Abstract
Background
Asciminib, a first-in-class, highly potent and specific ABL/BCR-ABL1 inhibitor, has shown superior efficacy compared to bosutinib in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase, treated with two or more tyrosine kinase inhibitors. This study aimed to describe pharmacokinetic (PK) properties of asciminib and to identify clinically relevant covariates impacting its exposure.
Methods
A population PK (PopPK) model was developed using a two-compartment model with delayed first-order absorption and elimination. The analysis included PK data from two clinical studies (Phases 1 and 3) involving 353 patients, with total daily dose of asciminib in the range of 20–400 mg.
Results
The nominal total daily dose was incorporated as a structural covariate on clearance (CL), and body weight (BW) was included as a structural covariate via allometric scaling on CL and central volume. Renal function and formulation were included as statistically significant covariates on CL and absorption (ka), respectively. The simulation results revealed a modest but clinically non-significant effect of baseline BW and renal function on ka. Correlations between covariates, such as baseline demographics and disease characteristics, heavy smoking status, hepatic function, and T315I mutation status, were not statistically significant with respect to CL, and they were not incorporated in the final model. Additionally, the final model-based simulations demonstrated comparable exposure and CL for asciminib 40 mg twice daily and 80 mg once daily (an alternative regimen not studied in the Phase 3 trial), as well as similar PK properties in patients with and without the T315I mutation.
Conclusions
The final PopPK model adequately characterized the PK properties of asciminib and assessed the impact of key covariates on its exposure. The model corroborates the use of the approved asciminib dose of 80 mg total daily dose as 40 mg twice daily, and supports the use of 80 mg once daily as an alternative dose regimen to facilitate patient’s compliance.
Trial Registration Number [Date of Registration]
First-in-human (CABL001X2101, Phase 1), ClinicalTrials.gov identifier: NCT02081378 [28 February 2014]; ASCEMBL (CABL001A2301, Phase 3), ClinicalTrials.gov identifier: NCT03106779 [10 April 2017].
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The population pharmacokinetics (PK) of asciminib, an FDA-approved drug for adult patients with chronic myeloid leukemia, is described by a non-linear mixed effects model using data from two clinical trials (First-in-Human (FIH) and ASCEMBL). |
The analysis finds that asciminib’s clearance is significantly affected by body weight, total daily dose and kidney function, while its absorption is significantly impacted by formulation (capsule or tablet). The PK properties of asciminib are not influenced by baseline factors such as race, age, sex, study effect, T315I mutation status, disease characteristics (chronic or acute phase), heavy smoking status, and liver function. |
The analysis indicates that both doses approved by the FDA, 80 mg once daily (evaluated in FIH study) and 40 mg twice daily (evaluated in both FIH and ASCEMBL studies) have the same clearance and overall PK properties, and further supports the use of 80 mg once-daily approved dose from the perspective of patients’ compliance. Moreover, the model shows similar asciminib PK properties in patients with/without T315I mutation. |
1 Introduction
Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML), which accounts for ~ 15% of newly diagnosed cases of adult leukemia [1], is driven by a novel fusion gene BCR-ABL1 that encodes a chimeric protein (P210BCRABL1) with constitutive tyrosine kinase activity [2]. The introduction of several adenosine triphosphate-competitive tyrosine kinase inhibitors (TKIs) has transformed the landscape of CML treatment, providing patients with near-normal life-expectancy. However, many patients either develop drug-resistance or experience intolerance to subsequent lines of TKI therapy [3]. Asciminib is a first-in-class inhibitor of BCR-ABL1 that specifically targets the ABL Myristoyl Pocket (STAMP) [4, 5]. Findings from ongoing trials highlight the potential of asciminib to transform the standard of care for patients with Ph+ CML in chronic phase (CP) [5,6,7].
