Abstract
Background
This analysis aimed to characterize the pharmacokinetics (PK) of the inhaled corticosteroid (ICS) fluticasone furoate (FF), the long-acting muscarinic antagonist umeclidinium (UMEC), and the long-acting β2-agonist (LABA) vilanterol (VI), administered as dual (FF/VI) or triple (FF/UMEC/VI) single-inhaler therapy to patients with asthma, and to identify covariates that may influence the PK of each analyte.
Methods
Blood samples were obtained from the phase IIIA CAPTAIN study (ClinicalTrials.gov: NCT02924688), which evaluated the efficacy and safety of once-daily FF/UMEC/VI versus FF/VI in patients with uncontrolled asthma taking ICS/LABA. Samples were collected at trough (defined as ≥ 20 h after the last dose) from all subjects randomized to the six treatment groups (FF/UMEC/VI 100/31.25/25 μg, 100/62.5/25 μg, 200/31.25/25 μg, 200/62.5/25 μg; FF/VI 100/25 μg, 200/25 μg) at week 24 or the early withdrawal visit. In a subset of patients, PK samples were obtained predose at week 12, and at 5–30 min, 45–90 min, and 2–3 h postdose. For each analyte, a population PK model was developed using non-linear mixed-effects modeling. The maximum likelihood method was utilized to incorporate data below the quantifiable limit (BQL). Final models were used to derive the area under the plasma concentration-time curve and maximum observed concentration at steady-state for each analyte.
Results
We obtained 4018, 2695, and 4032 samples from 1891, 1258, and 1891 patients, for FF, UMEC, and VI, respectively; 48%, 49%, and 50% of samples were reported as BQL for each analyte, respectively. The PK were adequately described by a two-compartment model with first-order absorption and elimination for FF, a two-compartment model with intravenous bolus input and first-order elimination for UMEC, and a three-compartment model with zero-order input and first-order elimination for VI. Statistically significant covariates were body weight on apparent inhaled clearance of FF, creatinine clearance on apparent clearance and body weight on apparent inhaled volume of distribution of the central compartment for UMEC, and race (East Asian, Japanese, and South East Asian heritage) on inhaled apparent volume of distribution of the central compartment for VI. However, the overall effects of covariates were marginal and thus do not warrant dose adjustment. Systemic exposures of FF or VI did not differ when administered as a single-inhaler triple (FF/UMEC/VI) or dual combination (FF/VI), and were similar to those reported for patients with chronic obstructive pulmonary disease.
Conclusion
Only marginal covariate effects were observed, and thus no dose adjustments are deemed necessary for FF, UMEC, or VI. There was no difference in FF or VI systemic exposure in patients with asthma when administered as either triple (FF/UMEC/VI) or dual therapy (FF/VI). Together with efficacy findings from the CAPTAIN study, our data support the use of single-inhaler FF/UMEC/VI triple therapy for patients with uncontrolled asthma currently receiving ICS/LABA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The pharmacokinetic profile of inhaled fluticasone furoate (FF) was adequately described by a two-compartment model with first-order absorption and first-order elimination; a two-compartment model with an intravenous-type bolus input and first-order elimination described umeclidinium (UMEC), and a three-compartment model with zero-order absorption and first-order elimination adequately described vilanterol (VI). |
Although weight, creatinine clearance and weight, and race were identified as significant covariates on the PK of FF, UMEC, or VI, respectively, their effects were marginal and thus dose adjustments were not warranted. |
Systemic exposures of FF or VI were similar whether administered as a triple therapy with UMEC via a single inhaler (FF/UMEC/VI) or as a dual combination (FF/VI); these findings were similar to those reported in patients with chronic obstructive pulmonary disease. |
1 Introduction
Despite adherence to inhaled corticosteroids/long-acting β2-agonist (ICS/LABA) maintenance therapy, approximately 30–50% of patients with asthma are still not well controlled [1,2,3,4]. Guidelines from the Global Initiative for Asthma [5] recommend a long-acting muscarinic antagonist (LAMA) as add-on treatment for patients with uncontrolled asthma currently taking medium- to high-dose ICS/LABA dual maintenance therapy.
