Abstract
Background and Objectives
Midazolam rectal gel is a novel rectal formulation that may be a promising and potential alternative to oral administration for pediatric sedation. The objective of this study was to evaluate the safety, pharmacokinetics, pharmacodynamics, and absolute bioavailability of midazolam rectal gel in healthy Chinese subjects.
Methods
An open-label, single-dose, randomized, two-period, two-treatment, crossover clinical study was conducted in 22 healthy subjects (16 males and six females), each receiving 2.5 mg intravenous midazolam in one period and 5 mg midazolam rectal gel in another period (the dosages here were calculated as active midazolam). Safety, pharmacokinetic, and pharmacodynamic assessments were conducted throughout the study.
Results
All of the subjects completed both treatment periods. The formulation of rectal gel was well tolerated, with no serious adverse events occurring. After a single rectal dose of 5 mg midazolam rectal gel, it was absorbed rapidly with a median value of time to peak concentration (Tmax) of 1.00 h, and mean values of the peak concentration (Cmax) and area under the concentration–time curve (AUC0–t) of 37.2 ng/mL and 137 h·ng/mL, respectively. The absolute bioavailability of rectal gel was 59.7%. The rectal gel exhibited a relatively delayed onset but a more stable sedative effect and a longer duration when compared with intravenous midazolam.
Conclusion
Midazolam rectal gel may be a feasible alternative with a high level of acceptance in pediatric sedation and enhanced bioavailability compared to an oral formulation. The modeling results may help to disclose out the exposure-response relationship of midazolam rectal gel and support the design of an escalating-doses study and pediatric extrapolation study.
Clinical trial registration
The study was registered at http://www.chinadrugtrials.org.cn (No. CTR20192350).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first study to characterize the pharmacokinetic and pharmacodynamic properties of a novel midazolam rectal gel formulation in humans. |
The bioavailability of midazolam rectal gel was 59.7%, which was slightly higher than the reported oral bioavailability of midazolam (40–50%). |
The pharmacodynamic results indicated that midazolam rectal gel has a relatively delayed onset but a more stable sedative effect and a longer duration when compared with intravenous midazolam. |
Pharmacokinetic-pharmacodynamic modeling in adults could provide a scientific basis for the design and development of future pediatric clinical trials. |
1 Introduction
Pediatric patients during diagnosis and treatment often suffer from intractable stress, anxiety, and tension, and may require pharmacological sedation to undergo examinations such as endoscopic examination, magnetic resonance imaging (MRI) examination, and induction of anesthesia. Midazolam, the representative agent used for sedation, is a benzodiazepine with rapid onset and a short duration of action [1, 2]. Human cytochrome P450 3A4 (CYP3A4) has been identified as the major enzyme responsible for the hepatic and intestinal metabolism of midazolam [3]. The major metabolite is 1 hydroxymidazolam, which also has pharmacological activity [4, 5]. The elimination half-life (t1/2) of midazolam ranges between 1.8 and 6.4 h [6]. Midazolam acts as an agonist on gamma-aminobutyric acid (GABA) receptors, which respond to the major inhibitory neurotransmitter in the mammalian brain [7].
For pediatric sedation, midazolam is available in various dosage forms. Oral dosage forms are the most common forms, but it is also common for children to spit them out or regurgitate them because of their bitter taste and difficulty in swallowing them [8]. Despite the syrup masking the bitter taste, midazolam hydrochloride syrup contains sorbitol, which could increase the risk of gastrointestinal adverse effects [9, 10]. The intravenous route causes pain, fear, and anxiety in pediatric patients. As one alternative, the transmucosal route (e.g., intranasal, intrarectal) could reduce the first-pass effect, increase bioavailability, and improve the acceptance of pediatric patients. Although intranasal administration is relatively painless, it may cause sneezing, coughing, and drug expulsion [11]. However, the intrarectal route currently has no formal dosage forms, only intravenous midazolam given as an enema [12].
As a candidate for administration via the intrarectal route, a novel midazolam rectal gel formulation was developed by Xinjiang Tefeng Pharmaceutical Co., Ltd. (Urumqi, China). The pre-clinical safety data from rectal administration in rabbits showed low pungency for this novel formulation. Moreover, the rectal gel exhibited a rapid onset of action, rapid distribution and elimination, and high bioavailability (74.1%) in rabbits [13]. Additionally, pharmacokinetic experiments in rats suggested that the time to peak concentration (Tmax) of midazolam rectal gel was shorter than that of the oral solution (0.17 h vs. 0.31 h), and the corresponding values for the peak concentration (Cmax) and area under the concentration-time curve (AUC) were higher than those for the oral solution (Cmax: 73.48 ng/mL vs. 26.62 ng/mL, AUC: 48.68 h·ng /mL vs. 28.85 h·ng/mL) (data not published). All these pre-clinical results support the hypothesis that midazolam rectal gel may produce a better curative effect in the clinic and has a certain developmental value. However, to date, the bioavailability and pharmacokinetic properties of the novel formulation in humans after rectal administration have not yet been reported.
