Abstract
Background and Objective
We aimed to develop a meropenem population pharmacokinetic model in critically ill children receiving continuous renal replacement therapy and simulate dosing regimens to optimize patient exposure.
Methods
Meropenem plasma concentration was quantified by high-performance liquid chromatography. Meropenem pharmacokinetics was investigated using a non-linear mixed-effect modeling approach. Monte Carlo simulations were performed to compute the optimal scheme of administration, according to the target of a 100% inter-dose interval time in which concentration is one to four times above the minimum inhibitory concentration (100% fT>1–4×MIC).
Results
A total of 27 patients with a median age of 4 [interquartile range 0–11] years, a median body weight of 16 [range 7–35] kg receiving continuous renal replacement therapy were included. Concentration–time courses were best described by a one-compartment model with first-order elimination. Body weight (BW) produced significant effects on volume of distribution (V) and BW and continuous renal replacement therapy effluent flow rate (Qeff) produced significant effects on clearance (CL): \({V}_{i}={V}_{pop }{x (\frac{BWi}{70})}^{1}\) and \({CL}_{i}={CL}_{pop }x ({\frac{BWi}{70})}^{0.75} x ({\frac{Qeffi}{1200})}^{0.337}\), where Vpop and CLpop estimates were 32.5 L and 5.88 L/h, respectively, normalized to a 70-kg BW and median Qeff at 1200 mL/h. Using this final model and Monte Carlo simulations, for patients with Qeff over 1200 mL/h, meropenem continuous infusion was adequate in most cases to attain 100% fT>1–4xMIC. For bacterial infections with a low minimum inhibitory concentration (≤2 mg/L), meropenem intermitent administration was appropriate for patients weighing more than 20 kg with Qeff <500 mL/h and for patients weighing more than 10 kg with Qeff <100 mL/h.
Conclusions
Meropenem exposure in critically ill children receiving continuous renal replacement therapy needs dosing adjustments to the minimum inhibitory concentration that take into account body weight and the continuous renal replacement therapy effluent flow rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first population PK model of meropenem in critically ill children undergoing CRRT highlighting the high between subject variability, and describing the impact of allometry and effluent flow rate on PK parameters. To ensure efficacy and avoid toxicity under CRRT, continuous administrations are useful to get more stable and adequate exposure but should be adjusted to the CRRT flow rate and MIC with doses adaptation and the usefulness of therapeutic drug monitoring in this vulnerable population. |
1 Introduction
Invasive bacterial infection in critically ill children is a source of morbidity and mortality [1]. In addition to the molecule adapted to the spectrum of the suspected or identified bacteria and to the infected site, the dosing must be optimal in order to quickly and sustainably reach an effective concentration [2]. In addition to growth and organ maturation, the critical illness increases the between-subject variability (BSV) in drug distribution and elimination [2,3,4]. Factors that may alter the pharmacokinetics in critically ill children include alterations in drug protein binding, modifications in body water and fat repartition, and drug elimination due to organ function changes.
The use of extracorporeal devices such as continuous renal replacement therapy (CRRT) amplify the pharmacokinetic (PK) parameter variability, already altered because of critical illness [5]. The need for CRRT in children with sepsis may occur given that acute renal failure has an incidence of 10–20% in such a clinical situation [6,7,8,9]. To date, no robust guideline is available for antibiotic dosing in children receiving CRRT while higher BSV is expected in this population. The dosing of drugs in critically ill children is mostly an extrapolation from adults or non-critically ill children [5, 10]. Many factors can impact drug clearance (CL) in critically ill children receiving CRRT, including the physicochemical properties of the drugs and the CRRT settings [11]. Specific pharmacological studies in children receiving CRRT are therefore mandatory to identify the optimal dosage of drugs [12], especially meropenem, which is one of the most widely used classes in pediatric critical care settings because of its broad-spectrum activity against multidrug-resistant bacterial infections [13]. As meropenem is hydrophylic and has a low molecular weight, it is expected to be eliminated by CRRT, depending on the dialysate and filtration rate [14,15,16,17]. Given the expected high BSV in critically ill children receiving CRRT, the resulting concentrations are unpredictable and usual dosing regimens of meropenem may not be adequate for all to reach the therapeutic target. Meropenem has a maximal efficacy when the free concentration is one to four times above the minimum inhibitory concentration (MIC) throughout the dosing interval (100% fT>1–4×MIC) [18, 19]. Inadequate concentrations with sub-therapeutic or supra-therapeutic exposure could lead to failure of the treatment, toxicity, and the emergence of multidrug-resistant bacteria. Pharmacokinetic studies using a population approach may be useful to optimize the dosing regimen for every patient [20]. This study aimed to develop a population PK model for meropenem in critically ill children receiving CRRT in order to individualize dosing regimens according to the selected PK target of 100% fT>1–4×MIC.
