Abstract
Introduction
Modeling and simulation approaches are increasingly being utilized in pediatric drug development. Physiologically based pharmacokinetic (PBPK) modeling offers an enhanced ability to predict age-related changes in pharmacokinetics in the pediatric population.
Methods
In the current study, adult PBPK models were developed for the renally excreted drugs linezolid and emtricitabine. PBPK models were then utilized to predict pharmacokinetics in pediatric patients for various age groups from the oldest to the youngest patients in a stepwise approach.
Results
Pharmacokinetic predictions for these two drugs in the pediatric population, including infants and neonates, were within a twofold range of clinical observations. Based on this study, linezolid and emtricitabine pediatric PBPK models incorporating the ontogeny in renal maturation describe the pharmacokinetic differences between adult and pediatric populations, even though the contribution of renal clearance to the total clearance of two drugs was very different (30 % for linezolid vs. 86 % for emtricitabine).
Conclusion
These results suggest that PBPK modeling may provide one option to help predict the pharmacokinetics of renally excreted drugs in neonates and infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Physiologically based pharmacokinetic (PBPK) models were developed for linezolid and emtricitabine to predict the pharmacokinetics of these drugs in adult and pediatric populations, including infants and neonates, and the predictions were within a twofold range of clinical observations. |
Linezolid and emtricitabine pediatric PBPK models incorporating the ontogeny of renal maturation reasonably describe the pharmacokinetic differences between adult and pediatric populations, which suggests that PBPK modeling may provide one option for predicting the pharmacokinetics of renally excreted drugs in neonates and infants. |
1 Introduction
The Food and Drug Administration Safety and Innovation Act (FDASIA) specifically mandates and provides incentives to conduct appropriate studies in neonates [1]. As clinical pharmacology studies are pivotal for dose selection in pediatric drug development, neonatal pharmacokinetics and dose-ranging studies are very challenging [2]. Considerable inter- and intra-patient variability in pharmacokinetics occurs in this age group because of the rapid growth and maturation in neonates. Within the neonatal population there is a 1 log size difference in weight (0.5–5 kg), which is analogous to the 1 log size spectrum (5–50 kg) in childhood. The first month of human life is characterized by a dynamic biological system where growth, maturation, and extensive variability are key issues [3]. Other difficulties inherent in neonatal trials include (a) ethical and practical constraints; (b) low study consent rates from the parents of vulnerable infants; (c) limited blood volume available to conduct pharmacokinetic studies; (d) lack of availability of sensitive drug concentration assays from very-small-volume specimens (e.g., dried blood spots); and (e) a lack of robust clinical endpoints [4].
The prediction of pediatric drug dosing has traditionally been scaled from adult doses using allometric scaling related to body weight, height, or age. However, due to rapid changes in the drug disposition processes, simple allometric approaches to dosing in infants and neonates are questionable and have poor prediction performance [5].
One approach to the difficulties in developing medicinal products for infants and neonates would be to use physiologically based pharmacokinetic (PBPK) modeling to develop effective dosing regimens [6]. In March 2012, the U.S. Food and Drug Administration (FDA) held a Clinical Pharmacology Advisory Committee meeting to discuss how to improve pediatric drug development through the use of modeling and simulation (M&S) [7]. The experts on the advisory committee unanimously recommended that M&S methods should be considered in all pediatric drug development programs for optimizing pediatric trial designs including dose selection. The traditional population pharmacokinetic modeling approach has limitations in extrapolating the prediction outside the age range studied [8]. With consideration of the physiological differences between adults and pediatric patients, several publications have demonstrated the advantage of using PBPK models in the prediction and simulation of drug exposure in different age groups within the pediatric population [9–12].
The application of PBPK in predicting drug exposure for the neonatal population has not been evaluated extensively. Recently, there has been an expanded use of M&S in drug development along with an increasing acceptance of M&S in pediatric drug development. A body of physiological, biological, and pharmacological data has subsequently become available to inform neonatal modeling related to the maturation of renal function [13, 14].
The goal of this study initially was to evaluate PBPK modeling for predicting the pharmacokinetics of candidate drugs in neonates, with a focus on renal maturation. After consideration, linezolid and emtricitabine were selected for the following reasons: (1) renal elimination is involved without significant hepatic metabolism, allowing a focus on the ontogeny of renal function uncomplicated by hepatic maturation; and (2) sufficient pharmacokinetic data were publically available from neonates, infants, children, and adults for the purpose of model evaluation. PBPK modeling of the two drugs with different extents of renal elimination could also be helpful to evaluate the sensitivity of PBPKs for the prediction of the effect of renal maturation in relation to neonatal drug clearance.
After a prior survey of neonatal drug development programs [2], linezolid was chosen as one of the drugs to assess with PBPKs. Although non-renal clearance accounts for approximately 65 % of the total clearance of linezolid, 30 % of the linezolid dose appears in the urine as parent compound. Linezolid is therefore considered to be substantially renally eliminated [15]. Linezolid is not a recognized substrate for any renal transporters, although renal reabsorption contributes overall to its renal clearance (CLR). Linezolid is only minimally metabolized by cytochrome P450 (CYP) 3A4 in vivo [16].