Therefore, we conducted this study to assess the safety, absolute bioavailability, pharmacokinetics, and pharmacodynamics of the novel midazolam rectal gel in humans. According to the guidance from the US Food and Drug Administration (FDA), the exposure-response relationship in adults should be clear before conducting clinical studies in the target pediatric population [14]. Thus, in the present study, the midazolam rectal gel was first trialed in healthy adults, and the leakage of the novel formulation after rectal administration was also estimated.
2 Methods
2.1 Subjects
The enrollment criteria for this study were healthy Chinese subjects, aged 18–45 years, with a body mass index (BMI) 19–26 kg/m2, and good health based on physical examination, measurement of vital signs, and clinical laboratory tests. Subjects were ineligible for enrollment if they had liver disorders or renal disease that might significantly alter metabolism and elimination, and were also excluded if they had used any medicines within 2 weeks or drugs that inhibit or induce hepatic metabolism within 1 month before screening. The oxygen saturation (SpO2) was to be higher than 95%. Respiratory disorder and anorectal disease were also included in the exclusion criteria. Subjects with a difficult airway were also excluded, including a limited mouth opening, restricted movement of the neck mentum and jaw, rheumatoid spondylitis, temporomandibular arthritis, and a high Mallampati score (≥ 3) [15]. Subjects had to abstain from alcohol, green tea, caffeine drinks, and grapefruit during the study.
2.2 Study Design
This was an open-label, single-dose, randomized, two-period, two-treatment crossover clinical study in healthy adults. The order in which each subject received intravenous midazolam or midazolam rectal gel was determined by a randomization table. The randomization table was generated by statisticians using SAS 9.4 software, and subjects were randomly assigned to one of the two administration sequences in a ratio of 1:1 to ensure equal arrangement of the order of the subjects. The study consisted of two phases, a pilot study and a pivotal study. The pilot study was conducted on four males, and the pivotal study included 12 males and six females. The study days were separated by a 7-day washout period in each phase. Subjects were required to fast overnight (at least 10 h) and were deprived of drinking water (at least 2 h) before administration, while standard meals (low-fat) and water intake were allowed and provided 4 h and 2 h post-dose, respectively. On the day of administration, subjects received either 5 mg midazolam rectal gel (5 g/bottle, each 1 g of gel contains 2 mg midazolam (0.2% w/w), Tefeng Pharmaceutical Co., Ltd, Xinjiang, China), or 2.5 mg intravenous midazolam (10 mg/2 mL, Roche Products Limited, Basel, Switzerland), and were allowed to get out of bed 4 h after dosing (note: the presented dosages here were calculated as active midazolam). For intravenous administration, intravenous midazolam was diluted with 0.9% sodium chloride solution to 0.25 mg/mL, and then the solution was administered via infusion pump at a constant infusion rate of 60 mL/h for 10 min. For rectal administration, subjects were requested to defecate (if possible) and clean their perianal region before dosing. The rectal tube connected to a syringe containing the drug was lubricated with paraffin oil, and then gently inserted into the anus at 6 cm. After dosing, subjects were required to contract the anus sphincter and maintain the lateral position. In order to evaluate the leakage of midazolam rectal gel, the perianal region was covered with sterile gauze fixed with medical tape for 2 h.
2.3 Safety Evaluation
All subjects were continuously observed and monitored by anesthesiologists on the day of drug administration. The bedside monitoring including ECG, blood pressure, respiration rate, and pulse oxygen saturation was performed within 2 h after administration to ensure the safety of the subjects. The safety was evaluated based on adverse events (AEs), physical examination, vital signs, 12-lead ECGs, subjects’ chief complaint, and other laboratory tests throughout the study. All AEs were recorded and reported in compliance with Good Clinical Practice (GCP). Based on the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, AEs were classified into five grades (grade 1: mild; grade 2: moderate; grade 3: severe or medically significant but not immediately life-threatening; grade 4: life-threatening consequences; grade 5: death related to AE). Subjects were to be followed up when AEs or unusual changes in clinical tests were noted.
2.4 Pharmacokinetic Evaluation and Statistics
2.4.1 Sample Collection for Pharmacokinetic Analysis
A series of venous blood samples (4 mL) were collected into tubes containing dipotassium ethylenediaminetetraacetic acid (K2EDTA) at 0 min (pre-dose), 5 min, 10 min, 15 min, 30 min, 45 min, 60 min, 75 min, 90 min, 2 h, 3 h, 4 h, 6 h, 8 h, and 12 h post-dose. Samples were centrifuged at 1500×g for 10 min (4 °C) within 1 h after collection. Plasma was separated and stored at −80 °C until analysis.
2.4.2 Bioanalytical Assay
The concentrations of midazolam and 1-hydroxymidazolam in plasma were analyzed using a validated liquid chromatography-tandem mass spectrometry assay (LC–MS/MS) method at the laboratory of Xihua Scientific Co., Ltd. (Shanghai, China). The LC–MS/MS system was conducted with a Sciex API 5000 tandem MS (Applied Biosystems, CA, USA) coupled with a Waters Acquity UPLC system (Waters, MA, USA). Samples were pre-purified by methanol, and the analytes were analyzed using midazolam-d4 and 1-hydroxymidazolam-d4 as internal standards. The calibration ranges were from 0.5 to 250 ng/mL, and 0.2 to 100 ng/mL for midazolam and 1-hydroxymidazolam, respectively. The intra-run and inter-batch precision were less than 14.2%, and accuracy was between –9.0 and 8.0%.