2 Methods
2.1 Patients and Settings
This study was conducted in a 32-bed pediatric intensive care unit (PICU) center and high-dependency unit at the Necker Hospital (Paris, France) from September 2015 to February 2018. All children aged less than 18 years receiving meropenem and undergoing CRRT were included. Baseline patient characteristics, actual body weight (BW), severity illness, residual diuresis, CRRT modalities and biological data were collected at every sampling time. The Ethics Committee of Necker Hospital approved the study, which was registered at www.clinicaltrials.gov (NCT02539407). Before any inclusion, the senior physician proposed the study to parental authority holders whose child received or were going to receive anti-infective agents during the follow-up or hospitalization. The senior physician also provided an information note to the parental authority holders, and to the child if the child was able to understand the information. The consent was obtained from children’s parent(s) and non-oral opposition for data retrieval and analysis was collected.
Clinical and microbiological success criteria were defined as (i) no escalation of therapy (change of antibiotic) for the treatment of the same infection and (ii) the disappearance of obvious infectious signs. For bacteremia: negative blood cultures at the supposed end of treatment (conventional duration 7–10 days) without recurrence within 14 days of stopping treatment. For pneumonia: no relapse within 14 days of stopping treatment. For deep infection: negative deep samples (if available) and no relapse within 21 days. We defined clinical failure as the persistence of clinical/biological infectious signs at the end of treatment and microbiological failure as the persistence of bacteria in the sterile sites at the end of treatment and/or the recurrence of infection with the same bacteria within the above-mentioned delay.
2.2 Study Design
According to local protocol, meropenem (1 g of powder, Viatris™; Paris, France) was diluted (glucose 5%, Freeflex™; Fresenius Kabi, Paris, France) to obtain 100-mg/mL standard solutions. Meropenem was prescribed at 60 mg/kg per day, administered by intravenous intermittent infusions every 8 h over 20 min or in continuous infusions (a loading dose of 20 mg/kg over 20 min then a maintenance dose of 60 mg/kg per day) using a programmable electronic syringe pump (Orchestra™ DPS; Fresenius Kabi, Paris, France). Syringes were changed every 6 h for continuous infusion modality. Dosing regimens could be modified during the treatment period at the discretion of the treating physician. Blood samples were collected before the entry line of CRRT (pre-filter) during routine laboratory tests and as part of the patient’s routine clinical care. Samples were centrifuged (4000×g, 5 min) to yield plasma, which was stored at – 20 °C before analysis. The samples were sent directly to the laboratory of Cochin Hospital and measured day by day. If it was during working days, it was immediately considered for assay. If not, the samples were buffered with 3-(N-morpholino) propanesulfonic acid before storage to minimize the degradation of antibiotics allowing a sensitive quantification.
2.3 Antimicrobial Susceptibility Determination
Minimum inhibitory concentrations were determined in the microbiology laboratory of Necker Hospital (Paris, France). Minimum inhibitory concentrations for meropenem were evaluated by a diffusion method on a solid-state culture, using the E-test methodology (ETEST; bioMérieux, Paris, France). When the MIC was not available, clinical breakpoints were used for identified pathogens according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations [21] such as the breakpoint for resistance regarding Pseudomonas aeruginosa, one of the most common bacteria encountered in PICU, of which 8 mg/L was taken for simulations.
2.4 CRRT
Continuous renal replacement therapy was performed using the PrismaflexTM (Gambro, Deerfield, IL, USA) with continuous venovenous hemofiltration, continuous venovenous hemodialysis (CVVHD), or continuous venovenous hemodiafiltration. Continuous renal replacement therapy was performed using a 0.2-m2 polyarylethysulfone filter (HF20; Gambro) or a 0.6-m2 or 1.5-m2 polyacrylonitrile filter (AN69 ST60 or ST150; Gambro) through a double-lumen catheter inserted into the femoral or internal jugular vein. Heparin or regional citrate anticoagulation was used either through the pre-blood pump or using a three-way stopcock attached to the arterial line. All flow rates, including dialysate fluid flow (QD), filtration replacement fluid flow (QRF), and ultrafiltration flow (QUF), were recorded from the PrismaflexTM machine during the study. These flow rates were added to obtain the total effluent flow rate (Qeff) resulting in the equation Qeff = QD + QRF + QUF.