Emtricitabine was selected as an additional candidate. Approximately 86 % of the emtricitabine dose is excreted in urine. The CLR of emtricitabine involves active tubular secretion, but the involvement of a specific renal transporter has not been demonstrated [17, 18]. Therefore, linezolid and emtricitabine have elimination pathways that include renal elimination and alternate clearance pathways that do not involve recognized drug transporters and CYP enzymes.
However, both linezolid and emtricitabine have clearance pathways that have not been fully elucidated. Emtricitabine is minimally transformed (13 %), including oxidation of the thiol moiety (9 %) and conjugation with glucuronic acid (4 %) [19]. However, the specific enzymes contributing to the biotransformation of emtricitabine have not been reported. Sixty-five percent of linezolid is cleared by a non-renal route through two pathways that form the oxidative metabolites. An in vitro study demonstrated that this is a non-enzymatic process but that the oxidants contributing to this non-enzymatic process are still unknown [20].
Since the metabolism of linezolid and emtricitabine are not fully elucidated, the effect of the maturation process on these pathways is unknown. This uncertainty can be reduced through PBPK modeling if maturation of renal elimination pathways alone can predict drug clearance in neonates. Therefore, the objective of this study was to determine whether a PBPK model could reasonably predict neonatal and infant drug clearance and dosing for linezolid and emtricitabine.
2 Methods
2.1 Drug Selection
The candidate drugs were selected based on the following criteria: abundant publically available pharmacokinetic data from different age groups of pediatric patients including infants and neonates; and primary renal elimination or a relatively simple mechanism of non-renal elimination (e.g., no involvement of drug transporters or CYPs).
2.2 Workflow
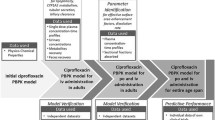
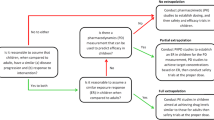
The strategy for the development of the linezolid and emtricitabine pediatric PBPK model followed the workflow described previously [21–23]. An adult model was initially developed and optimized by using the concentration–time profile after intravenous infusion administration (linezolid: intravenous 250 mg, single oral 600 mg tablet; emtricitabine: intravenous 200 mg, single oral solution 200 mg) in comparison with the observed data [24, 25]. The PBPK models were then further calibrated and validated by simulating the concentration–time profiles after different doses or formulations (linezolid: intravenous 500 mg; emtricitabine: single oral dose of 200 mg in capsule formulation) and compared with the prior clinical observations [24, 25]. The final adult model was then extended to children by switching to a pediatric population, which takes into consideration age-dependent anatomical and physiological parameters as well as the ontogeny of drug clearance pathways. This model was used to predict the pediatric linezolid and emtricitabine exposure across different age groups, including infants and neonates, following intravenous administration [24, 25]. The observed area under the plasma concentration–time curve (AUC) and maximum concentration (C max) values for each subject were determined by non-compartmental analysis (NCA), with the assumption that C max was the concentration at the end of the infusion.
PBPK models were developed by using the population-based PBPK software Simcyp® (Simcyp Ltd, a Certara company, Sheffield, UK; version 14.1).
2.3 Development of the Adult Linezolid Physiologically Based Pharmacokinetic (PBPK) Model
Linezolid drug-dependent parameters are listed in Table 1. Drug absorption was predicted by the advanced distribution, absorption, and metabolism (ADAM) model using P eff.man (human jejunum permeability) estimated from the Gastroplus® ADMET predictor (Simulation Plus., Inc., Lancaster, CA, USA) based on the structure of the drug and parameters shown in Table 1. Drug distribution of linezolid was described by a lumped minimal PBPK (mPBPK) model in Simcyp® with a volume of distribution at steady state (V ss) of 0.65 L/kg as reported previously [24, 26]. Since linezolid is extensively absorbed after oral dosing and the absolute bioavailability is approximately 100 %, the simulation of the linezolid 600 mg oral tablet was chosen as a solution (full absorption) in the formulation that was selected in the Simcyp® simulator. Sixty-five percent of linezolid is eliminated through a non-renal pathway, which was estimated with the whole-liver metabolic clearance model in Simcyp® since a detailed mechanism for liver metabolism is not known [16]. The intrinsic hepatic clearance was determined by the same retrograde method in Simcyp® as described above. Based on the linezolid drug label, the average total clearance after intravenous administration is approximately 8.76 L/h. The CLR after tubular reabsorption was reported to be in the range of 1.8–3.0 L/h [24]. With a CLR of 1.8 L/h, the predicted exposure change in AUC between renal impairment in an adult population (creatinine clearance <30 mL/min) and a healthy adult population was 1.3 L/h (95 % confidence interval [CI] 1.2–1.5), which was close to the clinical observation of 1.2 L/h (95 % CI 1.0–1.3) [24, 27] (Electronic Supplementary Material Fig. 1). If a CLR of 3.0 L/h was used in our model, it would significantly overpredict the ratio of the mean predicted AUC to the mean observed AUC (AUCR) (1.5; 95 % CI 1.3–1.7). Therefore, a CLR of 1.8 L/h was selected. The estimated liver intrinsic clearance (Hep CLint) using the retrograde method in Simcyp® was estimated to be 0.996 µL/min/mg protein. A non-enzymatic pathway plays a major role in non-renal clearance of linezolid, but the detailed mechanism is not clear [16]. Factors such as tissue volume, organ maturation, and blood flow have been considered in Simcyp® to contribute to the pharmacokinetic difference between pediatric and adult populations [14]. Since the major metabolism of linezolid is contributed by a non-enzymatic pathway, we assume there is no ontogeny associated with this pathway during the pediatric PBPK model development.