Following oral administration, asciminib is rapidly absorbed (median time to maximum concentration (Tmax) 2–3 h) in healthy participants and was mainly (80%) eliminated through the feces. Direct glucuronidation and oxidation were the major metabolic pathways in humans with relative contribution to the total clearance ranged to ~ 28–58% and ~ 37–64%, respectively [8]. Despite increased asciminib exposure in patients with impaired renal and hepatic function, there was no clinically meaningful effect on the efficacy or safety profile of asciminib [9]. The overall findings from the drug-drug interaction study in healthy humans found that asciminib [40 mg twice daily (b.i.d.)] is a weak inhibitor of cytochrome P3A (CYP3A) and CYP2C9, with no clinically relevant effect on CYP2C8, and also supported the concomitant use of CYP3A and P-glycoprotein inhibitors, CYP3A inducers, CYP2C9 and CYP2C8 substrates in patients treated with asciminib [10].
In the first-in-human (FIH) dose-escalation study (NCT02081378, Phase 1), patients with Ph+ CML in CP and accelerated phase (AP), who relapsed or were refractory to at least two prior TKIs, received asciminib between the doses of 10 and 200 mg once daily (q.d.) or 10–200 mg b.i.d. Asciminib showed durable clinical activity with major molecular responses (MMRs) achieved (MMR being defined as BCR-ABL1 < 0.1%) and maintained in 48% of patients over 12 months of treatment [11]. Furthermore, asciminib showed a favorable safety profile and meaningful clinical efficacy for patients harboring T315I mutation (mutation at residue 315 in the ABL kinase domain) [11]. In a Phase 3 study (ASCEMBL, NCT003106779), asciminib (40 mg b.i.d.) demonstrated statistically significant and clinically meaningful superior efficacy compared with the second-generation TKI bosutinib (500 mg q.d.) in resistant/intolerant patients with CML in CP treated with at least two prior TKIs. The MMR rate was nearly twofold higher with asciminib when compared to that of bosutinib at Week 24 (p = 0.029). In addition, asciminib demonstrated a favorable safety profile as discontinuation rate due to adverse events was nearly four times lower with asciminib than with bosutinib (5.8% vs. 21.1%) [12]. In October 2021, the US Food and Drug Administration (FDA) approved asciminib 80 mg q.d. or 40 mg b.i.d. for patients with Ph+ CML in CP, relapsed or refractory to two or more prior TKIs and asciminib 200 mg b.i.d. for patients with Ph+ CML-CP with the T315I mutation [13].
The aim of this study was to develop a population pharmacokinetic (PopPK) model to characterize the pharmacokinetics of asciminib and to identify potential intrinsic and extrinsic factors influencing its exposure. The analysis was based on pooled patient data from the FIH and ASCEMBL studies, which enrolled patients with Ph+ CML (in CP and AP) who were previously treated with at least two TKIs, including patients harboring the T315I mutation.
2 Methods
2.1 Studies Included in the Population Pharmacokinetic (PopPK) Analysis
This analysis was based on pooled data derived from patients enrolled in FIH and ASCEMBL studies and treated with asciminib in a dose range of 10–200 mg b.i.d., and 80 mg, 120 mg, and 200 mg q.d. The two studies are summarized in Table S1 of the Electronic Supplementary Material (ESM).
When generating analysis datasets for the PopPK model, the missing covariate values were imputed by the median value for continuous covariates and by the most frequent category for categorical covariates. Covariates that were only collected in a smaller subset of patients (heavy smoking status and hepatic function) or when there was only a small proportion of patients with a given covariate (e.g., AP), were not included as part of the covariate evaluation process, but were investigated through correlation with individual model parameters from the PopPK model.
In addition, positive concentration data prior to the first administration of asciminib were excluded from the analysis. Data from patients who switched from the bosutinib arm to the asciminib arm were also excluded.