A single-inhaler triple therapy containing the ICS/LABA/LAMA combination of fluticasone furoate (FF), umeclidinium (UMEC), and vilanterol (VI) [FF/UMEC/VI, via dry powder inhaler] was first approved for the treatment of chronic obstructive pulmonary disease (COPD) in 2017 [6]. The phase IIIA, randomized, double-blind CAPTAIN study (Clinical study in Asthma Patients receiving Triple therapy in A single INhaler) was designed to investigate the safety and efficacy of FF/UMEC/VI versus FF/VI in patients with uncontrolled asthma, despite ICS/LABA therapy [7]. The CAPTAIN study demonstrated that once-daily single-inhaler FF/UMEC/VI reduced airflow obstruction and enabled more patients to achieve asthma control, effectively reducing risk for patients whose asthma is inadequately controlled on ICS/LABA, with no additional safety concerns [7].
Population pharmacokinetic (PK) models using non-linear mixed-effects modeling have previously been developed in adults with asthma following administration of FF, UMEC, and VI as monotherapies or as dual therapy (FF/VI) [8, 9]. These studies reported minimal effects of creatinine clearance (CRCL), age, race, and body weight on overall plasma PK, and thus no relevant dosage adjustments are required [8, 9]. While the PK of FF, UMEC, and VI, administered as a single-inhaler triple therapy, have been characterized in patients with COPD [10], the same assessments have not been made in patients with asthma, as monotherapy with a long-acting bronchodilator would not be appropriate. Therefore, this analysis aimed to characterize the systemic exposure of FF, UMEC, and VI and to assess covariates that may influence the PK of the individual components when administered as a triple therapy via a single inhaler to patients with asthma.
2 Methods
2.1 Study Design
Data for this PK analysis were obtained from the CAPTAIN study (GSK: 205715; ClincialTrials.gov: NCT02924688) [7]. CAPTAIN was a phase IIIA, randomized, double-blind, 24- to 52-week variable duration, active-controlled, parallel-group, multicenter, superiority study evaluating once-daily FF/UMEC/VI versus FF/VI (both administered via the ELLIPTA dry powder inhaler) in patients with uncontrolled asthma receiving ICS/LABA [7]. Patients were randomly assigned to one of six treatment arms following a 5-week run-in/stabilization period: FF/VI 100/25, 200/25 μg; FF/UMEC/VI 100/31.25/25, 100/62.5/25, 200/31.25/25, 200/62.5/25 μg [7].
Eligble patients were adults ≥ 18 years of age, with a pre-bronchodilator forced expiratory volume in 1 s (FEV1) percent predicted ≥ 30% to < 85% and airway reversibility (defined as an increase in FEV1 of ≥ 12% and ≥ 200 mL 20–60 min following four inhalations of salbutamol) at screening, an Asthma Control Questionnaire-6 score of ≥ 1.5 (at screening and enrollment), and were required to have been receiving ≥ 12 weeks of maintenance ICS/LABA therapy (stable dose of daily fluticasone proprionate > 250 μg/day or equivalent for > 6 weeks) prior to the pre-screening clinic visit. In addition, in the year prior to screening, eligible patients were required to have a documented healthcare contact for acute asthma symptoms or a temporary change in asthma therapy for the treatment of acute asthma symptoms. Patients with a COPD diagnosis (based on the Global Initiative for Chronic Obstructive Lung Disease criteria [11]), or other concurrent respiratory disorders, including pneumonia and pneumonia risk factors, were excluded, along with those who had experienced an asthma exacerbation requiring a change in maintenance asthma therapy within 6 weeks prior to screening. Current smokers and former smokers with a smoking history of ≥ 10 pack years were also excluded [7].
The study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice, and applicable country-specific regulatory requirements. The protocol received approval from applicable central or local Institutional Review Boards or independent Ethics Committees. Written informed consent was obtained from all patients before participation.
A glossary of PK abbreviations is included as electronic supplementary Table 1.