2.5 Assays
Meropenem concentrations were quantified using a validated high-performance liquid chromatography with ultraviolet detection, based on a previous method [22]. The analysis was performed using the Ultimate 3000™ Chromatographic system (Dionex Corporation, Sunnyvale, CA, USA). The method was validated according to the European Medicines Agency guidelines for bioanalytical method validation [23]. The calibration range was from 0.5 to 200 mg/L. Inter-assay and intra-assay precision and accuracy were all between −5.8% and 6.3%.
2.6 Population PK Model
Meropenem time courses were modeled using a non-linear mixed-effect modeling software (MONOLIX, 2019R2 version) using the stochastic approximation expectation maximization algorithm. Both one-compartment and two-compartment structural models with first-order elimination were tested. The BSV (ω) was ascribed to an exponential distribution. The residual variability (σ) was tested for additive, proportional, or combined variability models. Outliers were defined as data points in the dataset that appear to be outside the norm for the dataset (e.g., data with conditional weighted residuals >5).
Given that meropenem was administered every 8 hours, steady state was assumed to be reached after the third injection. Several covariates were tested: BW, sex, post-natal age, albumin level, residual diuresis rates either in mL/h or in mL/kg/h, mechanical ventilation, catecholamines, Extra-Corporeal Membrane Oxygenation (ECMO) use, Paediatric Logistic Organ Dysfunction score, and number of organ dysfunctions. As meropenem is eliminated by the CRRT, we tested the effect on CL of the CRRT parameters: Qeff, QD, QRF, and QUF and blood flow rates in mL/h or in mL/kg/h and CRRT set sizes.
Categorical covariates were assessed as follows:
where \({\theta }_{i}\) is the individual PK parameter for the ith patient,\({\theta }_{pop}\) is the median population value for the parameter of the group for which the covariate is equal to 0, \(\theta\) is the covariate parameter, and \(cov\) is the category 0 or 1 for the covariate. Continuous covariates were associated using a power function:
where \({cov}_{i}\) is the covariate value for the ith patient, \(med\left(cov\right)\) is the median value of the covariate, and \(\beta\) is the exponent.
Body weight effect was assessed according to allometric rules and \(\beta\) values was fixed at 0.75 for CL and 1 for V. The Bayesian Information Criterion was used to test different hypotheses regarding the final model, covariate effect on PK parameters, residual variability model, and structure of the variance-covariance matrix for the BSV parameters. Pharmacokinetic parameters were properly estimated if the relative standard errors were <50%. The effect of a covariate was retained if it caused a decrease in the Bayesian Information Criterion and reduced the corresponding BSV. The goodness-of-fit plots (observed-predicted concentration scatter plots and normalized prediction distribution error vs time/predicted concentration scatter plots) of each model were evaluated by visual inspection. From the final model, 1000 Monte Carlo simulations per patient were performed to compute the prediction-corrected visual predictive check to evaluate the model [24].
2.7 Dosing Regimen Simulations
From the final model, 1000 Monte Carlo simulations were performed using the R software to determine the probability of target attainment (target of 100% fT>1–4×MIC) according to different dosing regimens and MICs. The simulated dosing regimens were: (1) 20 mg/kg every 12 h as a 20-min infusion, (2) 40 mg/kg every 12 h as a 20-min infusion, (3) 20 mg/kg every 8 h as a 20-min infusion, (4) 40 mg/kg every 8 h as a 20-min infusion, (5) 20 mg/kg of a loading dose then 60 mg/kg per day as a continuous infusion, and (6) 40 mg/kg of loading dose then 120 mg/kg per day as a continuous infusion. As the EUCAST clinical breakpoint for P. aeruginosa sensitivity is 2 mg/L and for resistance is 8 mg/L, we decided to consider the worst-case scenario corresponding to 8 mg/L and assumed the thresholds one and four times the breakpoint, i.e., 8 mg/L and 32 mg/L. [21]. The upper threshold for neurotoxicity was a trough concentration higher than 64 mg/L [25].