2.2 PopPK Model
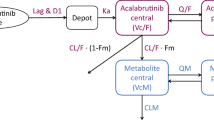
2.2.1 Base Model: Structural and Pharmacostatistical
The initial pharmacokinetic model was a two-compartment model with first-order absorption (ka) and lag time (Tlag), clearance (CL), volumes of central and peripheral compartments (V1 and V2, respectively), and inter-compartmental clearance (Q). Here, CL, V1, V2, and Q are apparent parameters, implicitly containing the 1/F term, where F refers to the bioavailability. The observed data (yij) were described using non-linear mixed effects models composed of both fixed and random effects (described by the population parameters vector \(\psi = \left\{{\beta }_{CL}, {\beta }_{V1}, {\beta }_{V2\dots \dots },\Omega ,\mathrm{ a},\mathrm{ b}\right\}\) such as:
\({{\varvec{y}}}_{{\varvec{i}}{\varvec{j}}}=f\left({t}_{ij};{\psi }_{i}\right)+{e}_{ij}\) where \({t}_{ij}\) represents the time of measurement \(j\) for patient \(i\), with \(i=1,\dots ,N\)(= total number of patients) and
\(j=1,\dots ,{n}_{i}\)(= number of observations for patient i), and the following assumptions are made:
-
\(f\left(t\right)\) is a (real-valued) function of time (\(t)\)
-
For each individual \(i\), \({\psi }_{i}\) is a (vector-valued) function \(M\left(\beta ,{\eta }_{i}\right)\), which depends on fixed effects
\(\beta =\{{\beta }_{CL}, {\beta }_{V1}, {\beta }_{V2}, \dots \}\) and inter-individual random effects \({\eta }_{i}=\{{\eta }_{i,CL},{\eta }_{i, V1},{\eta }_{i,V2},...\}\)
-
The \({\eta }_{i}\) values have a Gaussian multivariate distribution \(N(0,\Omega )\), \(\Omega\) being the variance-covariance matrix of all the random effects \({\eta }_{i}\), such as \(\Omega =\left\{\begin{array}{ccc}{\omega }_{CL}& \dots & {\omega }_{CL-{V}_{2}}\\ \vdots & \ddots & \vdots \\ {\omega }_{CL-{V}_{2}}& \cdots & {\omega }_{{V}_{2}}\end{array}\right\}\)
-
The \({e}_{ij}\) values are independent and normally distributed with a mean of 0 and variance described by a function, \(g(t,\varphi )\) which depends on time, and \(\psi\)
-
\({e}_{ij}\) and \({\eta }_{i}\) are independently distributed.
The inter-individual variability was assumed to follow log-normal distribution and the individual PK parameter \({\psi }_{i}\) can be described by the function \(M\left(\beta ,{\eta }_{i}\right)\):
The residual error model was described using the equation “combined1” in MONOLIX (Version 2019 R2), such that
where \(\alpha =\{a,b\}\) are estimated parameters defining the residual error of standard deviation \(g\). Other residual error models, such as additive, proportional and combined2 in MONOLIX, were tested when appropriate.
2.2.2 Covariate Model
The covariates (noted \({x}_{i}\)) tested in the PopPK model included baseline age, sex, baseline body weight, race (Asian vs. non-Asian), ethnicity (Japanese vs. non-Japanese based on the country of site – a requirement to support PMDA submission), T315I mutation status baseline renal function (measured as absolute glomerular filtration rate (aGFR)), drug formulation (capsule or tablet), and study effect (FIH vs. ASCEMBL). Renal function was classified as normal (aGFR ≥ 90 mL/min), mildly impaired (60 mL/min ≥ aGFR < 90 mL/min), or moderately impaired (30 mL/min ≥ aGFR < 60 mL/min). There were no patients with severe renal impairment recruited in either of the studies.
In cases where covariates were only collected in a small subset of patients (such as patients with heavy smoking status) or where there was only a small proportion of patients within a given covariate (e.g., hepatic function, and disease category (AP/CP)), the covariate evaluation was performed through investigating covariates’ correlation with individual PK parameter estimates from the PopPK model.
