-deficit/hyperactivity disorder symptoms associated with high-functioning pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):599–610.
Troost PW, Steenhuis MP, Tuynman-Qua HG, et al. Atomoxetine for attention-deficit/hyperactivity disorder symptoms in children with pervasive developmental disorders: a pilot study. J Child Adolescent Psychopharmacol. 2006;16(5):611–9.
Arnold LE, Aman MG, Cook AM, et al. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1196–205.
Mazzone L, Reale L, Mannino V, et al. Lower IQ is associated with decreased clinical response to atomoxetine in children and adolescents with attention-deficit hyperactivity disorder. CNS Drugs. 2011;25(6):503–9.
Fernández-Jaén A, Fernández-Mayoralas DM, Calleja Pérez B, et al. Atomoxetine for attention deficit hyperactivity disorder in mental retardation. Pediatr Neurol. 2010;43(5):341–7.
Shaywitz SE, Shaywitz BA, Wietecha LA, et al. Effects of atomoxetine on reading abilities in children with dyslexia and children with attention deficit/hyperactivity disorder and comorbid dyslexia. Ann Neurol. 2012;72:S204.
Sumner CR, Gathercole S, Greenbaum M, et al. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children with ADHD and dyslexia. Child Adolesc Psychiatry Ment Health. 2009;3:40.
Gau SSF, Huang YS, Soong WT, et al. A randomized, double-blind, placebo-controlled clinical trial on once-daily atomoxetine hydrochloride in Taiwanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):447–60.
Biederman J, Gao H, Rogers AK, Spencer TJ. Comparison of parent and teacher reports of attention-deficit/hyperactivity disorder symptoms from two placebo-controlled studies of atomoxetine in children. Biol Psychiatry. 2006;60(10):1106–10.
Bohnstedt BN, Kronenberger WG, Dunn DW, et al. Investigator ratings of ADHD symptoms during a randomized, placebo-controlled trial of atomoxetine: a comparison of parents and teachers as informants. J Atten Disord. 2005;8(4):153–9.
Vakula N, Vasyanina Y, Gorbunova Z, et al. Efficacy of Strattera in children and adolescents with attention deficit hyperactivity disorder. Neurosci Behav Physiol. 2010;40(9):1034–7.
Sumner CR, Haynes VS, Teicher MH, Newcorn JH. Does placebo response differ between objective and subjective measures in children with attention-deficit/hyperactivity disorder? Postgrad Med. 2010;122(5):52–61.
Kratochvil CJ, Milton DR, Vaughan BS, Greenhill LL. Acute atomoxetine treatment of younger and older children with ADHD: a meta-analysis of tolerability and efficacy. Child Adolesc Psychiatry Ment Health. 2008;2(1):25.
Acknowledgments and conflicts of interest
N.C. Savill, K. A. Day, E. Anand, T. Treuer, and H. P. Upadhyaya are full-time employees and stock holders of Eli Lilly and Co.
J. K. Buitelaar has been a consultant, a member of an advisory board, and/or a speaker for Janssen-Cilag BV, Eli Lilly, Bristol-Myers Squibb, Organon/Schering Plough, UCB, Shire, Medice, and Servier.
D. Coghill has served in an advisory/consultancy role for Flynn Pharma, Otsuka, Lilly, Janssen, Medice, Pfizer, Schering-Plough, Shire, and Vifor. He received conference attendance support, conference support, or speaker’s fees from Flynn Pharma, Eli Lilly, Janssen, Medice, Novartis, and Shire. He has been involved in clinical trials conducted by Eli Lilly and Shire and has received research funding from Eli Lilly, Janssen, Shire, and Vifor.
This review was funded by Eli Lilly and Company. K. A. Day performed the predefined literature database searches, and J. K. Buitelaar and N. C. Savill selected relevant literature from the search results. All authors contributed to the conception/design of the manuscript, and critically reviewed and thus participated in writing the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for this work. Michael Riley, PhD, from Trilogy Writing and Consulting GmbH, Frankfurt, Germany, provided medical writing support on behalf of Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 Strategy used in ‘Search 1’
Database: EMBASE <1974 to 2013 July 25>, Ovid MEDLINE® <1946 to July Week 3 2013>, Ovid MEDLINE® In-Process and Other Non-Indexed Citations <July 25, 2013>
1 exp atomoxetine/(3060)
2 exp attention deficit disorder/(54376)
3 1 and 2 (2079)
4 limit 3 to [human and (child <unspecified age> or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)] [Limit not valid in Ovid MEDLINE®, Ovid MEDLINE® In-Process; records were retained] (725)
5 limit 4 to year = “2001–Current” (722)
6 5 use oemezd (722)
7 atomoxetine.mp. (4248)
8 tomoxetine.mp. (146)
9 LY139603.mp. (17)
10 7 or 8 or 9 (4289)
11 exp Attention Deficit Disorder with Hyperactivity/(54376)
12 10 and 11 (2818)
13 limit 12 to [humans and year = “2001–Current” and “all child (0 to 18 years)”] [Limit not valid in EMBASE; records were retained] (2468)
14 13 use mesz, prem (476)
15 6 or 14 (1198)
16 remove duplicates from 15 (879)
Rights and permissions
About this article
Cite this article
Savill, N.C., Buitelaar, J.K., Anand, E. et al. The Efficacy of Atomoxetine for the Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Comprehensive Review of Over a Decade of Clinical Research. CNS Drugs 29, 131–151 (2015). https://doi.org/10.1007/s40263-014-0224-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0224-9