3 Results
3.1 Patients
Eighty-eight samples from 27 patients receiving CRRT were available. Two samples were discarded as outliers. The main characteristics are summarized in Table 1. Median [interquartile range (IQR)] Paediatric Logistic Organ Dysfunction-2 score and number of organ dysfunctions were respectively, 7 [5–10] and 4 [3–5], with 16 (59%) patients anuric. Eleven (41%) patients had comorbidities: hematological disease (n = 8), immune deficiency (n = 5), heart disease (n = 4), lung disease (n = 4), liver disease (n = 3), bowel disease (n = 2), and sickle cell disease (n = 1).
The median [IQR] number of meropenem concentrations per patient was three [2,3,4]. Continuous renal replacement therapy modalities were as follows: median [IQR] blood flow rate of 80 [50–120] mL/min corresponding to 5 [3–8] mL/kg/min; median [IQR] total effluent flow (Qeff) of 1025 [735–1644] mL/h corresponding to 77 [47–110] mL/kg/h. For the patients undergoing continuous venovenous hemodialysis or continuous venovenous hemodiafiltration, median [IQR] dialysate flow rate was 750 [500–2875] mL/h corresponding to 83 [53–100] mL/kg/h. For the patients undergoing continuous venovenous hemofiltration or continuous venovenous hemodiafiltration, median [IQR] filtration replacement fluid flow rate was 400 [100–900] mL/h corresponding to 29 [13–44] mL/kg/h. The median [IQR] meropenem daily dosing regimen was 60 [60–64] mg/kg/24 hours. Among the 86 measured plasma meropenem concentrations under CRRT, 10/43 (23%) were superior or equal to four times the MIC for documented bacterial infection and 12/45 (27%) for undocumented infection, which were superior or equal to four times the clinical breakpoint for P. aeruginosa corresponding to 32 mg/L.
Among the 27 patients, 17 infections were documented in 14 patients. Nine pathogens had a measurable MIC. In the cohort, 14 (52%) patients died. Six (22%) patients died from septic shock, among whom two (33%) were underexposed to meropenem and only one patient had a documented infection (extended spectrum beta-lactamase (ESBL) Escherichia coli in a tracheal sample with an MIC at 0.0016 mg/L) with a good exposure to meropenem.
3.2 Population PK Model
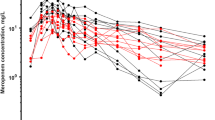
A one-compartment model with first-order elimination best described the data. Between-subject variability could be estimated for CL only and residual variabilities were estimated using a combined (proportional plus additive) model. The weight-based allometric approach decreased the Bayesian Information Criterion by 27 units and the BSV decreased from 0.79 to 0.45. Addition of the effluent flow rate (in mL/h) effect on CL further decreased its BSV to 0.37. The final PK parameter estimates are summarized in Table 2. The other covariates had no significant effect. The goodness-of-fit plots are depicted in Fig. 1. The normalized prediction distribution error metrics mean and variance were not different from 0 and 1 (p = 0.564 and p = 0.645), plus the normalized prediction distribution error distribution was not significantly different from normality (p = 0.9). The prediction-corrected visual predictive check is shown in Fig. 2.
Goodness-of-fit plots for the final meropenem population model. Black, red, and blue lines stand for the identity, regression, and spline curves. Normalized prediction distribution error (NPDE) mean and variance not different from 0 and 1 (p = 0.564 and p = 0.645), NPDE distribution not significantly different from normality (p = 0.9). DV meropenem concentration in mg/L, popPred and indivPred_mode population and individual predicted concentrations, mg/L, rho Spearman correlation coefficient
Prediction-corrected visual predictive check, meropenem concentration versus time. Lines depict the 10th, 50th, and 90th percentiles of observed data, and the blue and light-red areas represent the corresponding 90th confidence interval. Observation, prediction corrected meropenem concentration, blue circles, in mg/L; time in hours (h)
For patient i and BWi normalized by 70 kg, the final equations were:
-
CLi = \(5.88*{\left(\frac{BWi}{70}\right)}^{0.75}*{\left(\frac{\mathrm{Qeff}i}{1200}\right),}^{0.337}\) with CL in L/h and Qeffi in mL/h
-
Vi = \(32.5{* (\frac{BWi}{70})}^{1},\) with V in L
where i denotes the ith individual.