2.4 Development of the Adult Emtricitabine PBPK Model
The drug-dependent parameters of the emtricitabine PBPK model are listed in Table 1. The absorption of emtricitabine was predicted by the ADAM model using the P eff.man value from Xia et al. [28]. For the simulation of the emtricitabine 200 mg oral solution, “solution” was selected in the Formulation tab in the Simcyp® simulator. For the simulation of the emtricitabine 200 mg oral capsule, “immediate release” (IR) in solid formulation was selected in the Simcyp® simulator, and the intrinsic aqueous solubility was entered as 112 mg/mL [25]. The tissue distribution of emtricitabine was best described by a multi-compartmental full PBPK distribution model in Simcyp® by optimizing Log P vo:w (the logarithm of the olive oil:buffer distribution coefficient at pH 7.4) to 1.08 against the observed V ss, with method 2 published by Rodgers et al. [29–31] as the prediction model in Simcyp®. The majority (~86 %) of emtricitabine is renally eliminated. The CLR was assumed to be 14.9 L/h with a total clearance of 17.52 L/h [25]. With this CLR, the predicted exposure change for the AUC between a renal impairment adult population (creatine clearance <30 mL/min) and the healthy adult population was 2.2 L/h (95 % CI 2.2–2.3), which is close to the clinical observation of 2.8 L/h (95 % CI 2.3–3.7) [25] (Electronic Supplementary Material Fig. 1). Emtricitabine is minimally transformed (13 %) [19], and therefore the whole-organ metabolic clearance model was used to estimate the non-renal elimination of emtricitabine since the detailed enzyme pathway involved in liver metabolism is not clear. Using the retrograde tool in Simcyp®, which scales intravenous clearance or oral clearance values back to intrinsic clearance values corresponding to the in vitro system (i.e., any enzyme involved) [32], the estimated Hep CLint was determined to be 0.247 µL/min/mg protein. The biotransformation of emtricitabine includes oxidation of the thiol moiety (9 %) and conjugation with glucuronic acid (4 %) [19]. Since it is not clear which enzyme contributes to the biotransformation of emtricitabine, we assumed that the hepatic clearance pathway was associated with rapid maturational changes during our PBPK model development.
2.5 Development of Pediatric PBPK Models
The pediatric PBPK models were developed using the population-based PBPK software Simcyp®. Renal function in pediatric patients was calculated as the glomerular filtration rate (GFR) up to 20 years of age, which is in turn based on body surface area (BSA) [14] as in Eq. (1):
Renal function in the pediatric populations was expressed as the pediatric GFR relative to the standard adult value of 120 mL/min. This relative value was then subsequently used to calculate the CLR of each compound from the average value in adults. The GFR in adults (>20 years) is estimated using the Cockcroft–Gault equation [33].
In addition to the renal maturation [13, 34, 35] routine in Simcyp®, physiologic parameters such as organ size and cardiac output, liver blood flow, and age-related plasma protein binding also accounted for the difference between the pediatric and adult populations [14].
Real-time growth and maturation of individuals through time courses of drug exposure may need to be considered during neonatal studies, especially if they are prolonged. Time-varying physiology, which introduces age progression into a pediatric model, was considered during simulation whenever applicable (e.g., simulation of emtricitabine in a pediatric population 1–90 days old with a 6-day trial period; Fig. 5) by redefining individual subjects over the trial period in the Simcyp® simulator [36].
2.6 Simulation of Drug Pharmacokinetics in Pediatric Patients
The performance of the PBPK model was determined by the AUCR. A ratio within a twofold range (0.5–2.0 × mean) was considered to be a satisfactory prediction [37]. The CI of the ratio of the two means was calculated based on an equation developed by Fieller [38]. Virtual population simulations used ten trials with 50 subjects in each trial (500 subjects in total) for each age group by matching the demographic data to the actual clinical study data (e.g., age, female/male ratio, etc.). The cutoff for each age band, as shown in the figure legends in “Results” section, was based on the available observed data for each drug.