2.4.3 Pharmacokinetic Analysis
The pharmacokinetic parameters including Cmax, Tmax, t1/2, AUC to time infinity (AUC0–∞), and AUC from 0 h to the last measurable time point (AUC0–t) were calculated by Phoenix WinNonlin (version8.1, Certara, Co., Princeton, NJ, USA) using non-compartmental analysis. Cmax and Tmax were obtained from the observed plasma concentration-time profiles. AUC was estimated using the linear trapezoidal linear interpolation rule. AUC0–∞ was calculated as AUC0–t + Ct/λz, where Ct was the last detected concentration and λz was the slope of the log-linear regression of the terminal declining phase. The t1/2 value was calculated as ln2/λz using the best-fit model.
F (bioavailability) was calculated as (AUC0–t rectal/AUC0–t i.v.) × (dosei.v./doserectal). Rmetabolite/parent % was calculated as (AUC0–t 1-hydroxymidazolam/MW1-hydroxymidazolam)/(AUC0–t midazolam/MWmidazolam) × 100 (where MW is molecular weight). All the pharmacokinetic parameters were expressed as mean and standard deviation (SD) or median and range (Tmax).
2.5 Drug Leakage Evaluation
After rectal administration, the perianal region was gently covered with disposable medical gauze, which was fixed with medical adhesive tape. The gauze was removed 2 h after dosing and stored in a self-sealing bag at −20 °C until analysis.
The concentration of midazolam in gauze was also analyzed by the laboratory at Xihua Scientific Co., Ltd., using a validated liquid chromatography method. The gauze was repeatedly sonicated and soaked with a specific diluent solvent, and then the resulting solution was filtered and injected to determine the concentration of midazolam. The calibration range was from 0.05 to 102.67 μg/mL, and the lower limit of quantitation was 0.05 μg/mL. The leakage amount of the gel was calculated as the midazolam amount in the gauze/the dose amount·100%.
2.6 Pharmacodynamic Evaluation
Pharmacodynamic assessments were conducted at 0 (pre-dose), 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 100, 110, 120, 130, 140, 150, 160, 170, and 180 min after intravenous and rectal administration. Modified Observers Assessment of Alertness and Sedation (MOAA/S) score assessments and BIS (bispectral index) monitoring were selected for the pharmacodynamic analysis [16, 17].
The MOAA/S scale is widely used in clinical practice with regard to sedation, and is described as follows: 0 (does not respond to trapezius squeeze or electric simulation), 1 (responds to painful trapezius squeeze), 2 (purposeful response to mild prodding or shaking), 3 (responds after name called loudly or repeatedly), 4 (lethargic response to name), and 5 (responds readily to name spoken) [18]. A MOAA/S score of 3–4 has been determined to be an appropriate sedative score [19], which was also applied in our study (baseline: MOAA/S 4; threshold: MOAA/S 3). The MOAA/S recording was evaluated and recorded by the anesthesiologists.
The bispectral index (BIS) is an FDA-approved index for evaluating the level of sedation by integrating electroencephalographic descriptors into a digital signal [20]. The BIS index ranges from 0 to 100, where 0 means electroencephalographic silence and 100 means full wakefulness [21]. A BIS value between 80 and 60 has been considered medium sedation [22], and this was also applied in our study (Baseline: BIS 80; Threshold: BIS 60). The BIS monitor (BIS Vista, Medtronic; Boulder, CO, USA) conducted the BIS recording with a bilateral BIS sensor in this study.
The pharmacodynamic parameters were also calculated by Phoenix WinNonlin 8.1 using non-compartmental analysis, including maximum effect (Emax), time to reach Emax (TEmax), area under the effect curve that was below the baseline and above the effect curve (AUEC_Below_B), area under the effect curve that was below the threshold and above the effect curve (AUEC_Below_T), the total duration time when the effect was below the Baseline (Time_Below_B), and the total duration time when the effect was below the Threshold (Time_Below_T). AUEC was calculated using the linear trapezoidal linear interpolation rule. AUEC_Below_B and AUEC_Below_T can be understood as the total net drug effect with medium sedation and general anesthesia, respectively [23]. The Time_Below_B and Time_Below_T show the duration of sedation and general anesthesia, respectively. Emax and TEmax were directly obtained from the observed MOAA/S or BIS-time profiles. All pharmacodynamic parameters were expressed as mean and SD or median and range (TEmax).
2.7 Pharmacokinetic–Pharmacodynamic Evaluation
The pharmacokinetic/pharmacodynamic relationship was investigated by the sequential pharmacokinetic/pharmacodynamic modeling approach, which means developing the pharmacokinetic model first, then fixing post hoc pharmacokinetic parameters, and finally fitting the pharmacodynamic model with the predicted pharmacokinetic data and observed pharmacodynamic data [24]. The pharmacokinetic/pharmacodynamic model was built by maximum likelihood models of Phoenix NLME 8.1.