The list of pre-specified covariate-parameter relationships along with more details on the covariate model can be found in Table S2 of the ESM. The statistical significance of the covariates was determined using the stepwise covariate modeling method (SCM), an automated covariate selection algorithm implemented in MONOLIX (Version 2019 R2). Initially, the p-values of all the parameter-covariate relationships were calculated using Pearson’s correlation tests for continuous covariates and ANOVA for categorical covariates. A set of iterations of forward selection was then followed by a set of iterations of backward elimination. In the forward selection, at each step, each of the remaining (i.e., not yet included) parameter-covariate relationships was added to the model in a univariate manner (one model per relationship) based on the p-values of the correlations. The model that decreased the log-likelihood ratio the most was selected and carried forward to the next step. During backward elimination, parameter-covariate relationships were removed in a univariate manner based on log-likelihood ratio test. The log-likelihood ratio threshold for the forward selection is 0.05, and backward elimination is 0.01. Finally, the simplest model with the smallest BICc was selected as the final model.
2.3 Model Evaluation, Selection, and Validation
2.3.1 Assessment of Model Adequacy
The selection of candidate models at the key steps was influenced by convergence stability, biological plausibility, and precision of parameter estimates. The final model was assessed by examining the following diagnostic plots: (1) residual diagnostic, (2) observed versus predicted diagnostics (goodness-of-fit (GOF)), (3) empirical Bayes estimates diagnostics. The predictive performance of the model was assessed using visual predictive check (VPC). In VPC, the final model parameters were fixed and used to simulate 500 virtual trials of the original dataset. The median, 5th and 95th percentiles of the observations, along with the 95% confidence interval (CI) of the median, 5th and 95th percentiles of the predictions were determined. The model was considered adequate if the observed concentration data were within the 5th and 95th percentiles of the simulated data.
2.3.2 Effect of Covariates on Asciminib Exposure
To assess the influence of covariates on asciminib exposure, the simulated exposure was compared with that of a typical patient weighing 70 kg with normal renal function and treated with the tablet formulation. The steady-state area under the plasma concentration-time curve from time 0–24 h (AUC0–24h), maximum plasma drug concentration (Cmax), and minimum plasma drug concentration (Cmin) were determined. To account for inter-individual variability, the simulation was performed for 500 virtual patients, from which the median, 5th and 95th percentiles were calculated. The difference from the typical patient characteristics due to change in statistically significant covariates, such as baseline body weight, renal function, and switch to capsule formulation was assessed using the simulated PK metrics.
2.4 Software Package(s) Used for the Analysis
The analysis was performed using MONOLIX Suite version 2019R2 (Lixoft, Paris, France), a software for non-linear mixed-effects modeling. Data preparation, graphical exploration, GOF plots, simulations, and other model diagnostics were performed using R 3.6.1.
3 Results
3.1 Pharmacokinetic Data
The pooled PopPK dataset contained 6,603 PK concentration measurements from a total of 353 patients treated with asciminib in the FIH study (N = 199 (total daily dose of 20–400 mg given either q.d. or b.i.d.)) and the ASCEMBL study (N = 154 (40 mg b.i.d.)). The baseline demographic and disease characteristics of the patients, and the observed plasma drug concentration-time profiles from the FIH and ASCEMBL studies are provided in Table S3 and Fig. S1 of the ESM.
3.2 PopPK Analysis
3.2.1 Base PopPK Model and Evaluation
The observed data from the FIH study showed a slight over-proportional increase in exposure with increasing dose. Similarly, initial assessment of the base model revealed a dose-dependent bias in population predictions. Two approaches were tested to incorporate dose as a covariate into the structural model:
(1) Dose as a covariate on the apparent bioavailability F
where ED50 is the dose at which F equals 50% of the apparent bioavailability.
2) Dose as a covariate on CL, described using the following power function:
where CLref is the reference value of the parameter at the reference dose level, and the nominal total daily doseref is the reference total daily dose level of 80 mg (40 mg b.i.d.). Nominal total daily dose is defined as the first asciminib total daily dose received. The second approach was superior because it mitigated the correlation between total daily dose and the inter-individual variability in CL to a greater extent. Hence, the base model with total daily dose as a covariate on CL provided reasonable parameter estimates and was accepted as the final base model. This dose-dependent CL could also explain the nonlinearity observed based on non-compartmental analysis. Of note CL, V1, V2, and Q represented the apparent estimates in subsequent model development; the F term was removed for notational convenience.