The previously described PBPK models based on adult data were used to simulate pediatric concentration–time profiles. The pediatric models of each drug were first verified by simulating pharmacokinetics in the older age groups, which were then compared to the clinical observations before continuing to predict pharmacokinetics in younger children or neonates. Briefly, we used a pediatric linezolid model to predict pharmacokinetics in the pediatric population aged 13–17 years old, and evaluated the performance by visually checking and calculating the AUCR and the ratio of C max. We only continued our simulation in the younger age groups of 7–12 years old, 3–6 years old, 1–2 years old, 0.25–1 years old, and 1–28 days old if the prediction performance met our criteria. As stated previously, the acceptance criteria were that the observed concentration–time profiles need to be within the 90 % CI of the simulated concentration–time profiles, and the AUCR must be within a twofold range. Similarly, the emtricitabine pediatric PBPK model was used to predict the pharmacokinetics in the age group of 13–17 years old. If the AUCR and ratio of C max were within twofold, we then continued to predict the AUCR and the ratio of C max in the younger age groups of 7–12 years old, 2–6 years old, 0.2–2 years old, 43–90 days old, 22–42 days old, and 1–21 days old. During this stepwise verification process [21], the PBPK model was refined if the prediction in any older age groups did not meet our performance criteria, and then the whole stepwise verification process was repeated again starting in adult groups followed by the pediatric age groups. Both the emtricitabine and linezolid pediatric PBPK models were able to be extended from the adult model through the stepwise verification process without any refinement.
2.7 Evaluation of the Impact of Different Ontogeny Profiles of Whole-Liver Metabolic Clearance Pathways in Linezolid and Emtricitabine Pediatric PBPK Models
The detailed mechanism for the hepatic metabolism of linezolid and emtricitabine is not clear, and therefore the hepatic clearance of linezolid and emtricitabine were both defined by whole-liver intrinsic clearance in the respective PBPK model in Simcyp® as described earlier. Due to the uncertainty in maturation of this hepatic clearance pathway, the predicted impact of different ontogeny profiles on the hepatic clearance pathway and pharmacokinetics in neonates was evaluated by manually selecting ontogeny to no ontogeny, slow ontogeny, medium ontogeny, and fast ontogeny in the Simcyp® simulator. The default settings in Simcyp® for each different ontogeny profile were used.
3 Results
3.1 Simulations from the Adult Linezolid and Emtricitabine PBPK Models
The adult linezolid PBPK model could reasonably describe the concentration–time profiles of linezolid after the intravenous infusion of 250 or 500 mg, and a single oral dose of 600 mg compared to the observed data (Fig. 1). The mean ratio of observed versus predicted (95 % CI) for the C max were 1.08 (0.80–1.40) for the 250 mg intravenous dose, 1.00 (0.80–1.04) for the 500 mg intravenous dose, and 1.61 (1.10–2.35) for the 600 mg oral administration. These mean ratios were within the desired twofold range except for the upper bound of the oral 600 mg dose. Similarly, the mean ratio of observed versus predicted AUC was also within a twofold range. The mean ratios of observed versus predicted (95 % CI) for the AUC were 1.37 (1.11–1.55) for the 250 mg intravenous dose, 1.38 (1.15–1.62) for the 500 mg intravenous dose, and 1.61 (1.10–2.35) for the oral 600 mg dose. Furthermore, the clinical observations were within the 5–95 % of the mean simulated linezolid concentration–time profiles (Fig. 1).
Simulated linezolid concentration–time profiles after intravenous administration of a 250 mg, b 500 mg, or c a single oral administration of 600 mg. In a and b the solid squares denote mean values from the clinical studies; the error bars represents inter-individual difference (standard deviation) of the studied population in the clinical trial if available; in c the solid squares denote mean observed values from the clinical studies [24]. The thick line represents the mean value of the simulated concentration, while the upper and lower thin dashed lines represent 95 and 5 % of the mean simulated plasma concentration. The y-axis represents the drug concentration (µg/mL). i.v. intravenous
As shown in Fig. 2, the clinically observed emtricitabine AUC was within the 5–95 % of the mean simulated concentration–time profiles. The mean ratios of observed versus predicted (95 % CI) for the C max were 1.07 (1.02–1.12) for the 200 mg intravenous dose, 0.86 (0.74–1.00) for the 200 mg oral solution dose, and 1.33 (1.17–1.53) for the 200 mg oral capsule dose. The mean ratios of observed versus predicted (95 % CI) for the AUC were 1.01 (0.98–1.04) for the 200 mg intravenous dose, 0.78 (0.75–0.81) for the 200 mg oral solution dose, and 1.00 (0.97–1.03) for the 200 mg oral capsule dose.
Simulated versus observed plasma concentration–time profiles of a emtricitabine after intravenous infusion of 200 mg (the solid squares denote mean observed values from the clinical studies [25], the thick line represents the mean value of the simulated concentration, while the upper and lower thin dashed lines represent 95 and 5 % of the simulated mean plasma concentration), b emtricitabine after single oral administration of 200 mg oral solution (the solid squares denote mean observed values from the clinical studies [25], the thick line represents the mean values of the simulated concentration, while the upper and lower thin dashed lines represent 95 and 5 % of the simulated mean plasma concentration, respectively) and c emtricitabine after single oral administration of a 200 mg oral capsule (the solid squares denote mean observed values from the clinical studies [25], the thick line represents the mean values of the simulated concentration, while the upper and lower thin dashed lines represent 95 and 5 % of the simulated mean plasma concentration, respectively). The y-axis represents the drug concentration (µg/mL)
3.2 Predicting Pediatric Pharmacokinetics by Age Groups
The validated PBPK models were extended to corresponding pediatric models by switching the adult population to the built-in Simcyp® pediatric population, which takes into consideration the difference in anatomical and physiological processes and parameters, and the ontogeny of the metabolizing enzymes or transporters between the adult and pediatric populations. Since we already had pharmacokinetic data for emtricitabine and linezolid in various age groups of the pediatric population, we were able to evaluate the pediatric PBPK models using the stepwise approach as described in the Sect. 2. Both the linezolid and emtricitabine models went through this stepwise verification process without any model refinement. The simulated concentration–time profiles for linezolid and emtricitabine in different age groups that are shown in Figs. 3 and 5 were conducted by changing the age range of the pediatric population in the simulator.
