Abstract
Background and objective
Peficitinib is an orally administered, once-daily Janus kinase inhibitor in development for the treatment of rheumatoid arthritis. Peficitinib and its major metabolite H2 inhibit the hepatic uptake transporter organic anion transporting polypeptide 1B1 (OATP1B1) in vitro. This article reports a clinical study evaluating the effects of peficitinib on the pharmacokinetics of rosuvastatin, a substrate for the OATP1B1 transporter, and vice versa.
Methods
In an open-label, single-sequence clinical study, 24 healthy adults of East Asian and non-East Asian origin received a single dose of rosuvastatin 10 mg on days 1 and 10. On days 5–13, subjects received a daily dose of 150 mg peficitinib. Serial blood samples for pharmacokinetic assessment of rosuvastatin were collected up to 96 h post-dose on days 1 and 10, and for peficitinib were collected up to 24 h post-dose on days 9 and 10.
Results
Co-administration of peficitinib with rosuvastatin increased rosuvastatin area under the concentration-time curve (AUC) and maximum plasma concentration (C max) by 18 and 15%, respectively and increased peficitinib AUC and C max by 16 and 28%, respectively. In East Asian (n = 6) vs. non-East Asian subjects (n = 18), peficitinib mean AUC for a dosing interval was 45 and 21% higher, and mean C max was 67 and 34% higher, when administered alone or with rosuvastatin. Peficitinib was well tolerated with few adverse events overall.
Conclusion
In this study, once-daily oral administration of peficitinib had no clinically significant effect on the pharmacokinetics of rosuvastatin, a probe substrate for OATP1B1. Therefore, it is unlikely that peficitinib will have a clinically significant effect on the exposure of other substrates for OATP1B1.
ClinicalTrials.gov number
NCT01959399.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Co-administration of peficitinib, an orally administered, once-daily Janus kinase inhibitor, with rosuvastatin, a probe substrate for the organic anion transporting polypeptide 1B1, led to a small increase in rosuvastatin exposure (area under the concentration-time curve [AUC] from time zero to last measureable concentration [AUClast], 18%; maximum plasma concentration [C max], 15%), which was not considered clinically meaningful. |
Similarly, rosuvastatin had a small non-clinically meaningful effect on peficitinib exposure, increasing peficitinib AUC for a dosing interval (AUCtau) and C max by 16 and 28%, respectively. |
Peficitinib exposure (AUCtau and C max) was higher in East Asian subjects (n = 6) than in non-East Asian (n = 18) subjects by 45 and 67%, respectively; co-administration with rosuvastatin reduced these differences to 21 and 34%, respectively. |
When administered alone or in combination with peficitinib, rosuvastatin AUClast was 15% higher in East Asian subjects (n = 6) compared with non-East Asian subjects (n = 18). There was no difference in rosuvastatin C max between East Asian and non-East Asian subjects when administered alone, and rosuvastatin C max was 14% higher in East Asian vs. non-East Asian subjects when co-administered with peficitinib. |
Peficitinib administered alone and in combination with rosuvastatin was generally well tolerated. |
1 Introduction
Peficitinib is an orally bioavailable Janus kinase (JAK) inhibitor with selectivity for JAK1 and JAK3 that inhibits JAK1, JAK2, JAK3, and tyk2 enzyme activities, with inhibitory concentration 50% values of 3.9, 5.0, 0.7, and 4.8 nmol/L, respectively [1]. Peficitinib is being assessed as a treatment option for patients with rheumatoid arthritis and psoriasis. Several phase II trials have demonstrated the efficacy and tolerability of peficitinib in the treatment of psoriasis [2] and rheumatoid arthritis [1, 3, 4], with longer term and larger scale phase III studies of peficitinib 100- and 150-mg doses currently ongoing in Japan, Korea, and Taiwan (NCT02308163 and NCT02305849).
In vitro, peficitinib and its major metabolite H2 was shown to inhibit organic anion transporting polypeptide 1B1 (OATP1B1) [5]. OATP1B1 is a transporter that is specifically expressed on the basolateral membrane of hepatocytes and is involved in the transport of a wide range of compounds [6]. Inhibition of OATP1B1 in drug interaction studies has been shown to cause an increase in the plasma concentrations (area under the concentration-time curve [AUC] and maximum plasma concentration [C max]) of several drugs, including statins such as rosuvastatin [7–10].