Linear one- and two-compartment structural models were assessed for pharmacokinetics. The indirect models were considered for the pharmacodynamic structural model. The inter-individual variability (IIV) was evaluated on all parameters and assumed to follow a log-normal distribution. The residual error was tested with the additive, proportional, and combined (additive and proportional) models. The potential covariates included body weight, gender, age, and administration route (intravenous or rectal). The model selection criteria were the plausibility of parameter estimate, the coefficient of variation (%CV) of parameter estimate less than 40% with the smallest Akaike’s information criteria (AIC) value.
3 Results
3.1 Demographics
Twenty-two healthy subjects (16 males and 6 females) ranging in age from 18 to 41 years (mean age, 27 years), in body weight from 51.6 to 77.5 kg (mean weight, 65.0 kg), in body height from 153 to 181 cm (mean height, 167.9 cm), and in BMI from 19.9 to 25.4 kg/m2 (mean BMI, 22.9 kg/m2) were finally included in this study.
All subjects completed the study and were enrolled in the safety, pharmacokinetic, and pharmacodynamic analysis. No significant protocol deviations occurred during the study.
3.2 Safety
The midazolam rectal gel and intravenous midazolam appeared to be well tolerated throughout the study with no serious AEs occurring. For midazolam rectal gel, two subjects experienced four AEs (incidence rate 9.09%), including elevated levels of triglyceride and uric acid, diarrhea, and hypotension. For intravenous midazolam, two subjects experienced two AEs (incidence rate 9.09%), including urine occult blood positive and hypotension. Among the AEs, the hypotension included in the adverse reactions of intravenous midazolam (Hypnovel®) labeling occurred in both treatments and was considered to be drug related. All AEs were grade 1 (mild), and subjects recovered without any medical treatment by the end of the study. No unexpected adverse reactions were observed in the study. Mild sedation was noted in all subjects after drug administration.
3.3 Pharmacokinetic Results
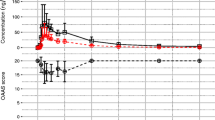
The mean plasma concentration-time curves of midazolam and 1-hydroxymidazolam after administration of 5 mg midazolam rectal gel or 2.5 mg intravenous midazolam are depicted in Fig. 1. The corresponding pharmacokinetic parameters are presented in Table 1. The mean Cmax and AUC0–t of midazolam for 5 mg treatment of midazolam rectal gel were 37.2 ng/mL and 137 h·ng/mL, respectively. The corresponding parameters for 2.5 mg treatment of intravenous midazolam were 120 ng/mL and 115 h·ng/mL, respectively. Furthermore, drug leakage after rectal administration was less than 0.01%, which was negligible. Thus, the mean absolute bioavailability for midazolam rectal gel was calculated as 59.7%. Additionally, the mean terminal elimination t1/2 for midazolam rectal gel was 3.45 h, which was consistent with intravenous midazolam (3.58 h).
Mean (SD) plasma concentration-time curves of midazolam (a linear ordinates, b logarithmic ordinates) and 1-hydroxymidazolam (c linear ordinates, d logarithmic ordinates) after intravenous (IV) administration of 2.5 mg midazolam (purple) or rectal administration of 5 mg midazolam rectal gel (yellow)
For the metabolite of 1-hydroxymidazolam, the mean Cmax and AUC0–t values for midazolam rectal gel were 9.81 ng/mL and 35.5 h·ng/mL, respectively. The corresponding parameters for intravenous midazolam were 4.30 ng/mL and 14.6 h·ng/mL, respectively. The ratio of the AUC of metabolite to that of parent (Rmetabolite/parent) for midazolam rectal gel was 25.5%, which was higher than intravenous midazolam (12.2%).
3.4 Pharmacodynamic Effects
The mean BIS and MOAA/S value-time curves after administration of 5 mg midazolam rectal gel or 2.5 mg intravenous midazolam are shown in Fig. 2. The main pharmacodynamic parameters are presented in Table 2. After rectal administration, the effect onset time was relatively delayed compared with that of intravenous administration, but the duration of the effect was longer (Fig. 2). For BIS monitoring analysis, the mean maximum effect (Emax) of midazolam rectal gel was 65.7, which was similar to that of intravenous midazolam. The TEmax (0.96 h) of rectal gel was slightly later than that of the injection (0.67 h). It has been reported that a BIS value between 80 and 60 is considered to be moderate sedation [22]. Therefore, when calculating the pharmacodynamic parameters using Drug Effect of Non-Compartmental Analysis in Phoenix WinNonlin 8.1, BIS values of 80 and 60 were set as “Baseline” and “Threshold,” respectively. The AUEC_Below_B and Time_Below_B values for the rectal gel were 7.65 and 1.09, respectively, which were a little higher and longer than for the injection (5.71 and 0.82 h). Additionally, the AUEC_Below_T and Time_Below_T values for the rectal gel were similar to that for the injection (0.21 vs. 0.21, 0.05 h vs. 0.03 h). Moreover, as shown in Table 3, the pharmacodynamic results of MOAA/S were roughly similar to those with BIS monitoring.