3.3 Dosing Regimen Optimization
We used our final model to perform Monte Carlo simulations to determine the probability to attain the target of 100% fT>1xMIC among different BW and CRRT effluent flow rates for MICs that ranged from 0.25 to 32mg/L (Fig. 3). Simulations curves of concentrations versus time among different CRRT effluent flow rates (Qeff at 100 mL/h, 500 mL/h, 1000 mL/h, and 1500 mL/h) are depicted in Fig. 4 showing treatment margins for efficacy and toxicity under (a) intermittent administrations corresponding to 20 mg/kg every 8 h as a 20-min infusion and 40 mg/kg every 8 h as a 20-min infusion or (b) continuous administrations corresponding to 20 mg/kg of a loading dose then 60 mg/kg per day as a continuous infusion and 40 mg/kg of a loading dose then 120 mg/kg per day as a continuous infusion. We suggested the best scheme to attain the target of 100% fT>4×MIC, at steady state according to MIC, BW, and the effluent flow rate (Table 3). According to these Monte Carlo simulations, for Qeff <100 mL/h corresponding to a very low CRRT effluent flow rate, intermittent infusion was the most appropriate regarding the risk of accumulation, especially for BW >10 kg, as shown in Fig. 4. For Qeff = 500 mL/h, for BW below 20 kg, continuous infusion was the most adapted dosing regimen to attain the target. For BW over 20 kg, intermittent infusion was sufficient except for MIC equal or over 4 mg/L. For the CRRT effluent flow rate over 1000 mL/h, continuous infusion was necessary to attain the target (superior or equal to four times the clinical breakpoint for P. aeruginosa corresponding to 32 mg/L) regardless of BW and MIC, but dosing was still dependent on MIC.
Probability of target attainment (PTA) for a target defined as 100% fT>1×MIC for a 16-kg patient. Blue vertical line: 1× Pseudomonas aeruginosa clinical breakpoint for resistance; red vertical line: 4× P. aeruginosa clinical breakpoint for resistance. CI continuous infusion, LD loading dose, MIC minimum inhibitory concentration (mg/L), q8h intermittent administration every 8 hours (h)
Concentration–time curves for various meropenem dosing schemes. (a) Intermittent infusion: 20 mg/kg every 8 h (h) as a 20-min infusion and 40 mg/kg every 8 h as a 20-min infusion. (b) Continuous administrations corresponding to a 20 mg/kg loading dose then 60 mg/kg per day as a continuous infusion and a 40-mg/kg loading dose then 120 mg/kg per day as a continuous infusion. (c) Intermittent infusion: 20 mg/kg every 12 h as a 20-minute infusion and 40 mg/kg every 12 h as a 20-minute infusion. For each figure, the simulations were performed for a different range of total effluent flow (Qeff) with, respectively, Qeff= 100mL/h, Qeff= 500mL/h, Qeff = 1000mL/h, and Qeff = 1500mL/h. Dotted lines: superior line corresponding to the neurotoxicity threshold at 64 mg/L and inferior lines at 4× EUCAST clinical breakpoint for Pseudomonas aeruginosa, which is at 8mg/L
4 Discussion
To the best of our knowledge, this is the first population PK model of meropenem in critically ill children undergoing CRRT, highlighting the high BSV, and describing the impact of allometry and effluent flow rate on PK parameters. A non-negligible variability has not been explained because there is a substantial residual (within-subject) variability that cannot be reduced that is expected from PICU patients. Therefore, our dosing guidance is here to avoid first overdosing or underdosing, but the therapeutic drug monitoring approach is still needed to adapt and personalize the dosing to potential modifications of the parameters (individual or CRRT).
Concentration–time courses were best described by a one-compartment model with first-order elimination. Body weight, on an allometric basis, was a significant predictor of individual CL and V. Moreover, CL was also positively related to the total effluent flow rate (Qeff) and further reduced ωCL. The population parameters were adjusted to an adult BW (70 kg) in order to facilitate comparison with the review provided in Table S1 of the Electronic Supplementary Material [26]. Most of the other covariates tested such as age, specific CRRT parameters, albumin level, residual diuresis, Extra-Corporeal Membrane Oxygenation (ECMO) use, Paediatric Logistic Organ Dysfunction score, and number of organ dysfunctions were not significant contrary to what was previously reported [26]. Indeed, some studies found different structural models, one or two compartments with an albumin effect [27] or residual diuresis [28] but rarely with a CRRT parameter impact [27,28,29,30]. We considered that the residual renal function was not dependent on Qeff. As most of the patients in our study did not have residual diuresis, we also considered that CL was dependent on Qeff only. The type of CRRT or the different values of QD, QRF, and QUF and blood flow rates in mL/h or in mL/kg/h or CRRT set sizes were not found significant. Meropenem is eliminated both by metabolism and excretion, while up to 70% was recovered in the urine of normal volunteers [31, 32]. Our study is consistent with that as both ‘endogenous’ (renal and hepatic) CL (reflected by age and BW) and CL by CRRT (reflected by the effluent flow rate) will determine the total CL and subsequent dosing. In our study, 41% of the patients had a residual diuresis (n = 11/27).