2.2 Pharmacokinetic (PK) Sample Collection and Bioanalysis
Blood samples for PK analysis of FF, UMEC, and VI were collected at trough (defined as ≥ 20 h after last dose) from all patients at the week 24 study visit, or, in patients discontinuing treatment prematurely, the early withdrawal visit. In a consenting subset of patients, PK samples were obtained predose on the day of the visit at week 12 and then postdose at each of the following three intervals: 5–30 min, 45–90 min, and 2–3 h postdose.
Plasma samples were analyzed using validated analytical methods based on solid-phase extraction followed by high-pressure liquid chromatography with tandem mass spectrometry, as used in previous studies [8,9,10], using a 150 µL aliquot of human plasma for FF and 250 µL aliquots for UMEC and VI. The lower limit of quantification (LLOQ) was 10 pg/mL for all three analytes, while the higher limit of quantification was 1000 pg/mL for FF and VI, and 2000 pg/mL for UMEC.
Quality-control samples, prepared at three different analyte concentrations, were analyzed with each batch of samples against separately prepared calibration standards. For the analysis to be acceptable, no more than one-third of the total quality control results and no more than one-half of the results from each concentration level were to deviate from the nominal concentration by more than 15%. The applicable analytical runs met all predefined run acceptance criteria.
2.3 PK Population Modeling
The population PK modeling and simulations were performed using NONMEM v7.4.3 (ICON Development Solutions, Ellicott City, MD, USA) under the Windows 7 Professional operating system with Intel Visual FORTRAN Complier Professional, version 11.1, interfaced with PDx-Pop v5.2.2 (ICON Development Solutions). Supporting applications for data handling, exploratory diagnostics, simulation, and data summary were conducted in R v3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria).
The previously developed final population PK models [8, 9] (with covariates included) for inhaled FF, UMEC, and VI in subjects with asthma were used as the starting point for the structural model development. Concentration data below the LLOQ [below the quantifiable limit (BQL)] were incorporated in the models using the maximum likelihood M3 methodology with the F_FLAG option in NONMEM [12]. Stochastic approximation expectation maximization with interaction was used as the estimation method. Specifically, the PK of FF were described by a two-compartment model with first-order absorption and first-order elimination, with race as a covariate on apparent clearance (CL/F); for VI, the PK were described by a three-compartment model with zero-order absorption and first-order elimination, with race as a covariate on apparent volume of distribution (V1/F); and the UMEC PK were described using a two-compartment model with intravenous bolus input, due to fast absorption following inhalation, with CRCL as a covariate on CL/F, and age and weight on V1/F. Monte Carlo simulations were undertaken to first assess the ability of these earlier PK models to describe the observed concentration versus time data from the present study for these analyses. Further model updates were considered by including new covariates or excluding non-significant covariates from the model.
2.4 Covariate Analysis
For each of the three compounds, a final population PK model including potential influential covariates was further investigated. The covariates of interest for this study included age, race, ethnicity (Hispanic or Latino vs. non-Hispanic or non-Latino), sex, weight, body mass index, and additional baseline characteristics, for example smoking status, CRCL, and lung function status (FEV1 and forced vital capacity). Race was grouped as East Asian versus non-East Asian (White, African American/African heritage, and others). The covariates were introduced into the models and selected using the same approach as described by Allen et al. [8] when describing the PK of FF and VI as dual therapy for patients with asthma, and by Mehta et al. [10], who reported on the PK of FF, UMEC, and VI as triple therapy for patients with COPD. Treatment group as a covariate was also tested in modeling the PK of FF or VI.
2.5 Model Evaluation
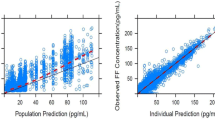
For each analyte, the final PK model was evaluated by prediction-corrected visual predictive checks (pcVPC) using the parameter estimates from each model [13]. One thousand replicates of the original datasets were simulated based on the model, and the 90% prediction interval (PI) was computed from these simulations. The observed concentration versus time data were overlaid onto the PI to assess the concordance between the simulated and observed data. For this evaluation, both observed concentrations reported as BQL and model-predicted concentrations below the LLOQ were set to a value of half the LLOQ (i.e. 5 pg/mL). Similarly, concordance between the observed and predicted proportion of BQL data over time was also assessed to further support model diagnostics.