Rosuvastatin is a selective and competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase that is used for the treatment of dyslipidemia, atherosclerosis, and hypercholesterolemia [11–14]. Rosuvastatin is also a probe substrate of OATP1B1 that has been used for in vivo drug–drug interaction studies as recommended by the US Food and Drug Administration [14–18]. Several known inhibitors of OATP1B1, including rifampicin and eltrombopag, have been reported to cause an increase in plasma rosuvastatin exposure upon co-administration [7, 10].
Reported here are the findings of a phase I, open-label, single-sequence, drug interaction study designed to evaluate the inhibitory effect of peficitinib on OATP1B1 in vivo by measuring the pharmacokinetics (PK) of rosuvastatin after repeat dosing with peficitinib in healthy East Asian and non-East Asian subjects. Peficitinib exposure has been shown to be higher in Japanese subjects; therefore, enrichment with East Asian subjects may increase the possibility of detecting an inhibitory effect of peficitinib on OATP1B1 [19]. In addition, population genetics indicate that some functionally significant haplotypes of SLCO1B1 single nucleotide polymorphisms (e.g., *1B [c.388G-c.521T], *5 [c.388A-c.521C], and *15 [c.388G-c.521C]) have higher frequencies in East Asian subjects [19]. Enriching the population of this study with East Asian subjects might also increase the possibility of enrolling subjects with OATP1B1 polymorphisms [20]. Rosuvastatin 10 mg was selected for this study because it is the starting dose in non-East Asian patients and is a mid-range dose in the approved clinical dose range, thus allowing for any increases in rosuvastatin exposure due to inhibition of OATP1B1-mediated hepatic uptake by peficitinib.
2 Methods
2.1 Study Design
The study was conducted according to an open-label, single-sequence design in healthy adult subjects at a single clinical site (PAREXEL International, Glendale, CA, USA). The institutional review board reviewed the ethical, scientific, and medical appropriateness of the study. Institutional review board approval of the protocol, informed consent, and subject information was obtained prior to the authorization of the drug shipment. The study was conducted in accordance with Good Clinical Practice, International Committee on Harmonization guidelines, and the Declaration of Helsinki.
Twenty-four subjects (six East Asian subjects [25%]) received a single dose of rosuvastatin 10 mg orally on days 1 and 10. On days 5–13, subjects received once-daily oral peficitinib 150 mg. Study drugs were administered in the morning under non-fasting conditions: subjects consumed a moderate-fat breakfast (19.5% total calories from protein, 49.5% from carbohydrates, and 32.4% from fat) approximately 30 min prior to drug administration. Elimination half-life of peficitinib has been shown to be 7–13 h [21], confirming that peficitinib plasma exposure was expected to reach steady state prior to the administration of the second dose of rosuvastatin on day 10.
Serial blood samples were collected for 96 h after dosing on days 1 and 10 for measurement of rosuvastatin concentrations in plasma, and for 24 h after dosing on days 9 and 10 for measurement of peficitinib and its metabolites. Samples were also collected immediately before dosing on days 7–10.
2.2 Study Population
Healthy male and female subjects of East Asian and non-East Asian origin, 18–55 years of age inclusive, with a body mass index of 18.5–32.0 kg/m2 and weighing at least 50 kg at screening were eligible to participate in this study; female participants must have been of non-child-bearing potential. East Asian subjects were first-generation Japanese/Chinese/Korean, born in Japan/China/Korea, with both parents and four grandparents of Japanese/Chinese/Korean descent. In addition, subjects must not have resided outside of Japan/China/Korea for longer than 5 years.
Key exclusion criteria included subjects with abnormalities in mean pulse rate and/or mean QT interval, positive serology test for hepatitis A, B, or C or human immunodeficiency virus type 1 or 2, a positive tuberculosis test, an absolute neutrophil count <2500 cells/mm3, or a creatinine phosphokinase >1.5 times the upper limit of normal at screening or at day −1. Subjects were excluded from the study if they had used any prescribed or non-prescribed drugs within the 2 weeks prior to study drug administration (except for hormone replacement therapy and intermittent acetaminophen), received any inducer of liver metabolism within the previous 3 months, received any vaccine within 60 days, had major gastrointestinal surgery, or had a medical condition that could inhibit the absorption and/or metabolism of the study drug. Furthermore, subjects were excluded if they anticipated an inability to abstain from xanthine, grapefruit, Seville oranges, star fruit, or any products containing these items for 72 h prior to day −1 and throughout the study. Additionally, they must not have been the recipient of any investigational drugs or participated in any interventional clinical study within 30 days, or five half-lives of the drug, prior to screening.