3.5 Pharmacokinetic–Pharmacodynamic Relationship
A two-compartment structure with linear elimination, IIV on volume of central compartment (Vc), clearance (CL), and volume of the peripheral compartment (Vp), proportional residual error, body weight on V, and administration route on CL, Vp and intercompartmental clearance (Q) was investigated to describe midazolam pharmacokinetic data well (see Eqs. 1–3). An effect compartment was used to link pharmacokinetic part and pharmacodynamic part (see Eq. 4). The sigmoid Emax model with IIV on Emax and half maximal effective concentration (EC50) and proportional residual error accurately described the relationship between the drug concentration in effective compartment and BIS (see Eq. 5).
The parameters were well estimated with CV% < 35% (see Table 3). The goodness-of-fit plots (in Fig. 3), the concordance plots, and the residual plots [see Figs. S1 and S2 in the Online Supplemental Material (OSM)] presented good agreement between the observed and predicted data. The visual predicted plots were used for validation (see Fig. 4), which indicated the final model is robust and stable. The key steps for building a pharmacokinetic/pharmacodynamic model are shown in Table S1 (OSM). The pharmacokinetic/pharmacodynamic model equations are shown in Eqs. (1–5):
where \({A}_{c}\) represents the drug amount in the central compartment, \({A}_{p}\) represents the drug amount in the peripheral compartment, \({A}_{a}\) represents the rectal dose, \({A}_{1}\) represents the intravenous dose, \({C}_{c}\) represents plasma concentration, \({c}_{e}\) represents drug concentration in the effect compartment, \({K}_{a}\) represents absorption rate constant, \({K}_{e0}\) represents equilibration rate constant,\(CL\) represents clearance, \(Q\) represents intercompartmental clearance, \({V}_{c}\) represents volume of central compartment, \({V}_{p}\) represents volume of the peripheral compartment, \(E\) represents the BIS value, \({E}_{0}\) represents the baseline of the BIS value, \({E}_{max}\) represents the minimal BIS value (maximum drug effect), \(EC50\) represents half maximal effective concentration, and hill represents the shape factor.
4 Discussion
Midazolam rectal gel is designed for pediatric sedation, and rectal drug delivery aims to improve adherence in pediatric patients. Currently, diazepam is the only rectally administered FDA-approved benzodiazepine for pediatric patients [25]. But pediatric patients treated with rectal diazepam have a risk of respiratory depression [26]. Additionally, rectal chloral hydrate and rectal thiopental can cause irritation in the pediatric population [27, 28]. Pentobarbital hydrogel for rectal administration is in the development pipeline, but the stability of a rectal drug formulation has limited its application in pediatric procedural sedation [29]. Hence, a safe, effective, and child-friendly sedation is urgently required. The aim of this prospective study was to evaluate the safety, pharmacokinetics, pharmacodynamics, and absolute bioavailability of a novel rectal gel formulation of midazolam in healthy Chinese subjects. The results of our study may help to figure out the exposure-response relationship of midazolam rectal gel and support pediatric extrapolation studies or the design of clinical studies in pediatric patients.
Rectal administration of a single dose of midazolam rectal gel was well tolerated and safe in healthy Chinese subjects. The rectal gel is easy to administer and causes minimal discomfort. This means midazolam rectal gel may be a suitable product for the pediatric population.
After a single rectal dose of 5 mg midazolam rectal gel, midazolam was absorbed rapidly with a median Tmax of 1.00 h, which was slightly slower than the oral administration of midazolam (Tmax: 30 min) [30]. The mean Cmax (37.2 ng/mL) of midazolam rectal gel was significantly lower than that (120 ng/mL) of intravenous midazolam, while the AUC0–t (137 h·ng/mL) value of midazolam rectal gel was higher than that of intravenous midazolam (115 h·ng/mL) and oral midazolam tablets (119 h·ng/mL, 7.5 mg) [30]. Furthermore, the mean AUC0–t and the t1/2 values after intravenous administration in our study were similar to those (109.2 h·ng/mL, 4.03 h) reported in the literature [31]. The determination results of drug leakage evaluation indicated that the drug leakage after rectal administration was negligible. Therefore, the mean absolute bioavailability for midazolam rectal gel was calculated as 59.7%, which is slightly higher than the oral bioavailability of midazolam (40–50%) [32]. From a physiological point of view, the rectal gel was inserted 6 cm into the rectum in our clinical study, meaning that the bulk of midazolam would be delivered to the superior rectal vein, and then delivered to the portal vein, and subsequently into the liver. Only a small amount of midazolam would avoid hepatic first-pass elimination by transportation of the inferior rectal vein [33]. The mean AUC0–t (35.5 h·ng/mL) and Cmax (9.81 ng/mL) values of 1-hydroxymidazolam after rectal administration were higher than those (14.6 h·ng/mL, 4.30 ng/mL) of 1-hydroxymidazolam after intravenous administration. After oral administration of the 7.5 mg midazolam tablet, the mean AUC0-t and Cmax values of 1-hydroxymidazolam were 56.0 h·ng/mL and 39.0 ng/mL, respectively [30]. Comparing the Rmetabolite/parent values of the 2.5 mg treatment of intravenous midazolam (12.2%), 5 mg treatment of midazolam rectal gel (25.5%), and 7.5 mg treatment of midazolam tablet (44.8%), it can be inferred that the first-pass effect of oral administration was slightly higher than that of rectal administration. Moreover, the improved bioavailability for midazolam rectal gel was consistent with our previous study [34], in which we found that 9.9% midazolam rectal gel could avoid hepatic first-pass metabolism. The pharmacokinetic results indicated that midazolam rectal gel might be more modest and safer than intravenous midazolam, which is especially important for the pediatric population.