2.6 Model-Predicted Systemic Exposure
For each of the three analytes (FF, UMEC, and VI), the final model was used to predict steady-state exposure over a 24-h period (area under the concentration–time curve from time zero to 24 h [AUC24]) and the maximum plasma concentration (Cmax) in patients with asthma. Individual AUC24 values were derived as the ratio of dose divided by the individual post hoc estimate of CL/F from the final population PK model [AUC = dose/CL/F × 1000 (pg·h/mL)]. More intense concentration–time profiles were simulated using the parameter estimates from the final model to derive Cmax (pg/mL) estimates for each patient. The AUC24 and Cmax estimates for each analyte were summarized by treatment group, and, for VI, by race as well.
Comparisons of the individual derived exposures for each treatment group and FF and UMEC dose level were performed using linear mixed-effects models to obtain a point estimate for each comparison and the corresponding 90% confidence intervals (CI). The comparison of interest was assigned as a fixed effect with subject as a random variable.
3 Results
3.1 Patient Demographics and Baseline Characteristics
The final dataset for population PK analysis comprised a total of 4018 samples from 1891 patients for FF, 2695 samples from 1258 patients for UMEC, and 4032 samples from 1891 patients for VI. The week 12 PK subset comprised 579, 395, and 582 patients in the three groups, respectively. Baseline demographics were similar across all PK datasets. The majority of patients were White, female, and non-smokers, with a median age of 55 years (Table 1). FEV1 and CRCL at baseline were also similar for all treatment groups.
3.2 Fluticasone Furoate (FF), Umeclidinium (UMEC), and Vilanterol (VI) Concentration–Time Data
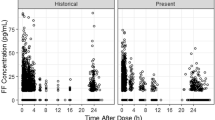
Of the samples obtained, 48%, 49%, and 50% were reported as BQL (i.e. below the LLOQ of 10 pg/mL for FF, UMEC, and VI), respectively. However, at least one quantifiable concentration was obtained from 962, 696, and 916 subjects for the FF, UMEC, and VI datasets, while 429, 267, and 480 subjects had more than one quantifiable concentration, respectively; 65%, 61%, and 73% of predose concentrations (samples taken >20 h after the previous dose) were BQL for FF, UMEC, and VI, respectively (Table 2).
Overall, the distribution and range of the observed plasma concentration–time data for FF was similar for both doses across both the dual- and triple-therapy treatment groups. For UMEC, observed plasma concentration–time data were also similar in the triple-therapy groups for each of the two UMEC doses. The observed plasma concentration–time data for VI were similar across the dual- and triple-therapy treatment groups (Fig. 1).
3.3 Final PK Models for FF, UMEC, and VI
A pcVPC plot with the parameter estimates from the previously reported FF model for subjects with asthma [8] showed that the majority of the observed FF PK concentration–time data from the CAPTAIN study was within the 90% PI derived from the respective PK models for FF (electronic supplementary Fig. 1a, b). FF concentrations were adequately described by a two-compartment model with first-order absorption and elimination [8] and there was also a good agreement between observed BQL data and the model-predicted BQL (Table 2). A review of the interindividual variability (NONMEM interindividual error [ETA]) versus covariate plots indicated that weight was a significant covariate on FF CL/F. When weight was included in the model, race was no longer a statistically significant covariate; therefore, only weight was retained in the final model for FF (Table 3).
The observed distribution and range of UMEC plasma concentration–time data, as well as the observed and model-predicted BQL data, were also consistent with the previous PK model for UMEC [9], as demonstrated in Table 2 and by the pcVPC plots (electronic supplementary Fig. 1c, d). UMEC concentrations were adequately described by a two-compartment model with intravenous bolus input and first-order elimination [9]. The effects of weight on V1/F and CRCL on CL/F, as well as the relative bioavailability of the lower dose, were incorporated into the final model for UMEC (Table 3); the effect of age was not statistically significant and was thus removed from the model.