Prior to the covariate search, the base model incorporated body weight as a covariate of CL and V1 in the form of allometric scaling with fixed exponents of 0.75 and 1 on CL and V1, respectively. Of note, weight as identified to have statistically significant impact on CL and V1 during the initial explorations of the base models. The parameter estimates and the diagnostic plots of the model are provided in the ESM (Table S4 and Fig. S2). Overall, the parameter estimates (Table S4 of the ESM) showed good precision with reasonable percentage relative standard error and the diagnostic plots (Fig. S2 of the ESM) show a good alignment between the observed and predicted data, without any apparent bias.
3.2.2 Covariate Selection
The covariate screening process showed that baseline aGFR, and body weight had statistically significant effects on CL, while formulation had a statistically significant effect on ka (Fig. 1). Asian race, Japanese ethnicity, study effect, sex, and T315I mutation status had no statistically significant effects on CL or ka and were not included as covariates in the final model. The T315I mutation was not identified as a statistically significant covariate on CL, which suggests that the PK properties of asciminib are comparable between patients with or without the T315I mutation.
Model-estimated correlation between covariates and clearance and absorption rate constant. Baseline body weight versus CL (a), baseline aGFR versus CL (b) and different formulations versus ka (c). Solid circles depict the individual data points, the red line indicates smooth regression, and the grey areas represent 95% CIs around the smooth line (a, b). The lower and upper ends of the boxes represent the 25th and 75th percentiles, respectively, horizontal lines inside the boxes indicates median, and the filled circles represent outliers (c). aGFR absolute glomerular filtration rate, CI confidence interval, CL clearance, ka absorption rate constant
3.2.3 Evaluation of the Final Covariate Model
The final model featured a two-compartmental structure with delayed first-order oral absorption and dose-dependent clearance. Inter-individual variabilities were applied following a log normal distribution on the parameters ka, CL, V1, and V2, with a correlation between CL and V1. Nominal total daily dose, baseline body weight, and baseline renal functions were incorporated as covariates on CL, baseline weight as covariate on CL, and formulation as covariate on ka. Asciminib’s CL in the typical patient was 6.31 L/h and combined V1 and V2 was approximately 111 L. The PK parameters of the final covariate model are listed in Table 1.
There was good agreement between the observed data and the population-fitted or individual-fitted data for the final covariate model (Fig. 2a, b). The residual (normalized prediction distribution error (NPDE)) versus time since the first dose and population fit plots (Fig. 2c, d) showed a horizontal line centered around zero across time and increasing concentrations, respectively, implying no systemic bias with respect to time or concentration (the NDPE quantiles plot is presented in Fig. S3 of the ESM). The final PopPK model was qualified through VPC, which graphically compared the 90% CI of the predicted 5th, 95th, and 50th percentiles and the observed 5th, 95th, and 50th percentiles (Fig. 2e). The overall predictions captured most of the observed data, indicating that the PopPK model described the observations reasonably well for the relevant doses evaluated in the patient population.