2.3 Study Objectives
The primary objective of the study was to assess the effect of multiple doses of peficitinib on the PK of rosuvastatin after single-dose administration. The secondary objectives of the study were to evaluate the effect of single-dose rosuvastatin on the PK of peficitinib and to assess the safety and tolerability of peficitinib administered alone and in combination with rosuvastatin. An exploratory analysis was conducted to examine the effects of peficitinib on the PK of rosuvastatin in East Asian and non-East Asian subjects.
2.4 Sample Collection and Pharmacokinetic Analysis
Blood samples for rosuvastatin were collected on days 1 and 10, pre-dose and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12, 24, 36, 48, 60, 72, and 96 h post-dose. For pharmacokinetic analysis of peficitinib and its metabolites, blood samples were collected on days 7 and 8 prior to dosing, day 9 pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 16 h post-dose, and on day 10 pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h post-dose. Venous blood samples were collected in K3 EDTA tubes for peficitinib and in di-potassium EDTA vacutainer tubes for rosuvastatin.
The plasma PK parameters were estimated through non-compartmental analysis (Model 200 for extravascular administration) for each subject using Phoenix® WinNonlin® 6.2 (Certara, Lincoln Park, NJ, USA). Parameters measured included: AUC from time zero to last measurable concentration (AUClast), AUC within a 24-h dosing interval (AUCtau) after multiple doses of peficitinib, C max, time to C max (t max), and elimination half-life. Non-compartmental analysis used the linear trapezoidal rule for all AUC calculations.
2.5 Bioanalytical Methods
Plasma samples were analyzed for peficitinib and its metabolites, H1, H2, and H4 at a central laboratory (Advion BioServices, Inc., Ithaca, NY, USA) using a validated high-performance liquid chromatography tandem mass spectrometry method with a limit of quantification of 0.25 ng/mL. Sample purification for peficitinib and the H4 metabolite was accomplished via supported liquid extraction (Isolute 200 mg SLE+, 96-well; Biotage, Charlotte, NC, USA), whereas the metabolites H1 and H2 were purified using protein precipitation (Ostro, 96-well; Waters Corporation, Milford, MA, USA).
Plasma samples were analyzed for rosuvastatin using a validated high-performance liquid chromatography tandem mass spectrometry method with a limit of quantification of 0.50 ng/mL. Sample purification used solid-phase extraction (Isolute® C18, 100 mg, 96-well; Biotage, Charlotte, NC, USA) followed by chromatographic separation on a Luna® C18 [2], 100 × 2.0 mm, 3 µM, high-performance liquid chromatogrpahy column (Phenomenex, Torrance, CA, USA).
2.6 Safety Assessments
Safety and tolerability was assessed through adverse event (AE) reporting (coded using the Medical Dictionary for Regulatory Activities, [MedDRA®] version 14.0) [22]; safety was evaluated via physical examinations and monitoring for changes in vital signs, clinical laboratory evaluations (hematology, chemistry, and urinalysis), and 12-lead electrocardiogram.
2.7 Statistical Analysis
The safety analysis set (SAF), which consisted of all enrolled subjects who received at least one dose of peficitinib, was used for the statistical summary of the safety data. The pharmacokinetic analysis set (PKAS) included the subjects from the SAF for whom sufficient plasma concentration data were available to facilitate derivation of at least one primary pharmacokinetic parameter.
To assess the effect of peficitinib on the PK of rosuvastatin, the natural log-transformed rosuvastatin AUClast and C max were analyzed using a mixed-effects model with treatment as a fixed effect and subject as a random effect. The 90% confidence intervals (CIs) were constructed around the geometric least-squares (LS) mean ratio of rosuvastatin and peficitinib (day 10) to rosuvastatin alone (day 1) for the primary pharmacokinetic parameters. As an exploratory analysis to assess the effect of rosuvastatin on the PK of peficitinib, the natural log-transformed plasma AUCtau and C max for peficitinib were analyzed. Treatment ratios were calculated by taking the anti-logarithm of the difference between treatment LS means. A 90% CI for each treatment ratio was obtained by taking the anti-logarithm of the 90% CI for each mean difference.