As with FF and UMEC, the majority of the observed VI concentration–time data from the CAPTAIN study were within the 90% PI from the previously reported model [8] (electronic supplementary Fig. 1e). The PK of VI were adequately described by a three-compartment model with zero-order absorption and first-order elimination. Furthermore, race remained a significant covariate on VI V1/F, and was thus included in the final VI model (Table 3).
3.4 Model-Predicted Systemic Exposure
Using the final PK models, the predicted systemic exposures for FF or VI were comparable for FF/UMEC/VI single-inhaler triple therapy and FF/VI single-inhaler dual therapy. A dose-dependent increase in exposure with FF 200 μg versus 100 μg (Table 4) was observed. For UMEC, exposures associated with the 62.5 μg versus 31.25 μg were slightly higher than dose-proportional: the ratio of the geometric mean AUC24 (95% CI) for the 31.25 μg UMEC dose versus the 62.5 μg dose was 0.879 (0.853–0.907), while for Cmax, the ratio was 0.851 (0.815–0.889).
3.5 Effect of Covariates
Population PK models identified weight as a significant covariate on CL/F for FF, and race as a significant covariate on V1/F for VI (Table 3). For UMEC, CRCL and weight identified as significant covariates on CL/F and V1/F, respectively. However, the overall effects of the identified covariates on the systemic exposure of FF, UMEC, and VI were marginal (electronic supplementary Fig. 2), with the exception of the observable difference on VI Cmax between East Asian and non-East Asian patients (Table 5). The model-predicted systemic exposure of VI following FF/VI or FF/UMEC/VI administration according to race showed that the overall geometric mean (95% CI) Cmax in the East Asian group (n = 275) was 147 pg/mL (143–151) compared with 53.3 pg/mL (52.4–54.2) and 50.0 pg/mL (44.9–55.7) for White (n = 1507) or African American (n = 77) groups, respectively (Table 5).
4 Discussion
This population PK analysis using data from the CAPTAIN study demonstrated that the distribution and range of the observed plasma concentration–time data were similar whether FF, UMEC, or VI were administered as single-inhaler triple therapy (FF/UMEC/VI), or FF and VI as a single-inhaler dual therapy (FF/VI). In addition, this analysis showed that the structure of the previously derived PK models established for FF, UMEC, and VI during their development as asthma therapies [8, 9] adequately described the data from the CAPTAIN study, as demonstrated by pcVPC stimulation-based diagnostics, where the majority of the observed FF, UMEC, and VI PK concentration–time data lay within the 90% PI from the previously reported models for each analyte.
FF concentrations were described by a two-compartment model with first-order absorption and elimination [8]; UMEC concentrations were described by a two-compartment model with intravenous bolus input and first-order elimination [9]; and VI concentrations were described by a three-compartment model with zero-order input and first-order elimination [8]. The model-predicted systemic exposure of FF, UMEC, or VI demonstrated good agreement for each analyte between treatment groups. For FF, there was a dose-proportional increase in predicted systemic exposure with 100 μg versus 200 μg doses in both the dual- and triple-therapy groups, as expected. As observed previously by Yang et al. [9], the systemic exposure of UMEC was slightly higher than dose proportional when comparing 62.5 μg versus 31.25 μg, although the reasons for this are unclear. However, this observation was not considered to be clinically relevant, since the safety profiles of both doses of UMEC were similar [7].
For analysis of the covariates affecting the PK of each analyte, the majority of the fixed-effect parameters in the current models were estimated with sufficient precision, and the model parameter estimates were similar to those previously reported following either FF, UMEC, or VI monotherapy or dual therapy (FF/VI) in subjects with asthma [8, 9], further confirming the robust nature of the current models. For FF, body weight was a statistically significant covariate on CL/F, and, for UMEC, CRCL was a statistically significant covariate on CL/F. While the effect of body weight on V1/F was marginal, with 95% CI including 0, this covariate was retained in the model. Race (East Asian, Japanese, and South-East Asian heritage) was a statistically significant covariate on V1/F for VI.