The final PopPK model: observation versus population fit (a) and individual fit (b) of asciminib plasma concentrations), the residual NDPE versus population fit (c), time since first dose (d), and VPC plots (e). The black open circles depict the individual asciminib plasma concentrations, the red solid line captures the correlation between observed and predicted concentration through a smooth line while the solid black line depicts the identity line (a, b). The solid dots represent NPDE at each population estimate or time point since first dose, the red line represent a smooth line capturing the correlation between NPDE and population fit, or NPDE with time and the solid line represents residual NPDE equals to zero (c, d). The solid and dotted black lines depict the 50th, 5th, and 95th percentiles of observed data, while the red and blue shaded areas represent the 90% CI around the same percentiles of the simulated data (e). CI confidence interval, NPDE normalized prediction distribution error, PopPK population pharmacokinetics, VPC visual predictive check
3.2.4 Evaluation of Significant Covariates
The final covariate model was used to perform simulations for patients receiving asciminib 40 mg b.i.d. As evident from the forest plots (Fig. 3), the difference in median exposure between a typical patient and a patient with a change in covariate was within 20% for all three PK metrics at steady state, AUC0–24h, Cmax, and Cmin; consequently, the differences were not considered clinically relevant. The 90% prediction interval (PI) overlap with that of a typical patient suggests that the effects of these covariates were negligible considering the inter-individual variability
Forest plots showing the median and 90% PI of the steady-state PK parameters at the 25th and 75th percentiles of significant covariates relative to the median steady-state PK parameters of a typical individual following administration of 40 mg asciminib b.i.d. AUC0–24h (a), Cmax (b), and Cmin (c). AUC0-24h area under the plasma concentration-time curve from time zero to 24 h, b.i.d. twice daily, BW body weight, Cmax maximum drug concentration, Cmin minimum drug concentration, CSF clinical service formulation (capsule), PI prediction interval, PK pharmacokinetic, RI renal impairment
The empirical Bayes estimates of CL from the final covariate model were plotted for different categories of patient characteristics (Fig. S4 of the ESM). Overall, there was no correlation between CL and disease category (CML-AP vs. CML-CP), smoking status (heavy vs. non-heavy smokers), or hepatic function (normal vs. mild hepatic impairment). However, CL was slightly lower in patients with mild or moderate renal impairment than in those with normal renal function.
3.2.5 Comparison of 40 mg b.i.d. with 80 mg q.d. Dose Regimen
Once daily dosing is known to encourage adherence and compliance to TKI therapy. Thus, an 80 mg q.d. dosing regimen was evaluated as an alternative to the 40 mg b.i.d. [14]. The final PopPK model was used to predict the steady state PK profiles of the two regimens for 500 simulated typical patients (Fig. 4 and Table 2). The average steady-state Cmax and Cmin values of 80 mg q.d. were 1.61-fold higher and 0.72-fold lower than those of 40 mg b.i.d., respectively, but the average steady-state AUC0-24h values were comparable between the two regimens. The empirical Bayes estimates of CL were also similar between 40 mg b.i.d. (N = 188) and 80 mg q.d. (N = 18).
Comparison of simulated PK time course (a) and CL (b) between asciminib 40 mg b.i.d. and 80 mg q.d. using the final PopPK model and empirical Bayes estimates on CL. The solid lines represent the median values and the shaded areas depict the 10th–90th percentile of the simulations (a). The lower and upper ends of the boxes represent the 25th and 75th percentiles, respectively, horizontal lines inside the boxes indicate medians, and the filled circles depict outliers (b). b.i.d. twice daily, PK pharmacokinetic PopPK population pharmacokinetics, q.d. once daily
4 Discussion
The two-compartment PopPK model developed for asciminib adequately described the PK dataset collected from 353 patients with CML in AP/CP, who participated in the FIH and ASCEMBL studies, over a wide range of doses. The GOF plots indicated a good alignment between the observed and predicted concentrations, and the lack of systemic bias confirms the accuracy of the PopPK model in characterizing the concentration-time profile of asciminib. Based on the VPC plots, the observed median of the 5th, 50th, and 95th percentiles was well captured by the CIs for the corresponding predicted 5th, 50th, and 95th percentiles, which further confirms the robustness of the current PopPK model.
To address the slightly more than dose-proportional increase in asciminib exposure, the PopPK model included nominal total daily dose as a structural covariate on CL. This approach significantly improved the model as the correlation between the dose and inter-individual variability of CL was mitigated. In addition to nominal total daily dose, baseline body weight was incorporated in the structural model, specifically on CL and V1, in the form of allometric scaling with fixed exponents of 0.75 and 1 on CL and V1, respectively. Although the impact of body weight on CL and V1 was statistically significant, the difference in PK exposure between an individual representing the 25th and 75th percentiles of weight distribution and a typical individual weighing 70 kg was < 20%, and thus was not considered clinically relevant.