Simulated concentration–time profiles after intravenous administration of linezolid 10 mg/kg in different age groups of pediatric patients. The validated linezolid physiologically based pharmacokinetic model was applied to predict pediatric pharmacokinetics by switching to the built-in pediatric population in Simcyp®. The simulated versus observed plasma concentration–time profiles of an intravenous infusion of linezolid 10 mg/kg in different pediatric age groups according to the age grouping in the actual clinical trials [24] (Electronic Supplementary Material Table 1) (a 13–17 years old [12 subjects], b 7–12 years old [15 subjects], c 3–6 years old [14 subjects], d 1–2 years old [12 subjects], e 3–12 months old [ten subjects], and f 1–28 days old neonates [29 subjects]) were then conducted by changing the respective age range in the pediatric population. The solid squares denote mean observed values from the clinical studies [24]; the error bars represent the inter-individual difference of the studied population (standard deviation) in the clinical trial if available. The thick lines represent the mean values of the simulated concentration, while the upper and lower thin dashed lines represent 95 and 5 % of the mean simulated plasma concentration, respectively. The y-axis represents the drug concentration (µg/mL). i.v. intravenous
For linezolid, as shown in Fig. 3, the simulated pharmacokinetic profiles in the pediatric age groups, including children 13–17, 7–12, 3–6, and 1–2 years old, were consistent with the observed data [24] (Electronic Supplementary Material Table 1). The mean ratio of the observed to predicted value for AUC and C max was within the twofold range (Fig. 4). When using this pediatric model to predict the linezolid exposure in infants (0.25–1 years old), the prediction slightly under-estimated the clearance, but the observation was still within 5–95 % of the mean simulated concentration–time profiles. The mean ratio of the AUC or C max observed versus predicted values was within the twofold range. For the simulation of linezolid pharmacokinetics in the neonatal population (age group 1–28 days in Fig. 3), most of the observed neonates were within 5–95 % of the mean simulated plasma concentration. The mean ratios of C max and AUC with 95 % CIs were within a twofold range (Fig. 4).
Comparison between the linezolid observed and predicted value of the ratio of the area under the plasma concentration–time curve (left) and the ratio of the maximum concentration (right). Results are presented as mean ratios (red solid circles) in each age group with the 95 % confidence interval (horizontal lines). The y-axis represents the age groups of pediatric patients as grouped in actual clinical studies. AUC area under the plasma concentration–time curve, C max maximum concentration
For the simulation of emtricitabine in the age groups of 22–42 and 43–90 days of age, the clinically observed individual data were generally within 5–95 % of the mean simulated plasma concentration. In the age group of 1–21 days of age, the simulated pharmacokinetic profiles were consistent with the majority of the individual observed data, with some outliers at earlier sampling points (Fig. 5) [25] (Electronic Supplementary Material Table 2). The mean ratios of the emtricitabine C max and AUC with 95 % CIs were within the twofold range for all age groups (Fig. 6).
The validated emtricitabine physiologically based pharmacokinetic model was applied to predict pediatric pharmacokinetics by switching to the built-in pediatric population in Simcyp®. The simulated versus observed plasma concentration–time profiles after administration of emtricitabine oral solution 3 mg/kg in different pediatric age groups according to the age grouping in the actual clinical trials [25] (Electronic Supplementary Material Table 2) (a 43–90 days old [12 subjects], b 22–42 days old [10 subjects], and c 1–21 days old [18 subjects]) were then conducted by changing the age range in the pediatric population of the software. The solid squares represent the observed concentration of emtricitabine in individuals in the actual clinical trial [25]. The thick line represents the mean values of the simulated concentration, while the upper and lower thin dashed lines represent 95 and 5 % of the mean simulated plasma concentration, respectively. The y-axis represents the drug concentration (µg/mL)
Comparison between the emtricitabine observed and predicted value of the ratio of the area under the plasma concentration–time curve (left), and the ratio of the maximum concentration (right). Results are presented as mean ratios (red solid circles) in each age group with the 95 % confidence interval (horizontal lines). The y-axis represents the age groups of pediatric patients as grouped in actual clinical studies. AUC area under the plasma concentration–time curve, C max maximum concentration
3.3 Evaluation of the Impact of Different Ontogeny Profiles of Uncertain Metabolic Pathways in Linezolid and Emtricitabine
Although linezolid and emtricitabine have relatively simple metabolism and elimination pathways, both drugs have unidentified metabolism or elimination mechanisms. Sixty-five percent of linezolid is cleared via a non-renal route through an unidentified non-enzymatic pathway. No ontogeny was assumed with this non-enzymatic pathway in whole-liver drug clearance. The predicted data using this approach were consistent with clinical observations in the various pediatric age groups. We also evaluated the impact of different types of ontogeny profiles (fast and slow) on predicted AUCR within neonates, which should be the population mostly affected by the different ontogeny profiles. With any ontogeny, this pathway would under-estimate the clearance and the predicted AUCR (Fig. 7a). Another possibility is that the ontogeny associated with this pathway is so rapid that the clearance reaches the adult level immediately after the birth.