In the present study, the effects of race were not statistically significant on FF CL/F after including body weight on CL/F, in contrast to the results reported by Allen et al. [8], where body weight was not a significant covariate on FF CL/F but race was. This is not surprising as patients of East Asian origin are typically lighter than other patients, and the impact of the weight effect on FF CL/F in the present analysis was similar to that of the previously reported race effect on FF CL/F, with slightly higher FF systemic exposures for subjects of East Asian origin [8].
CRCL was a significant covariate for CL/F of UMEC, and this effect was consistent with the previous model by Yang et al. [9]. However, the impact was marginal over the range of CRCL (37.2–424 mL/min) in this study. In addition, a previously reported study using 125 μg of UMEC or UMEC/VI in patients with severe renal impairment (CRCL < 30 mL/min) reported no clinically relevant increases in systemic exposure compared with healthy volunteers, confirming that UMEC dose adjustments on the basis of CRCL are not warranted [14]. These findings further support our observation that the effects of CRCL on UMEC PK are unlikely to be clinically relevant [14]. Moreover, whereas Yang et al. [9] reported that age was a significant predictor of UMEC V1/F in patients with asthma, in the present analysis, where subjects tended to be older (median age 55 years vs. 45 years in the previous analysis), the covariate selection indicated that age was not a significant covariate on V1/F after adjusting for body weight. In addition, substantially more patients were included in this study (n = 1258) compared with the previous study (n = 128), and thus a more robust covariate analysis was performed with this analysis.
With regard to VI, the explanation behind the higher Cmax in patients of East Asian origin is unclear; however, this finding is consistent with what was previously reported [8]. The exact reason for this difference is unclear, but it could be attributed to differences in the rate of lung absorption of VI between different race groups [15]. Furthermore, there was no evidence of a difference in adverse events, including increased heart rate, among patients in the East Asian race category in the CAPTAIN study [7], negating the need for any compensatory dose adjustments to be made on account of this observation in the East Asian group. Therefore, despite being statistically significant, the effects of weight, CRCL, and race on the PK of FF, UMEC, or VI were all considered to be marginal and not clinically relevant, thus no dose adjustments are deemed necessary for each of the three analytes based on these factors.
Although it was not straightforward to directly compare the population PK models derived in this study with those derived from patients with COPD [10], systemic exposure (AUC24 and Cmax) of FF, UMEC, and VI following administration of FF/UMEC/VI 100/62.5/25 was generally similar and overlapping between asthma and COPD populations.
For a number of individuals, FF, UMEC, and VI concentrations measured at predose were unexpectedly high, despite the assessment time point (≥ 20 h postdose) being assumed to be a trough measurement. However, no data were available to verify the relative time from dosing for these measurements due to the nature of the study. A similar effect was reported in a study using data from two phase III clinical trials, investigating the PK of UMEC and VI when administered as single-inhaler dual therapy or as individual monotherapies to patients with COPD [16]. The sensitivity analysis performed in that study demonstrated that excluding such data did not significantly alter the PK estimates for either analyte. Consequently, by applying that inference, all available data were utilized for the current analyses.
5 Conclusion
The population PK analysis of data from the CAPTAIN study showed only marginal covariate effects and no dose adjustment requirements for FF, UMEC, or VI. There was no difference in FF or VI systemic exposure when administered either as a single-inhaler triple therapy (FF/UMEC/VI) or as the dual combination of FF/VI. Observed exposures for FF, UMEC, or VI in patients with asthma from this study were also similar to those previously reported in patients with COPD. Alongside the favorable efficacy and safety data reported from the CAPTAIN trial [7], this PK analysis further supports the use of FF/UMEC/VI single-inhaler triple therapy without the need for dose adjustments, in patients with uncontrolled asthma, despite current treatment with ICS/LABA.
References
Bernstein DI, Bateman ED, Woodcock A, Toler WT, Forth R, Jacques L, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52(10):1073–83.