The BIS value and MOAA/S score were recorded and applied to assess the sedation of subjects. Compared with intravenous administration, the effect onset time of rectal administration was relatively delayed (BIS: 0.96 h vs. 0.67 h; MOAA/S: 0.75 h vs. 0.33 h), but the effect was more stable, and the duration was longer. The TEmax values of BIS and MOAA/S for midazolam rectal gel were consistent with the time to reach Cmax of plasma midazolam. The mean Emax of midazolam rectal gel was similar to that of intravenous midazolam (BIS: 65.7 vs. 68.0; MOAA/S: 2.91 vs. 3.05). The sedative effects were similar after rectal and intravenous administration. BIS values were < 90 in 100% of the subjects after rectal and intravenous administration; < 70 in 68.2% of the subjects after rectal administration and in 54.6% of the subjects after intravenous administration; < 60 and > 40 in 31.8% of the subjects after rectal administration and in 27.3% of the subjects after intravenous administration. For MOAA/S analysis, MOAA/S values were ≤ 4 in 100% of the subjects after rectal and intravenous administration; ≤ 3 in 81.1% of the subjects after rectal administration and in 59.1% of the subjects after intravenous administration; ≤ 2 in 36.4% of the subjects after rectal administration and in 40.9% of the subjects after intravenous administration. Based on the bioavailability of midazolam rectal gel (59.7%), the system exposure of 5 mg midazolam rectal gel was about 2.99 mg, which is higher than 2.5 mg intravenous midazolam. The calculated AUEC_Below_B and Time_Below_B (the effective duration of sedation) values for BIS after rectal administration were 7.65 and 1.09, respectively, which were a little higher and longer than intravenous administration (5.71 and 0.82 h). Moreover, the AUEC_Below_T and Time_Below_T values for BIS after rectal administration were similar to those for intravenous administration (0.21 vs. 0.21, 0.05 h vs. 0.03 h), suggesting that higher efficacy and safety might be achieved with midazolam rectal gel. As shown in Table 2, the pharmacodynamic parameters of MOAA/S were roughly similar to those of BIS, and the results indicate that compared with intravenous administration, rectal administration has a relatively delayed onset but a more stable sedative effect and a longer duration.
The BIS index is subjective and continuous, which is appropriate to establish the exposure-response relationship with plasma midazolam concentrations [35]. As mentioned in the FDA guidance, a known exposure-response relationship can be used to support different routes of administration [36]. Our study integrated the concentrations of midazolam-BIS profiles for intravenous and rectal administration to develop the pharmacodynamic model. Considering the time course of pharmacokinetics and pharmacodynamics, the sequential pharmacokinetic/pharmacodynamic modeling approach was used in our study. The final pharmacokinetic/pharmacodynamic model was a two-compartment pharmacokinetic model with effect compartment and sigmoid Emax pharmacodynamic model. The “elimination phase” and “rapidly distributed phase” were shown in the intravenous pharmacokinetic profile, which indicated a two-compartment model as the structural model. However, it is hard to find biphasic distribution from the rectal pharmacokinetic profile. The potential reason is that the absorption phase could make a “rapidly distributed phase” indistinct. So, the administration route could be the covariate on CL, Vp, and Q. Body weight was taken as the covariate on Vc, which could be interpreted through the high lipid solubility of midazolam [32]. The effect compartment was added in our pharmacokinetic-pharmacodynamic model to describe the transport of midazolam from the plasma to the brain. The indications for midazolam rectal gel are for the pediatric population. Based on the completed pharmacokinetic/pharmacodynamic data of rectal administration in adults, population pharmacokinetic or physiological-based pharmacokinetic/pharmacodynamic models should be developed to simulate the blood concentration-effect time curve of midazolam rectal gel in a pediatric population with different dosages and different age groups, so as to provide reference for pediatric dosage selection. When extrapolating this pharmacokinetic/pharmacodynamic model to a pediatric population, the following points should be paid attention to when performing pediatric extrapolation. Firstly, the estimated Emax was 15.6, which means the lowest BIS value can reach 15.6 (deep hypnotic state) [37]. However, some physiological changes would result in differences in pharmacokinetic-pharmacodynamic behaviors in the pediatric population. Benzodiazepines that act on the GABAA receptor are the mainstay of treatment for neonatal epileptic seizures. However, neonates have fewer GABAα1 subunits and more GABAα2/3 subunits than adults, which makes neonates less responsive to benzodiazepines [38]. Secondly, the EC50 was 22.06 ng/mL, which is the total plasma concentration of midazolam, since only the free drug is pharmacologically active. Therefore, the age-related differences in the unbound fraction of plasma (fup) of midazolam should be taken into consideration when pediatric extrapolation is conducted [39]. Furthermore, the development of metabolizing enzymes could affect pediatric pharmacokinetics. CYP3A4 was identified as the major enzyme responsible for the metabolism of midazolam. The development of CYP3A4 is age-related; CYP3A4 shows low activity in fetuses and reaches adult values at about 1 year of age [40].