The present study is one of the largest population PK studies on PICU patients and the first pediatric population PK study to highlight the impact of CRRT settings. Compared to adults, the impact of CRRT parameters on CL may be due to a relatively high effluent flow used on critically ill children. Yet, this was expected, as meropenem is an hydrophilic molecule (molecular weight of 437.52 Da) with negligible protein binding (2%) and a sieving coefficient reported in the literature superior to 0.90, meaning that CL is nearly proportional to the total effluent flow rate, CL = 0.9 × Qeff [33, 34]. These properties facilitate the diffusion through the dialysate membrane, regardless of dialysis or filtration mode [35] with negligible bound meropenem. In recent ex vivo studies, both the high dialysate and filtrate flow rates influenced meropenem elimination [36, 37]. Continuous renal replacement therapy has already been included in a meropenem population PK model in critically ill children but without reflecting the values of the flow rates [30, 38].
To ensure efficacy and avoid toxicity under CRRT, continuous administrations are useful to obtain more stable and adequate exposure but should be adjusted to the CRRT flow rate and MIC. Therefore, reduced daily intermittent doses every 12 hours when Qeff is low seemed to be sufficient for an adequate exposure. Tables 3 and 4 summarize the best scheme to attain the target of 100% fT>1–4×MIC, respectively, at steady state according to MICs that ranged from 0.125 to 8 mg/L, BW and effluent flow rate as tools to guide phycisian for an a priori dosing initiation that should be associated with therapeutic drug monitoring to adjust the dosing during the whole period of the treatement. In the current literature, there is some variability on the chosen MIC target to attain (from a EUCAST clinical breakpoint for sensitivity of 2 mg/L to a EUCAST clinical breakpoint for resistance of 8 mg/L). We considered a 8-mg/L MIC target. This may seem like an ambitious goal, but it is relevant to achieve the best PK target for the most severe cases in this critically ill population where inefficacy could be harmful. This is consistent with previous studies that use the 8-mg/L threshold for target attainment and dosing simulations [29, 39,40,41]. Furthermore, in our practice, meropenem is administered even when P. aeruginosa has an MIC of 8 mg/L. As we could only measure the plasma concentration for the treatment of deep and severe infections, it would be useful to consider this PK target to be achieved at least in plasma.
The CL increased with Qeff; therefore, failure to attain the target could be avoided with a continuous infusion, especially with high Qeff and high MIC. The meropenem threshold concentration for which there is 50% risk of developing a neurotoxicity or nephrotoxicity event is a trough concentration higher than 64 mg/L and 44 mg/L, respectively [25], forgetting to adjust the dosing especially if the CRRT is discontinued could lead to an accumulation with a risk of toxicity. This supports therapeutic drug monitoring and prospective recording of adverse effects with our suggested dosing regimens, to ensure both efficacy and safety exposure.
Our study had some limitations. Because of the small size of our cohort, we could not describe the association between meropenem concentrations and clinical or bacterial outcomes. Owing to the lack of documentation, we could not provide all MICs and therefore the observed suboptimal exposure rate with actual dosing was likely overestimated. We also could not use the concentrations of meropenem in the effluent, which may improve the model, but the very few patients who had a dosage in the effluent would limit the interpretation even in a population PK approach. Conversely, this study improved the knowledge of meropenem pharmacokinetics in critically ill children, confirming the high variability of CRRT parameters and their associated impact on CL and highlighting the benefit of dose adaptation to the CRRT flow rate and the usefulness of therapeutic drug monitoring in this vulnerable population.