3 Results
3.1 Subject Demographics
Subject demographics and baseline characteristics are presented in Table 1. In total, 24 subjects were enrolled in the study (six East Asian [25%] and 18 non-East Asian subjects); one East Asian subject withdrew from the study on day 4 (informed consent withdrawn). All 24 subjects were included in the SAF and the PKAS.
3.2 Effect of Peficitinib on the Pharmacokinetics of Rosuvastatin
Co-administration of rosuvastatin with peficitinib did not affect the median t max; however, the AUClast and C max of rosuvastatin increased by 18 and 15%, respectively, compared with rosuvastatin alone, as judged by the rosuvastatin geometric LS mean ratios (90% CI 100.4–139.3% for AUClast and 100.7–130.9% for C max) (Table 2). For the majority of the subjects, it was not possible to determine the elimination half-life due to not enough concentration data in the terminal phase. Therefore, the AUCinf and elimination half-life were only determined in a small number of subjects (n = 6 for rosuvastatin alone and n = 8 for rosuvastatin plus peficitinib), which precluded a conclusive assessment on these parameters.
3.3 Effect of Rosuvastatin on the Pharmacokinetics of Peficitinib
Although co-administration of peficitinib with rosuvastatin did not affect the median t max, peficitinib AUCtau and C max were increased by 16 and 28%, respectively, compared with peficitinib administered alone, as judged by the peficitinib geometric LS mean ratios (90% CI 106.0–127.9% for AUClast and 113.2–145.4% for C max) (Table 2). Peficitinib AUCtau and C max showed low variability.
3.4 Plasma Pharmacokinetics in East Asian and Non-East Asian Subjects
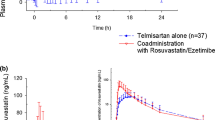
The mean plasma concentration-time profiles for rosuvastatin in East Asian and non-East Asian subjects are shown in Fig. 1. Mean AUClast for rosuvastatin was 15% higher in East Asian subjects (n = 6) than in non-East Asian subjects (n = 18) when administered both alone and in combination with peficitinib (Table 3). Mean C max for rosuvastatin administered alone was similar between East Asian and non-East Asian subjects, whereas co-administration with peficitinib appeared to increase the mean C max for rosuvastatin by 14% in East Asian subjects compared with non-East Asian subjects. Median t max for rosuvastatin administered alone was 2.5 h longer in East Asian subjects than in non-East Asian subjects. In non-East Asian subjects, median t max for rosuvastatin when co-administered with peficitinib was 0.7 h longer than in East Asian subjects.
The mean plasma concentration-time profiles for peficitinib are shown in Fig. 2. Either administered alone or in combination with rosuvastatin, peficitinib exposure appeared to be higher in East Asian subjects compared with non-East Asian subjects. The mean AUCtau and C max for peficitinib was 45 and 67% higher in East Asian subjects than in non-East Asian subjects, respectively, when peficitinib was administered alone (Table 4). The differences in peficitinib plasma exposure between East Asian and non-East Asian subjects appeared to be reduced when peficitinib was co-administered with rosuvastatin, with mean AUCtau and C max being 21 and 34% higher in East Asian subjects than in non-East Asian subjects, respectively. Median t max was similar in East Asian and non-East Asian subjects.
3.5 Effect of Rosuvastatin on the Pharmacokinetics of Peficitinib Metabolites H1, H2, and H4
An increase in AUCtau and C max was observed for all three metabolites, H1, H2, and H4, after co-administration with rosuvastatin, which was consistent with the increases observed with administration of the parent compound, peficitinib (Supplementary Table 1). The median t max appeared to be unchanged.
3.6 Safety
Overall, nine of the 24 subjects (38%) reported 13 AEs; eight AEs were reported by five subjects receiving rosuvastatin alone, five AEs were reported by four subjects receiving peficitinib alone (four of which were considered to be drug related: eye irritation [n = 1], nausea [n = 1], and headache [n = 2]), and none were reported by subjects receiving peficitinib co-administered with rosuvastatin (Table 5). The most frequently reported AEs overall were vessel puncture site swelling (n = 3), vessel puncture site hematoma (n = 2), vessel puncture site pain (n = 2), and headache (n = 2). All AEs were grade 1 and were followed until resolved.
No subjects died, experienced a serious AE, or discontinued because of an AE. There were no clinically significant abnormalities in any of the clinical laboratory, vital signs, or electrocardiogram assessments performed during the study.