a Predicted AUCR obtained with the linezolid pediatric physiologically based pharmacokinetic model after intravenous infusion of 10 mg/kg in neonates (1–28 days) under three different ontogeny profiles for the whole-liver clearance pathway (error bars represent 95 % CIs of predicated AUCR). b Predicted AUCR obtained with the emtricitabine pediatric physiologically based pharmacokinetic model after oral administration of 3 mg/kg oral solution in neonates (1–21 days) under three different ontogeny profiles for the whole-liver clearance pathway (error bars represent 95 % CIs of predicated AUCR). For both a and b the three different ontogeny profiles (no ontogeny, fast ontogeny, and slow ontogeny) are the profiles built in the Simcyp® simulator. The predicted AUC after simulation was compared with clinical observations shown in Electronic Supplementary Material Table 1 (a) and Electronic Supplementary Material Table 2 (b), and the 95 % CI of the AUCR was calculated in a similar manner as is described in the Sect. 2. AUC area under the plasma concentration–time curve, AUCR ratio of the mean predicted AUC to the mean observed AUC, CI confidence interval
Approximately 86 % of the emtricitabine dose is excreted in urine, and emtricitabine is minimally transformed (13 %). However, it is not clear which specific enzymes contribute to the biotransformation of emtricitabine. During model development, we assumed that the non-renal clearance of emtricitabine was contributed by whole-liver clearance with rapid ontogeny. Our predictions in the pediatric age groups were generally consistent with clinical observations. In the established PBPK model, we further evaluated the impact of ontogeny in this pathway on model prediction. Although changing the ontogeny profile for this pathway in Simcyp® to slow or no ontogeny slightly changed the predicted AUCR for neonates, the predicted AUCR is still within the twofold range (Fig. 7b), as expected by the relative minor contribution of this pathway to the overall clearance of emtricitabine.
4 Discussion
Our study provides two cases to illustrate that PBPK modeling can serve as an alternative approach for predictions of pediatric drug clearance for all age groups when the drug clearance is related to renal function maturation.
Both emtricitabine and linezolid are relatively rapidly and extensively absorbed following oral administration. The mean observed absolute bioavailability of the emtricitabine oral solution was 75 %, while the absolute bioavailability for linezolid was approximately 100 %. Linezolid is not known to be a substrate of any renal transporters, although renal reabsorption may affect its CLR. Linezolid has previously been found to be only minimally metabolized by CYP3A4 in vivo [16]. Therefore, renal transporters and CYP3A4 enzyme kinetics are not considered in the current linezolid PBPK model. The CLR of emtricitabine involves active tubular secretion, but it is still not clear which specific transporter is involved [17, 18]. Therefore, renal transporters are not considered in the current emtricitabine PBPK model. Since hepatic enzyme metabolism played a minimal role with both drugs, the ontogeny in both PBPK models is mainly focused on the renal pathway.
As pediatric pharmacokinetic data for both drugs are available, we are able to confirm that the pediatric PBPK model developed is appropriate as it was validated through a stepwise verification process by simulating pharmacokinetics in older pediatric age groups first [21]. Both pediatric models simulated pharmacokinetics in different age groups down to neonates without any model refinement. The AUCR and C max ratio are within a twofold range, and the simulated concentration–time profiles are close to clinical observations.
Our results suggest that a pediatric PBPK model incorporating renal maturation, along with our assumptions on ontogeny of the metabolism and elimination pathways of both drugs, can sufficiently describe pharmacokinetic differences of primarily renally excreted drugs between pediatric and adult populations. The relatively good prediction of pharmacokinetics in neonates with our current models may not apply to pharmacokinetic prediction of other drugs in neonates or infants, especially if they involve complex elimination pathways in addition to renal elimination. However, linezolid and emtricitabine were selected as candidates for PBPK models not only because both of them are renally eliminated, but also because they have relatively simple elimination pathways with no transporters and no CYP enzymes involved. Therefore, these models allow an evaluation of the performance of these PBPK models in neonates with a focus on renal maturation. If future model drugs have complex metabolic pathways in addition to renal elimination, then we may have confidence in the renal maturation pathway based on our current study, and only focus on the ontogeny of other pathways in neonates when building a more complex model.