5 Conclusions
In conclusion, as the first study to evaluate the safety, pharmacokinetics, and pharmacodynamics of a novel rectal gel formulation of midazolam, the absolute bioavailability of midazolam rectal gel was calculated as 59.7%. which is slightly higher than the oral bioavailability of midazolam. The pharmacodynamic results indicated that rectal administration of midazolam rectal gel has a relatively delayed onset but a more stable sedative effect and a longer duration when compared with intravenous administration of midazolam. Midazolam rectal gel was well tolerated and had an acceptable safety profile in healthy subjects. Therefore, midazolam rectal gel may be a feasible and safe alternative for pediatric patients requiring sedation. A phase I clinical study would support a future study design with escalating doses and pediatric extrapolation.
References
Pecking M, Montestruc F, Marquet P, Wodey E, Homery M-C, Dostert P. Absolute bioavailability of midazolam after subcutaneous administration to healthy volunteers. Br J Clin Pharmacol. 2002;54(4):357–62.
Conway A, Rolley J, Sutherland JR. Midazolam for sedation before procedures. Cochrane Database Syst Rev. 2016;2016(5):CD009491.
Kabi F. Midazolam injection, USP Rx only. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208878Orig1s000lbl.pdf. Accessed 3 May 2023.
Balk M, Hentschke H, Rudolph U, Antkowiak B, Drexler B. Differential depression of neuronal network activity by midazolam and its main metabolite 1-hydroxymidazolam in cultured neocortical slices. Sci Rep. 2017;7(1):3503.
Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther. 1992;51(6):715–28.
Singh SK, Dixit T. Pharmacogenomics in anesthesia. In: Handbook of pharmacogenomics and stratified medicine. Elsevier Inc.; 2014. p. 815–33. https://doi.org/10.1016/B978-0-12-386882-4.00035-9.
Sigel E, Steinmann ME. Structure, function, and modulation of GABAA receptors. J Biol Chem. 2012;287:40224–31.
Salman S, Tang EKY, Cheung LC, Nguyen MN, Sommerfield D, Slevin L, et al. A novel, palatable paediatric oral formulation of midazolam: pharmacokinetics, tolerability, efficacy and safety. Anaesthesia. 2018;73:1469–77.
Geiger CM, Sorenson B, Whaley PA. Stability of midazolam in syrspend SF and syrspend SF cherry. Int J Pharm Compd. 2013;17(4):344–6.
Johnston KR, Govel LA, Andritz MH. Gastrointestinal effects of sorbitol as an additive in liquid medications. Am J Med. 1994;97(2):185–91.
Nelson T, Xu Z. Pediatric dental sedation: challenges and opportunities. Clin Cosmet Investig Dent. 2015;7:97–106.
Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37(3):267–72.
Duan YX, Li Q, Zheng AP, Zhu XW. Pharmacokinetics and the absolute bioavailability of midazolam rectal gel in rabbits. J Int Pharm Res. 2013;40(3):338–43.
Administration USF and D. Draft guidance for industry—general considerations for pediatric studies for drugs and biological products. 2014. https://www.fda.gov/media/90358/download. Accessed 05 May 2023.
Thorpy MJ, Ahmed I. Chapter 2—Approach to the patient with a sleep disorder. In: Barkoukis TJ, Matheson JK, Ferber R, Doghramji KBT-T in SM, editors. Philadelphia: W.B. Saunders; 2012. p. 10–27. https://www.sciencedirect.com/science/article/pii/B9781437717037100027.
Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–51.
Bower AL, Ripepi A, Dilger J, Boparai N, Brody FJ, Ponsky JL. Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc. 2000;52(2):192–6.
Kim TK, Niklewski PJ, Martin JF, Obara S, Egan TD. Enhancing a sedation score to include truly noxious stimulation: the Extended Observer’s Assessment of Alertness and Sedation (EOAA/S). BJA Br J Anaesth. 2015;115(4):569–77.
Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, et al. Safety and efficacy of Remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–46.
Litman RS, Cohen DE, Sclabassi RJ. Chapter 9—Pediatric anesthesia equipment and monitoring. In: Motoyama EK, Davis PJ, editors. Smith’s anesthesia for infants child. 7th Ed. Philadelphia: Mosby; 2006. p. 272–318. https://www.sciencedirect.com/science/article/pii/B978032302647550014X
Agrawal D, Feldman HA, Krauss B, Waltzman ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004;43(2):247–55.
Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94(1):125–9.
Krzyzanski W, Jusko WJ. Application of moment analysis to the sigmoid effect model for drug administered intravenously. Pharm Res. 1997;14(7):949–52.
Mukashyaka MC, Wu C-L, Ha K, Zhang J, Wood J, Foley S, et al. Pharmacokinetic/pharmacodynamic modeling of a cell-penetrating peptide phosphorodiamidate morpholino oligomer in mdx mice. Pharm Res. 2021;38(10):1731–45.
Nickels KC. Less effective and more expensive: is it time to move on from rectal diazepam? Epilepsy Curr. 2018;18(1):27–8.