4 Discussion
This open-label, single-sequence study in healthy adult subjects was designed to evaluate the inhibitory effect of peficitinib on OATP1B1 in vivo by measuring the PK of rosuvastatin after long-term dosing with peficitinib in healthy East Asian (n = 6) and non-East Asian subjects (n = 18) subjects. The study also aimed to evaluate the safety and tolerability of peficitinib administered alone, rosuvastatin administered alone, and peficitinib co-administered with rosuvastatin.
The study population was enriched by enrolling 25% East Asian subjects. One of the purposes of including East Asian subjects was to increase the frequencies of some functionally significant OATP1B1 genotypes, in particular SLCO1B1 haplotypes *1B [c.388G-c.521T], *5 [c.388A-c.521C], and *15 [c.388G-c.521C]) [20]. The demographic data confirmed that all six East Asian subjects in the study were either *1B [c.388G-c.521T] (83.3%) or *15 [c.388G-c.521C] (16.7%). Despite the physiological diversity between subjects of Japanese, Korean, or Chinese origin [19, 20, 23], the East Asian subjects enrolled in the current study had similar OATP1B1 genotypes.
Co-administration of peficitinib with rosuvastatin increased both peficitinib and rosuvastatin plasma exposures, which is consistent with the results of other trials assessing the effect of OATP1B1 inhibitors on the PK of different statins [7, 8]. Plasma concentration-time profiles for rosuvastatin were similar in East Asian and non-East Asian subjects (for rosuvastatin administered alone and co-administered with peficitinib), and rosuvastatin AUClast was 15% higher in East Asian subjects compared with non-East Asian subjects (for rosuvastatin administered alone and co-administered with peficitinib). These data are consistent with data previously shown in other studies [19], and could be explained by the different genetic polymorphisms that encode drug-metabolizing transporters [24]. There was no difference in C max between East Asian and non-East Asian subjects when rosuvastatin was administered alone or with peficitinib. When administered alone and with rosuvastatin, respectively, peficitinib mean AUCtau was 45 and 21% higher, and mean C max was 67 and 34% higher in East Asian subjects compared with non-East Asian subjects. However, drawing firm conclusions regarding the pharmacokinetic differences between East Asian and non-East Asian subjects are difficult owing to the low and disproportionate number of subjects in each group (East Asian [n = 6], non-East Asian subjects [n = 18]). Despite this, we believe that the East Asian vs. non-East Asian comparison is informative for clinicians, but further studies should be conducted using a larger number of subjects to investigate whether there are statistically significant differences in the PK of peficitinib and rosuvastatin between these two populations.
In terms of safety, peficitinib administered alone or in combination with rosuvastatin was well tolerated and consistent with previous trials assessing the effects of OATP1B1 inhibition on statins [7]. All AEs were grade 1 and the most common AE was vessel puncture site swelling (n = 3). This indicates that peficitinib is well tolerated when patients are taking concomitant OATP1B1 inhibitors such as rosuvastatin.
The limitations of the current study include the use of healthy subjects rather than the target patient population, the single-dose administration of rosuvastatin, the low and disproportionate number of East Asian and non-East Asian subjects in each group, and that OATP1B1 polymorphisms were not assessed. Peficitinib administered alone and in combination with rosuvastatin was generally well tolerated in this study.
5 Conclusion
Co-administration of orally administered, once-daily peficitinib led to a small non-significant increase in rosuvastatin exposure. Therefore, it is unlikely that peficitinib will have a clinically significant effect on the exposure of other substrates for OATP1B1 upon co-administration.
References
Takeuchi T, Tanaka Y, Iwasaki M, et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015 K) monotherapy in moderate to severe rheumatoid arthritis patients in Japan: a 12-week, randomized, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75(6):1057–64.
Papp K, Pariser D, Catlin M, et al. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2015;173(3):767–76.
Genovese M, Greenwald M, Codding C, et al. A phase 2b, randomized, double-blind, parallel-group, placebo-controlled, dose-finding, multi-center study to evaluate the safety and efficacy of ASP015K in moderate-to-severe rheumatoid arthritis subjects not on concomitant methotrexate. Arthritis Rheumatol. 2014;66(S10):S1234–5.