5 Conclusions
Optimal meropenem exposure in critically ill children receiving CRRT needs an individualization of the dosing schemes depending on the MIC, BW, and CRRT effluent flow rate with reduced intermittent doses under a low CRRT effluent flow rate and continuous infusion under a high MIC and/or high CRRT effluent flow rate.
References
Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis. 2015;15:46–54.
Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43:223–30.
Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, et al. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523–8.
Felton TW, Hope WW, Roberts JA. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis. 2014;79:441–7.
Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical pharmacology studies in critically ill children. Pharm Res. 2017;34:7–24.
Groupe E, Van Vong L, Osman D, Vinsonneau C. Épuration extrarénale en réanimation adulte et pédiatrique.: recommandations formalisées d’experts sous l’égide de la Société de Réanimation de Langue Française (SRLF), avec la participation de la Société Française d’Anesthésie-Réanimation (Sfar), du Groupe Francophone de Réanimation et Urgences Pédiatriques (GFRUP) et de la Société Francophone de Dialyse (SFD): Société de Réanimation de Langue Française. Experts recommendations. Réanimation. 2014;23:714–37.
Xu X, Nie S, Zhang A, Mao J, Liu H-P, Xia H, et al. Acute kidney injury among hospitalized children in China. Clin J Am Soc Nephrol. 2018;13:1791–800.
Plötz FB, Hulst HE, Twisk JWR, Bökenkamp A, Markhorst DG, van Wijk JAE. Effect of acute renal failure on outcome in children with severe septic shock. Pediatr Nephrol. 2005;20:1177–81.
Barletta G-M, Bunchman TE. Acute renal failure in children and infants. Curr Opin Crit Care. 2004;10:499–504.
Udy AA, Roberts JA, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013;39:2070–82.
Bridges BC, Askenazi DJ, Smith J, Goldstein SL. Pediatric renal replacement therapy in the intensive care unit. Blood Purif. 2012;34:138–48.
Shaw AR, Mueller BA. Antibiotic dosing in continuous renal replacement therapy. Adv Chronic Kidney Dis. 2017;24:219–27.
Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008;68:803–38.
Beltramo F, Dicarlo J, Gruber JB, Taylor T, Totapally BR. Renal replacement therapy modalities in critically ill children. Pediatr Crit Care Med. 2018;2:2.
Wong W-T, Choi G, Gomersall CD, Lipman J. To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: the eternal doubt. Curr Opin Pharmacol. 2015;24:68–78.
Böhler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int. 1999;56:S24–8.
Beumier M, Casu G, Hites M, Seyler L, Cotton F, Vincent J-L, et al. β-lactam antibiotic concentrations during continuous renal replacement therapy. Crit Care. 2014;18:R105.
Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther. 2004;26:1187–98.
Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am. 2003;17:479–501.
Cohen J. Confronting the threat of multidrug-resistant Gram-negative bacteria in critically ill patients. J Antimicrob Chemother. 2013;68:490–1.
EUCAST. Clinical breakpoints and dosing of antibiotics. Available from: https://eucast.org/clinical_breakpoints/. [Accessed 22 Jun 2020].
Legrand T, Chhun S, Rey E, Blanchet B, Zahar J-R, Lanternier F, et al. Simultaneous determination of three carbapenem antibiotics in plasma by HPLC with ultraviolet detection. J Chromatogr B. 2008;875:551–6.
Schuster C, Sterz S, Teupser D, Brügel M, Vogeser M, Paal M. Multiplex therapeutic drug monitoring by isotope-dilution HPLC-MS/MS of antibiotics in critical illnesses. J Vis Exp. 2018;2:58148.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Imani S, Buscher H, Marriott D, Gentili S, Sandaradura I. Too much of a good thing: a retrospective study of β-lactam concentration-toxicity relationships. J Antimicrob Chemother. 2017;72:2891–7.
Béranger A, Oualha M, Urien S, Genuini M, Renolleau S, Aboura R, et al. Population pharmacokinetic model to optimize cefotaxime dosing regimen in critically ill children. Clin Pharmacokinet. 2018;57:867–75.
Onichimowski D, Będźkowska A, Ziółkowski H, Jaroszewski J, Borys M, Czuczwar M, et al. Population pharmacokinetics of standard-dose meropenem in critically ill patients on continuous renal replacement therapy: a prospective observational trial. Pharmacol Rep. 2020;72:719–29.
Ulldemolins M, Soy D, Llaurado-Serra M, Vaquer S, Castro P, Rodríguez AH, et al. Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother. 2015;59:5520–8.