Lee LK, Obi E, Paknis B, Kavati A, Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55(2):208–19.
Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51(1):1701126.
Davis J, Trudo F, Siddall J, Small M. Burden of asthma among patients adherent to ICS/LABA: a real-world study. J Asthma. 2019;56(3):332–40.
GINA. Global Initiative for Asthma guidelines 2020. https://ginasthma.org/. Accessed 3 Aug 2020.
GSK. Trelegy Ellipta US prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209482s000lbl.pdf. Accessed 8 Jul 2020.
Lee LA, Bailes Z, Barnes N, Boulet L, Edwards D, Fowler A, et al. Efficacy and safety of once-daily single-inhaler triple therapy (fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma: a double-blind, randomised phaseIIIA trial (CAPTAIN study). Lancet Respir Med. 2021;9(1):69–84.
Allen A, Siederer S, Yang S. Population pharmacokinetics of inhaled fluticasone furoate and vilanterol in adult and adolescent patients with asthma. Int J Clin Pharmacol Ther. 2016;54(4):269–81.
Yang S, Lee L, Pascoe S. Population pharmacokinetics modeling of inhaled umeclidinium for adult patients with asthma. Eur J Drug Metab Pharmacokinet. 2017;42(1):79–88.
Mehta R, Farrell C, Hayes S, Birk R, Okour M, Lipson DA. Population pharmacokinetic analysis of fluticasone furoate/umeclidinium bromide/vilanterol in patients with chronic obstructive pulmonary disease. Clin Pharmacokinet. 2020;59(1):67–79.
GOLD. Global Initiative for Chronic Obstructive Lung Disease GOLD Report. 2020. https://goldcopd.org/gold-reports/. Accessed 3 Aug 2020.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35(4):401–21.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Mehta R, Hardes K, Brealey N, Tombs L, Preece A, Kelleher D. Effect of severe renal impairment on umeclidinium and umeclidinium/vilanterol pharmacokinetics and safety: a single-blind, nonrandomized study. Int J Chron Obstruct Pulmon Dis. 2015;10:15–23.
Korotzer B, Ong S, Hansen JE. Ethnic differences in pulmonary function in healthy nonsmoking Asian–Americans and European–Americans. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1101–8.
Goyal N, Beerahee M, Kalberg C, Church A, Kilbride S, Mehta R. Population pharmacokinetics of inhaled umeclidinium and vilanterol in patients with chronic obstructive pulmonary disease. Clin Pharmacokinet. 2014;53(7):637–48.
Acknowledgements
Editorial support in the form of preparation of the first draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, was provided by Rebecca Dawson, Ph.D., of Fishawack Indicia, Ltd, UK, part of Fishawack Health, and was funded by GSK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the GlaxoSmithKline (study number GSK 205715), NCT02924688.
Conflict of Interest
SY, NS, AF, and GP are all employees of GlaxoSmithKline (GSK) and own stocks and shares in the company. LAL was an employee of GSK at the time of this study. GP also owns stocks and shares in Novartis.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice, and applicable country-specific regulatory requirements. The protocol received approval from applicable central or local Institutional Review Boards or independent Ethics Committees.
Consent to Participate
Written informed consent was obtained from all participants before participation.
Consent for Publication
Not applicable.
Availability of Data and Material
Anonymized individual participant data and study documents can be requested for further research from http://www.clinicalstudydatarequest.com.
Code Availability
Not applicable.
Author Contributions
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. SY, LL, and GP were involved in the conception and design of the study and the data analysis and interpretation. AF and NS were involved in the data analysis and interpretation.
Additional information
Laurie A. Lee: Affiliation at the time of this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, S., Lee, L.A., Sule, N. et al. Population Pharmacokinetic Modeling of Fluticasone Furoate, Umeclidinium Bromide, and Vilanterol in Patients with Asthma, Using Data from a Phase IIIA Study (CAPTAIN). Clin Pharmacokinet 60, 887–896 (2021). https://doi.org/10.1007/s40262-021-00988-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-00988-1