These two cases suggest that PBPKs are a viable option to provide useful information for pharmacokinetic prediction or dose selection in neonates. The impact of different ontogeny profiles on model prediction was evaluated to verify our assumptions (Electronic Supplementary Material Figs. 1, 2). These practices suggest that PBPK modeling provides a convenient platform on which to evaluate different scenarios in ontogeny when uncertainties exist. This is particularly important for model prediction for neonates because of the rapid physiological development in this pediatric population. Even when ontologic data for a pathway are available from in vitro data or from a prediction based on in vivo data, it may still be necessary to evaluate different ontogeny profiles in PBPK models due to the possible gaps in in vitro–in vivo extrapolation or the effect of a disease state on maturation of a pathway.
There are several limitations to our study. First, renal function maturation predicted in Simcyp® using Eq. (1) is not completely validated for neonates with low birth weight, especially preterm neonates. Once the BSA drops below 0.18 m2, Eq. (1) generates negative GFR values. Also, the current Simcyp® simulator does not consider the physiology and maturation process for preterm neonates. Therefore, the simulations of linezolid and emtricitabine in the neonatal population are only for full-term neonates. Better characterization of the physiology and ontogeny in preterm and extreme preterm neonates is needed in order to predict the drug clearance in this critically ill pediatric population. Second, our study focused on the maturation of renal function in pediatric patients with normal renal function for their developmental stage. Renal impairment produced by disease or drug toxicity in pediatric patients warrants further investigation [39, 40]. Another limitation is that the possible involvement of transporters in the renal reabsorption of linezolid and active tubular secretion of emtricitabine are not considered in the current models. The involved renal transporters might have a different ontogeny profile than renal maturation. The maturation of a renal transporter for the reabsorption of linezolid might contribute to the faster clearance observed in neonates (1–28 days) shown in Fig. 3. The simulation of emtricitabine in neonates in comparison to clinical observations (Fig. 5) may suggest that the ontogeny profile of a renal transporter involved in the active secretion of emtricitabine might have a similar ontogenic profile to GFR maturation. Therefore, the possible contribution of transporter ontogeny to renal maturation cannot be excluded by this work. Finally, we focused only on ontogeny studies of elimination pathways, but the impact of ontogeny on drug absorption in infants is discussed elsewhere [41].
5 Conclusions
In summary, our linezolid and emtricitabine PBPK models could describe the pharmacokinetics of these drugs in the adult population. When adult models were extended to the pediatric population, the mean ratios of the observed to the predicted C max and AUC with a 95 % CI per age group were within a twofold range of error. Our two case studies suggest that PBPK models incorporating renal maturation ontogeny may provide a viable option for predicting the pharmacokinetics of renally eliminated drugs in the pediatric patient populations including neonates. For compounds primarily excreted renally and having a relative simple elimination pathway with no transporter or CYPs involved, PBPK modeling may provide an alternative approach for predicting neonatal and pediatric pharmacokinetics based on adult data.
References
FDA. Food and Drug Administration Safety and Innovation Act (FDASIA). Silver Spring: FDA; 2012.
Wang J, Avant D, Green D, Seo S, Fisher J, Mulberg AE, et al. A survey of neonatal pharmacokinetic and pharmacodynamic studies in pediatric drug development. Clin Pharmacol Ther. 2015;98(3):328–35.
van den Anker J, Allegaert K. Clinical pharmacology in neonates and young infants: the benefit of a population-tailored approach. Expert Rev Clin Pharmacol. 2012;5(1):5–8.
Laughon MM, Avant D, Tripathi N, Hornik CP, Cohen-Wolkowiez M, Clark RH, et al. Drug labeling and exposure in neonates. JAMA Pediatr. 2014;168(2):130–6.
Johnson TN. The problems in scaling adult drug doses to children. Arch Dis Child. 2008;93(3):207–11.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacomet Syst Pharmacol. 2014;3:e150.
Advisory Committee for Pharmaceutical Science and Clinical Pharmacology. Clinical pharmacology aspects of pediatric clinical trial design and dosing to optimize pediatric drug development. Silver Spring: FDA; 2012.
Wang J, Edginton AN, Avant D, Burckart GJ. Predicting neonatal pharmacokinetics from prior data using population pharmacokinetic modeling. J Clin Pharmacol. 2015;55(10):1175–83.
Johnson TN, Zhou D, Bui KH. Development of physiologically based pharmacokinetic model to evaluate the relative systemic exposure to quetiapine after administration of IR and XR formulations to adults, children and adolescents. Biopharm Drug Dispos. 2014;35(6):341–52.
Khalil F, Laer S. Physiologically based pharmacokinetic models in the prediction of oral drug exposure over the entire pediatric age range-sotalol as a model drug. AAPS J. 2014;16(2):226–39.
Jiang XL, Zhao P, Barrett JS, Lesko LJ, Schmidt S. Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacomet Syst Pharmacol. 2013;2:e80.
Emoto C, Fukuda T, Johnson TN, Adams DM, Vinks AA. Development of a pediatric physiologically based pharmacokinetic model for sirolimus: applying principles of growth and maturation in neonates and infants. CPT Pharmacomet Syst Pharmacol. 2015;4(2):e17.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45(9):931–56.