Isla A, Rodríguez-Gascón A, Trocóniz IF, Bueno L, Solinís MÁ, Maynar J, et al. Population pharmacokinetics of meropenem in critically ill patients undergoing continuous renal replacement therapy. Clin Pharmacokinet. 2008;47:173–80.
Tan WW, Watt K, Boakye-Agyeman F, Cohen-Wolkowiez M, Mok YH, Yung CF, et al. Optimal dosing of meropenem in a small cohort of critically ill children receiving continuous renal replacement therapy. J Clin Pharmacol. 2020. https://doi.org/10.1002/jcph.1798.
Mouton JW, van den Anker JN. Meropenem clinical pharmacokinetics. Clin Pharmacokinet. 1995;28:275–86.
Tegeder I, Neumann F, Bremer F, Brune K, Lötsch J, Geisslinger G. Pharmacokinetics of meropenem in critically ill patients with acute renal failure undergoing continuous venovenous hemofiltration. Clin Pharmacol Ther. 1999;65:50–7.
Craig WA. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis. 1997;24(Suppl. 2):S266–75.
Pistolesi V, Morabito S, Di Mario F, Regolisti G, Cantarelli C, Fiaccadori E. A guide to understanding antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob Agents Chemother. 2019;63:e00583-e619.
Goldstein SL, Murry DJ, May S, Aleksic A, Sowinski KM, Blaney S. Meropenem pharmacokinetics in children and adolescents receiving hemodialysis. Pediatr Nephrol. 2001;16:1015–8.
Isla A, Maynar J, Sánchez-Izquierdo JÁ, Gascón AR, Arzuaga A, Corral E, et al. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J Clin Pharmacol. 2005;45:1294–304.
Sime FB, Pandey S, Karamujic N, Parker S, Alexander E, Loutit J, et al. Ex vivo characterization of effects of renal replacement therapy modalities and settings on pharmacokinetics of meropenem and vaborbactam. Antimicrob Agents Chemother. 2018;62:e01306-e1318.
Saito J, Shoji K, Oho Y, Kato H, Matsumoto S, Aoki S, et al. Population pharmacokinetics and pharmacodynamic implementation of meropenem in critically ill pediatric patients. Antimicrob Agents Chemother. 2021;65:e01909-e1920.
Cojutti P, Maximova N, Pea F. Pharmacokinetics and pharmacodynamics of continuous-infusion meropenem in pediatric hematopoietic stem cell transplant patients. Antimicrob Agents Chemother. 2015;59:5535–41.
Ehmann L, Zoller M, Minichmayr IK, Scharf C, Huisinga W, Zander J, et al. Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents. 2019;54:309–17.
Selig DJ, Akers KS, Chung KK, Pruskowski KA, Livezey JR, Por ED. Meropenem pharmacokinetics in critically ill patients with or without burn treated with or without continuous veno-venous hemofiltration. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.15138.
Acknowledgments
The authors thank the PICU team (physicians and nurses) that selected the children and collected the samples, making this work possible. They also thank the pharmacology laboratory of the Cochin Teaching Hospital, which analyzed the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
MT collected the data and drafted the manuscript. MT and MO conceived the study and critically revised the manuscript. EB identified pathogen agents and related MIC and also critically revised the manuscript. MT, SU, FF, and NB contributed to the acquisition, analysis, and interpretation of data and also critically revised the manuscript. IG, AB, JT, RB, FL, SR, JMT, and SB critically revised the manuscript.
Funding
Michael Thy received a grant from the “Société de Reanimation de Langue Française” supporting research on this topic.
Conflicts of Interest
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The Ethics Committee of Necker Hospital approved the study, which was registered at www.clinicaltrials.gov (NCT02539407).
Consent to Participate
Before any inclusion, written consent was obtained from children’s parent(s).
Consent for Publication
Not applicable.
Availability of Data and Material
All data generated or analyzed during this study are included in this study or its supplementary material files. Further inquiries can be directed to the corresponding author.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thy, M., Urien, S., Bouazza, N. et al. Meropenem Population Pharmacokinetics and Dosing Regimen Optimization in Critically Ill Children Receiving Continuous Renal Replacement Therapy. Clin Pharmacokinet 61, 1609–1621 (2022). https://doi.org/10.1007/s40262-022-01179-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01179-2