Baseline renal function, measured as aGFR, was also found to be a statistically significant covariate on CL. However, the difference in PK exposure between an individual with mild/moderate renal impairment and a typical individual with normal renal function was relatively small, and was therefore not considered clinically meaningful, as the CIs of the individual PK metrics were largely overlapping between normal versus mild/moderate. Based on these results, no dose adjustment is required for patients with mild/moderate renal impairment. As there were no patients with severe renal impairment in our analysis, no conclusions could be made for this group of population.
The formulation was found to be a statistically significant covariate on ka. However, the prediction intervals for the PK metrics were largely overlapping between the CSF (capsule) and FMI (tablet) formulations, and was therefore considered clinically insignificant. Of note, the capsule formulation was an earlier trial formulation that will not be available to patients.
Other covariates, such as sex, hepatic function, heavy smoking status, disease category (CP/AP), Asian origin, Japanese ethnicity, and T315I mutation status were also tested, but they were not statistically significant. Based on these results, no dose adjustment is required for patients with mild/moderate hepatic impairment and for the Asian patients and for patients of Japanese ethnicity.
Asciminib represents a potentially beneficial treatment option for patients with the T315I mutation, thus it is very important for the PopPK model to evaluate whether PK exposure in this population is impacted by the mutation status and to assess the necessity for a dose adjustment. To ensure sufficient efficacy, the recommended asciminib dosing for patients with the T315I mutation is 200 mg b.i.d. [13]. The results of the covariate analysis suggest that there is no correlation between T315I mutation status and PK exposure; hence, no further dose adjustment should be required for this patient population based on PK exposure but should only be based on efficacy.
Finally, simulations based on the PopPK model were used to compare asciminib 40 mg b.i.d. (used in the ASCEMBL study) and 80 mg q.d. (an alternative, more patient-centric regimen). While there were differences in Cmax and Cmin, the overall asciminib exposure (AUC0-24h) was very similar between the two regimens [15].
5 Conclusions
Asciminib is a first-in-class highly potent inhibitor of the ABL kinase activity of BCR-ABL1, which is currently recommended by the FDA as a treatment option for patients with relapsed and refractory CML in CP as well as for those with T315I mutation. Our analysis demonstrates the robustness of the PopPK model in adequately describing the PK properties of asciminib and characterizing the covariate effects on exposure. While baseline body weight, aGFR, and formulation were identified as statistically significant covariates on CL and ka, the impact on exposure was relatively small, and therefore not considered clinically meaningful. Other covariates, such as the T315I mutation, renal/hepatic impairment, disease status, ethnicity, and race were not statistically significant, suggesting no dose adjustment for special populations. In addition, simulation based on the PopPK model demonstrates comparable daily exposure between the 40 mg b.i.d. and the approved alternative regimen of 80 mg q.d., which represents a more patient-centric treatment option. Taken together, our work highlights the importance of PopPK analysis in understanding the PK properties of asciminib and assessing the impact of covariates on asciminib PK and the feasibility of regimen switch.
Change history
17 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40262-022-01166-7
References
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95(6):691–709. https://doi.org/10.1002/ajh.25792.
Houshmand M, Simonetti G, Circosta P, Gaidano V, Cignetti A, Martinelli G, et al. Chronic myeloid leukemia stem cells. Leukemia. 2019;33(7):1543–56. https://doi.org/10.1038/s41375-019-0490-0.
Cortes J, Lang F. Third-line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14(1):44. https://doi.org/10.1186/s13045-021-01055-9.
Manley PW, Barys L, Cowan-Jacob SW. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk Res. 2020;98: 106458. https://doi.org/10.1016/j.leukres.2020.106458.
Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. 2018;61(18):8120–35. https://doi.org/10.1021/acs.jmedchem.8b01040.
Breccia M, Colafigli G, Scalzulli E, Martelli M. Asciminib: an investigational agent for the treatment of chronic myeloid leukemia. Expert Opin Investig Drugs. 2021;30(8):803–11. https://doi.org/10.1080/13543784.2021.1941863.
Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543(7647):733–7. https://doi.org/10.1038/nature21702.
Tran P, Hanna I, Eggimann FK, Schoepfer J, Ray T, Zhu B, et al. Disposition of asciminib, a potent BCR-ABL1 tyrosine kinase inhibitor, in healthy male subjects. Xenobiotica. 2020;50(2):160–70. https://doi.org/10.1080/00498254.2019.1594449.
Hoch M, Sato M, Zack J, Quinlan M, Sengupta T, Allepuz A, et al. Pharmacokinetics of asciminib in individuals with hepatic or renal impairment. J Clin Pharm. 2021;61(11):1454–65. https://doi.org/10.1002/jcph.1926.
Hoch M, Sengupta T, Hourcade-Potelleret F. Pharmacokinetic drug interactions of asciminib with the sensitive cytochrome P450 probe substrates midazolam, warfarin, and repaglinide in healthy participants. Clin Transl Sci. 2022. https://doi.org/10.1111/cts.13252 (Epub ahead of print).
Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. NEJM. 2019;381(24):2315–26. https://doi.org/10.1056/NEJMoa1902328.
Hochhaus A, Boquimpani C, Rea D, Minami Y, Lomaia E, Voloshin S, et al. Efficacy and safety results from ASCEMBL, a multicenter, open-label, phase 3 study of asciminib, a first-in-class STAMP inhibitor, vs bosutinib (BOS) in patients (Pts) with chronic myeloid leukemia in chronic phase (CML-CP) previously treated with ≥2 tyrosine kinase inhibitors (TKIs). Blood. 2020;136(2):LBA-4. https://doi.org/10.1182/blood-2020-143816.
The Food and Drug Administration, USA. Highlights of asciminib prescribing information, in, October 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215358s000Orig1lbl.pdf. Accessed 11 Nov 2021.
Geissler J, Sharf G, Bombaci F, Daban M, De Jong J, Gavin T, et al. Factors influencing adherence in CML and ways to improvement: Results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol. 2017;143(7):1167–76. https://doi.org/10.1007/s00432-017-2372-z (Epub 2017 Mar 13 PMID: 28289895).
Combes F, Li YF, Sy S, Lorenzo S, Dasgupta K, Kapoor S, et al. Dose Justification for asciminib in patients with Ph+ chronic myeloid leukemia in chronic phase with or without the T315I mutation previously treated with two or more tyrosine kinase inhibitors. In: ePoster Presented at The American Association of Pharmaceutical Scientists (AAPS), October 17–20, 2021. Philadelphia, Pennsylvania. Hybrid event. Poster# T6107V
Acknowledgements
The authors thank Wenping Wang and Farkad Ezzet for their scientific expertise/contribution during the model development along with Twarita Chakraborty, PhD, Novartis Healthcare Private Limited, Hyderabad for providing medical writing/editorial support in accordance with Good Publication Practice (GPP3) guidelines (https://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflicts of Interest
All authors are employees of Novartis. SL, SS, YFL, Y-YH, MH, and FPC own Novartis shares. FPC also owns Simulation Plus shares.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data sharing statement
Anonymized patient‐level data from clinical trials may be shared by Novartis in a consortium called ClinicalStudyDataRequest.com (CSDR) in accordance with Novartis’ policy for sharing clinical trial data (https://www.clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Novartis.aspx).
Code availability
Not applicable.
Author contributions
All the authors contributed to designing and performing the research, data analysis, and drafting and review of the manuscript. All authors read and approved the final manuscript.
Additional information
The original Online version of this article was revised: The figure Fig. 1(a) has been incorrectly published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y.F., Combes, F.P., Hoch, M. et al. Population Pharmacokinetics of Asciminib in Tyrosine Kinase Inhibitor-Treated Patients with Philadelphia Chromosome-Positive Chronic Myeloid Leukemia in Chronic and Acute Phases. Clin Pharmacokinet 61, 1393–1403 (2022). https://doi.org/10.1007/s40262-022-01148-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01148-9