Kivitz A, Zubrzycka-Sienkiewicz A, Guttierez-Urena S, et al. A phase 2b, randomized, double-blind, parallel-group, placebo-controlled, dose-finding, multi-center study to evaluate the safety and efficacy of ASP015 K in moderate-to-severe rheumatoid arthritis subjects who have had an inadequate response to methotrexate. Arthritis Rheumatol. 2014;66(S10):S421–2.
Zhu T, Parker B, Wojtkowski T, et al. Drug interactions between ASP015 K (ASP) and rosuvastatin (R) in Asian and non-Asian subjects [abstract no. 1-42-2000353]. Clin Pharmacol Drug Dev. 2014;3(S1):1–59.
Sharma P, Butters CJ, Smith V, et al. Prediction of the in vivo OATP1B1-mediated drug-drug interaction potential of an investigational drug against a range of statins. Eur J Pharm Sci. 2012;47(1):244–55.
Allred AJ, Bowen CJ, Park JW, et al. Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers. Br J Clin Pharmacol. 2011;72(2):321–9.
Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B1 transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81(2):194–204.
Menon RM, Badri PS, Wang T, et al. Drug–drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J Hepatol. 2015;63(1):20–9.
Prueksaritanont T, Chu X, Evers R, et al. Pitavastatin is a more sensitive and selective organic anion-transporting polypeptide 1B clinical probe than rosuvastatin. Br J Clin Pharmacol. 2014;78(3):587–98.
Davidson M, Ma P, Stein EA, et al. Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. Am J Cardiol. 2002;89(3):268–75.
Davidson MH, Abate N, Ballantyne CM, et al. Ezetimibe/simvastatin compared with atorvastatin or rosuvastatin in lowering to specified levels both LDL-C and each of five other emerging risk factors for coronary heart disease: non-HDL-cholesterol, TC/HDL-C, apolipoprotein B, apo-B/apo-A-I, or C-reactive protein. J Clin Lipidol. 2008;2(6):436–46.
Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152–60.
Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–65.
Simonson SG, Raza A, Martin PD, et al. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004;76(2):167–77.
Kulmatycki K, Hanna I, Meyers D, et al. Evaluation of a potential transporter-mediated drug interaction between rosuvastatin and pradigastat, a novel DGAT-1 inhibitor. Int J Clin Pharmacol Ther. 2015;53(5):345–55.
Li R, Barton HA, Maurer TS. Toward prospective prediction of pharmacokinetics in OATP1B1 genetic variant populations. CPT Pharmacomet Syst Pharmacol. 2014;3:e151.
Ebner T, Ishiguro N, Taub ME. The use of transporter probe drug cocktails for the assessment of transporter-based drug-drug interactions in a clinical setting: proposal of a four component transporter cocktail. J Pharm Sci. 2015;104(9):3220–8.
Lee E, Ryan S, Birmingham B, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78(4):330–41.
Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–81.
Zhu T, Sawamoto T, Valluri U, et al. Pharmacokinetics, safety, and tolerability of ASP015K, a novel janus kinase inhibitor, in healthy volunteers. Ann Rheum Dis. 2013;72(Suppl 3):A898–9.
MedDRA. Introductory guide MedDRA v14.0. 2011. http://www.meddra.org/sites/default/files/guidance/file/intguide_14_0_english.pdf. [Accessed 17 Jan 2016].
Choi JH, Lee MG, Cho JY, et al. Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin Pharmacol Ther. 2008;83(2):251–7.
Smith NF, Figg WD, Sparreboom A. Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination. Exp Opin Drug Metab Toxicol. 2005;1(3):429–45.
Acknowledgements
Medical writing support was provided by Matthew Reynolds and Victoria Jones of Choice Healthcare Solutions and funded by Astellas Pharma Inc. We acknowledge all other investigators for their participation in this trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Astellas Pharma Inc.
Conflict of interest
Tong Zhu, Tomasz Wojtkowski, Tetsuya Nishimura, and James Keirns are employees of Astellas Pharma Inc. Barbara Parker, Jay Garg, and Ogert Fisniku were employees of Astellas Pharma Inc. during the study. David Han is an employee of PAREXEL International, contracted by Astellas Pharma Inc. to conduct the clinical assessments throughout this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, T., Parker, B., Wojtkowski, T. et al. Drug Interactions Between Peficitinib, an Orally Administered, Once-Daily Janus Kinase Inhibitor, and Rosuvastatin in Healthy Subjects. Clin Pharmacokinet 56, 747–757 (2017). https://doi.org/10.1007/s40262-016-0474-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0474-4