US Food and Drug Administration. Guidance for Industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. In: Center for Drug Evaluation and Research (CDER), editor. Silver Spring: US FDA; 2010. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM204959.pdf. Accessed 12 Aug 2016.
Wynalda MA, Hauer MJ, Wienkers LC. Oxidation of the novel oxazolidinone antibiotic linezolid in human liver microsomes. Drug Metab Dispos. 2000;28(9):1014–7.
Nakatani-Freshwater T, Taft DR. Renal excretion of emtricitabine I: effects of organic anion, organic cation, and nucleoside transport inhibitors on emtricitabine excretion. J Pharm Sci. 2008;97(12):5401–10.
Gutierrez F, Fulladosa X, Barril G, Domingo P. Renal tubular transporter-mediated interactions of HIV drugs: implications for patient management. AIDS Rev. 2014;16(4):199–212.
Molina JM, Cox SL. Emtricitabine: a novel nucleoside reverse transcriptase inhibitor. Drugs Today (Barc). 2005;41(4):241–52.
Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42(13):1129–40.
Leong R, Vieira ML, Zhao P, Mulugeta Y, Lee CS, Huang SM, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91(5):926–31.
Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–9.
Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455–64.
Drugs@fda. Clinical pharmacology and biopharmaceutics review for NDA 021130. FDA; 2000. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21130_Zyvox_biopharmr.pdf.
Drugs@fda. Clinical pharmacology and biopharmaceutics review on NDA 021896. FDA; 2005. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021896s000_ClinPharmR.pdf.
Gandelman K, Zhu T, Fahmi OA, Glue P, Lian K, Obach RS, et al. Unexpected effect of rifampin on the pharmacokinetics of linezolid: in silico and in vitro approaches to explain its mechanism. J Clin Pharmacol. 2011;51(2):229–36.
Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O’Grady M, et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 2003;47(9):2775–80.
Xia B, Heimbach T, Gollen R, Nanavati C, He H. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J. 2013;15(4):1012–24.
Rodgers T, Leahy D, Rowland M. Tissue distribution of basic drugs: accounting for enantiomeric, compound and regional differences amongst beta-blocking drugs in rat. J Pharm Sci. 2005;94(6):1237–48.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res. 2007;24(5):918–33.
Vieira ML, Zhao P, Berglund EG, Reynolds KS, Zhang L, Lesko LJ, et al. Predicting drug interaction potential with a physiologically based pharmacokinetic model: a case study of telithromycin, a time-dependent CYP3A inhibitor. Clin Pharmacol Ther. 2012;91(4):700–8.
Pierrat A, Gravier E, Saunders C, Caira MV, Ait-Djafer Z, Legras B, et al. Predicting GFR in children and adults: a comparison of the Cockcroft–Gault, Schwartz, and modification of diet in renal disease formulas. Kidney Int. 2003;64(4):1425–36.
T’Jollyn H, Snoeys J, Vermeulen A, Michelet R, Cuyckens F, Mannens G, et al. Physiologically based pharmacokinetic predictions of tramadol exposure throughout pediatric life: an analysis of the different clearance contributors with emphasis on CYP2D6 maturation. AAPS J. 2015;17(6):1376–87.
Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21(39):5688–98.
Abduljalil K, Jamei M, Rostami-Hodjegan A, Johnson TN. Changes in individual drug-independent system parameters during virtual paediatric pharmacokinetic trials: introducing time-varying physiology into a paediatric PBPK model. AAPS J. 2014;16(3):568–76.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–37.
Fieller EC. Some problems in interval estimation. J R Stat Soc. 1954;16(2):175–85.
Agras PI, Tarcan A, Baskin E, Cengiz N, Gurakan B, Saatci U. Acute renal failure in the neonatal period. Ren Fail. 2004;26(3):305–9.
Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009;24(2):265–74.
Yu G, Zheng QS, Li GF. Similarities and differences in gastrointestinal physiology between neonates and adults: a physiologically based pharmacokinetic modeling perspective. AAPS J. 2014;16(6):1162–6.
Acknowledgments
We thank Dr. Gerri Baer, Office of Pediatric Therapeutics, US FDA, for her critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used for this work or the preparation of the manuscript.
Disclosure
Dr Kenta Yoshida was supported in part by an appointment to the Research Participation Program at the Center for Drug Evaluation and Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the US Food and Drug Administration (FDA).
Conflict of interest
Peng Duan, Jeffery W. Fisher, Kenta Yoshida, Lei Zhang, Gilbert J. Burckart, and Jian Wang have no conflicts of interest that are directly relevant to the content of this manuscript.
Disclaimer
The contents of this manuscript reflect the views of the authors and should not be interpreted as representing the US FDA’s views or policies. No official support or endorsement by the FDA is intended or should be inferred. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the FDA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, P., Fisher, J.W., Yoshida, K. et al. Physiologically Based Pharmacokinetic Prediction of Linezolid and Emtricitabine in Neonates and Infants. Clin Pharmacokinet 56, 383–394 (2017). https://doi.org/10.1007/s40262-016-0445-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0445-9