Dieckmann RA. Rectal diazepam for prehospital pediatric status epilepticus. Ann Emerg Med. 1994;23(2):216–24.
Nie Q, Hui P, Ding H, Wang Z. Rectal chloral hydrate sedation for computed tomography in young children with head trauma. Medicine (Baltimore). 2021;100(9): e25033.
Lam SHF, Li DR, Hong CE, Vilke GM. Systematic review: rectal administration of medications for pediatric procedural sedation. J Emerg Med. 2018;55(1):51–63.
Belo S, Touchard J, Secretan P-H, Vidal F, Boudy V, Cisternino S, et al. Stability of pentobarbital hydrogel for rectal administration in pediatric procedural sedation. Hosp Pharm. 2021;56(4):332–7.
Shao F, Zhang H, Xie L, Chen J, Zhou S, Zhang J, et al. Pharmacokinetics of ginkgolides A, B and K after single and multiple intravenous infusions and their interactions with midazolam in healthy Chinese male subjects. Eur J Clin Pharmacol. 2017;73(5):537–46.
Wermeling DP, Record KA, Archer SM, Rudy AC. A pharmacokinetic and pharmacodynamic study, in healthy volunteers, of a rapidly absorbed intranasal midazolam formulation. Epilepsy Res. 2009;83(2–3):124–32.
Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997;15(3):357–65.
de Boer AG, Moolenaar F, de Leede LGJ, Breimer DD. Rectal drug administration: clinical pharmacokinetic considerations. Clin Pharmacokinet. 1982;7(4):285–311.
Zhu J, Zhao Y, Wang L, Zhou C, Zhou S, Chen T, et al. Physiologically based pharmacokinetic/pharmacodynamic modeling to evaluate the absorption of midazolam rectal gel. Eur J Pharm Sci. 2021;167:106006.
Peeters MYM, Prins SA, Knibbe CAJ, Dejongh J, Mathôt RAA, Warris C, et al. Pharmacokinetics and pharmacodynamics of midazolam and metabolites in nonventilated infants after craniofacial surgery. Anesthesiology. 2006;105(6):1135–46.
FDA. Guidance for industry: exposure–response relationships—study design, data analysis and regulatory applications. FDA Guidance. 2003. https://www.fda.gov/media/71277/download. Accessed 05 May 2023.
Mathur S, Patel J, Goldstein S et al. Bispectral index. Treasure Isl. StatPearls Publishing. 2022. https://www.ncbi.nlm.nih.gov/books/NBK539809/. Accessed 05 May 2023.
Chudomel O, Herman H, Nair K, Moshé SL, Galanopoulou AS. Age- and gender-related differences in GABAA receptor-mediated postsynaptic currents in GABAergic neurons of the substantia nigra reticulata in the rat. Neuroscience. 2009;163(1):155–67.
Ignjatovic V, Lai C, Summerhayes R, Mathesius U, Tawfilis S, Perugini MA, et al. Age-related differences in plasma proteins: how plasma proteins change from neonates to adults. PLoS ONE. 2011;6(2): e17213.
van Groen BD, Nicolaï J, Kuik AC, Van Cruchten S, van Peer E, Smits A, et al. Ontogeny of hepatic transporters and drug-metabolizing enzymes in humans and in nonclinical species. Pharmacol Rev. 2021;73(2):597–678.
Acknowledgements
The authors would like to thank the healthy subjects involved in the study and their families.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Sufeng Zhou, Jinying Zhu, Xiaodi Sun, Lijun Xie, Yuqing Zhao, Sijia Ding, Lu Wang, Juan Chen, Bei Zhu, Chen Zhou, and Feng Shao are employees of the First Affiliated Hospital with Nanjing Medical University. Aiping Zheng is an employee of the Institute of Pharmacology and Toxicology of Academy of Military Medical Sciences. Yajuan Li is an employee of Xinjiang Tefeng Pharmaceutical Company, Ltd. The authors report no other conflicts of interest.
Funding
This study was funded by Xinjiang Tefeng Pharmaceutical Company, Ltd.
Availability of data and material
The data are not available in a repository, but reasonable requests can be directed to Feng Shao at jsphshaofeng@hotmail.com.
Ethical approval
This study was conducted according to Good Clinical Practice (GCP) following the tenets of the Declaration of Helsinki. The study was registered at chinadrugtrials.org.cn (CTR20192350). The protocol and informed consent form were approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (Nanjing, China).
Informed consent
All subjects provided written informed consent before any study-related procedures were conducted.
Consent for publication
Not applicable.
Code availability
Not applicable
Author contributions
Conceptualization: FS, CZ, SZ. Methodology, material preparation and data collection: SZ, XS, LX, SD, LW, JC, BZ, AZ. Data analysis: JZ. Writing-original draft preparation: JZ. Writing-review and editing: SZ, JZ. Resources: YL. Supervision: FS. All authors approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, S., Zhu, J., Sun, X. et al. Safety, Pharmacokinetics, and Pharmacodynamics of Midazolam Gel After Rectal Administration in Healthy Chinese Subjects. Clin Drug Investig 43, 421–433 (2023). https://doi.org/10.1007/s40261-023-01276-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01276-5